Abstract
Background and Aims
To review published studies on the effectiveness of combining cognitive-behavioural therapy (CBT) and motivational interviewing (MI) to treat comorbid clinical and subclinical alcohol use disorder (AUD) and major depression (MDD) and estimate the effect of this compared with usual care.
Methods
We conducted systematic literature searches in PubMed, PsycINFO and Embase up to June 2013 and identified additional studies through cross-references in included studies and systematic reviews. Twelve studies comprising 1721 patients met our inclusion criteria. The studies had sufficient statistical power to detect small effect sizes.
Results
CBT/MI proved effective for treating subclinical and clinical AUD and MDD compared with controls, with small overall effect sizes at post-treatment [g = 0.17, confidence interval (CI) = 0.07–0.28, P < 0.001 for decrease of alcohol consumption and g = 0.27, CI: 0.13–0.41, P < 0.001 for decrease of symptoms of depression, respectively]. Subgroup analyses revealed no significant differences for both AUD and MDD. However, digital interventions showed a higher effect size for depression than face-to-face interventions (g = 0.73 and g = 0.23, respectively, P = 0.030).
Conclusions
Combined cognitive-behavioural therapy and motivational interviewing for clinical or subclinical depressive and alcohol use disorders has a small but clinically significant effect in treatment outcomes compared with treatment as usual.
Keywords: Alcohol use disorders, cognitive-behavioural therapy, comorbidity, major depression, meta-analysis, motivational interviewing, randomized controlled trials, treatment effect
Introduction
Alcohol use disorders (AUD) often co-occur with major depressive disorder (MDD), both in treatment and in general populations 1,2. Among AUD treatment populations, comorbid depression can mount to 50% 3. Similarly, MDD treatment populations have up to 40% life-time probability of developing AUD 4,5. Co-occurrence of AUD and MDD results in even greater disease burdens than the separate disorders 6. Some of the burdens experienced by people with comorbid AUD and MDD are high morbidity and mortality levels, functional impairment and increased suicide risk 7. Not surprisingly, the costs to society are substantial, owing to high levels of health-care consumption, inadequate treatment outcomes, high work absenteeism and lost productivity 8,9.
Combined treatment of comorbid AUD and MDD could hence be vitally important from a clinical and a public health viewpoint 10. Combined treatment has never been common clinical practice 11. The comorbid disorder was either not recognized or was not treated, under the assumption that it would resolve once the primary disorder was treated 12. Today, a growing number of combined treatments for comorbid AUD and MDD are available; these include psychotherapeutic treatments either as an adjunct to treatment as usual (TAU) or as an alternative to it 7,13,14.
Studies that have evaluated the impact of various psychotherapies—including cognitive-behavioural therapy (CBT 15), Twelve-Step facilitation (TSF 16) and motivational interviewing (MI 17)—on MDD or on AUD alone have found them effective. These therapies have also proved effective for subclinically depressed populations 18 and for populations that do not fulfil the DSM-IV criteria 19 for AUD but still experience problems with alcohol, such as those drinking beyond guidelines for low-risk drinking 20–22. Integrated treatment approaches are often based on components of these CBT and/or MI interventions 23, mainly with a focus on both depression and alcohol, and with or without pharmacological intervention 14. Brown and colleagues 24, for example, evaluated the effectiveness of a treatment intervention made up of components of the evidence-based CBT course Coping with Depression 25 and the cognitive-behavioural alcohol skills training components identified by Project Match 16.
Studies on the effectiveness of psychotherapy for people with comorbid AUD and MDD have shown promising results. A first indication of effectiveness came indirectly from the seminal meta-analysis by Nunes & Levin 26. Their review of 14 studies assessed the impact of antidepressant medication in the treatment of comorbid MDD and substance use disorders (SUD) compared to placebo controls, with or without adjunct psychotherapies (some of which were manual-guided CBT). Antidepressant medication appeared effective for depression [d = 0.38; confidence interval (CI) = 18–58] and for alcohol reduction (d = 0.25; CI = 0.08–0.42). Subgroup analyses revealed that studies adding psychotherapeutic interventions in both experimental and control groups, such as the study by Roy-Byrne and colleagues in 2000 27, showed a higher placebo response in the control groups than the set of studies without added psychotherapies, such as the study by Altamura and colleagues in 1990 28. The higher placebo response in the study by Roy-Byrne et al. and thus a lower between-group effect size was hence potentially explained by the psychotherapeutic interventions. Hides and colleagues 29 included 12 studies in their review, eight of which focused on comorbid alcohol and depression and four on depression and SUD. They found positive results for the effectiveness of psychotherapies, including CBT (either alone 30 or in combination with antidepressant medication 31). They concluded, however, that the evidence was not yet strong enough, due to the small numbers of studies they had for their review, the diversity among them and the low methodological qualities of some. Such diversity was also seen in the systematic review by Baker and colleagues 32. The Randall et al. study 33, for instance, evaluated the effectiveness of a CBT procedure focused on alcohol only in comparison with a combined CBT alcohol and social phobia treatment. Markowitz et al. 34 focused on the effectiveness of interpersonal psychotherapy for populations with comorbid AUD and dysthymia.
The results of these reviews suggest some preliminary evidence that psychological interventions (in particular CBT/MI) may be effective for treating co-occurring clinical or subclinical MDD and AUD. As no meta-analysis was yet available, we performed a review to gain evidence on the effectiveness of CBT/MI for treating such comorbid conditions. On the basis of the existing literature, we also expected that depression improvement would mediate an effect of CBT on alcohol improvement and vice versa 14,26. We therefore also examined associations between depression and alcohol effect sizes.
To the best of our knowledge, this is the first meta-analysis of the impact of CBT/MI on the treatment of comorbid depression and AUD.
Method
Identification and selection of studies
We used a database of 1344 studies on the psychological treatment of depression. Details of this database have been described elsewhere 35. It has been used in more than 30 published meta-analyses (see also http://www.evidencebasedpsychotherapies.org). It is updated continuously through comprehensive literature searches (from 1966 to January 2013). We examined a total of 13 407 abstracts in Pubmed (3320 abstracts), PsycInfo (2710), Embase (4389) and the Cochrane Central Register of Controlled Trials (2988). The abstracts were identified by combining terms indicative of psychological treatment and depression (both MeSH terms and text words). Primary studies from 42 meta-analyses of psychological treatment for depression were also examined for our database to ensure that no published studies were missed. From the 13 407 abstracts (9860 after removal of duplicates), 1344 full-text papers were retrieved for possible inclusion in the database. From these [which included 351 randomized controlled trials (RCTs)], we selected those papers that were suitable for our meta-analysis (see Fig. 1). We also identified studies on the basis of cross-references in these studies and references found in additional systematic reviews. We included studies that examined effects of CBT/MI on alcohol use (AUD, abuse or dependence) as assessed by diagnostic interviewing or by screening for scores above a cut-off point on a self-report alcohol measure (such as those based on guidelines for low-risk drinking; see Table 1). We followed a similar procedure for the assessment of depression. We did not apply any age or language restrictions. Our original search concentrated on randomized controlled trials focused on comorbid alcohol and depression. However, in due course it emerged that the number of RCTs in that domain was still limited and that a number of high-quality non-randomized studies were available. We therefore decided to include the latter in the meta-analysis. We included only randomized and non-randomized controlled studies in which (i) CBT/MI was compared with TAU or (ii) CBT/MI was compared with another psychological treatment. Comparative controlled studies were included only if allocation was not influenced by individual patients, therapists or researchers.
Figure 1.
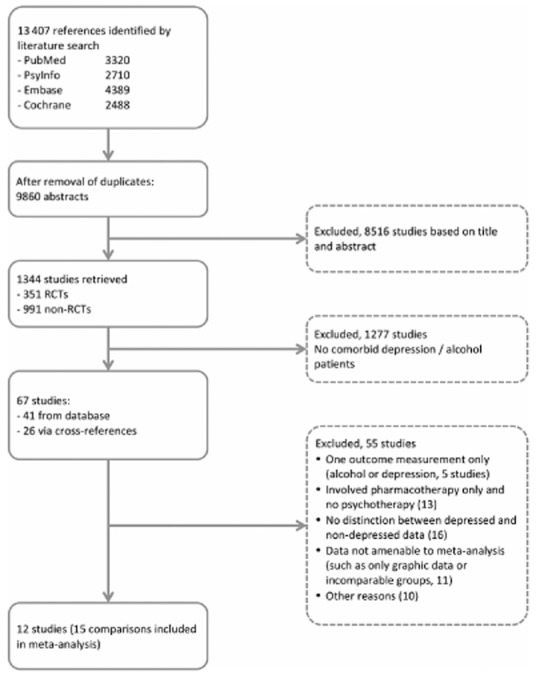
Flow-chart of study inclusion
Table 1.
Characteristics of included studies comparing cognitive-behavioural therapy/motivational interviewing (CBT/MI) plus treatment as usual (TAU) with TAU alone, or brief other psychological treatments
| Author, year, country | Target group | Recruitment source | Inclusion criteria | Conditions | Intensity of treatment | n (% male) | Symptoms of depression outcome measures | Alcohol consumption outcome measures | Post-treatment (pt) and follow-up (fu) assessments | Study attrition (%) | Methodological qualities |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agyapong 2012, 2013 57,76 Ireland | Adults 18+ who completed in-patient double diagnosis programme | Clinic | 1. DSM-IV MDD 2. DSM-IV AUD 3. MMSE ≥ 24 | 1. ICBT/MI mobile text messages + TAU 2. Placebo mobile text messages + TAU | 1. 2 per day, 3 months 2. 2 per month, 3 months | 1. 26 2. 28 (46%) | BDI-II | 1. No. of days abstinent 2. No. of days to first drink 3. No. of drinks per drinking day 4. No. of patients abstinent | pt: 3 m fu: 6 m | pt: 7.4% fu:11% | 1. Yes 2. Yes 3. Yes 4. Yes |
| Baker 2010 30 Australia | Young adults ≥16 | Media and referrals by health professionals | • BDI-II ≥ 17 • m ≥ 4/f ≥ 2 drinks per day in past month | 1. CBT/MI-D 2. CBT/MI-A 3. iCBT/MI 4. Brief (control) | 1. 9 × 1 hour 2. 9 × 1 hour 3. 9 × 1 hour 4. 1.5 hours | 1. 71 2. 68 3. 75 4. 70 (53%) | BDI-II | 1. Mean drinks p.w. 2. Mean drinking days p.w. 3. Max. no. of drinks per day 4. Mean drinks per day | pt: 4.5 m | pt: 16 | 1. Yes 2. Yes 3. Yes 4. Yes |
| Battersby 2013 77 Australia | Adults 18+, out-patients (Vietnam veterans) | Referrals by health-care settings and media | • AUDIT ≥ 8 • Chronic condition (mental or physical) | 1. CBT/MI + TAU TAU | 6 × 2.5 hour –gr self-care manual | 1. 46 2. 31 (100%) | HADS | 1. AUDIT | pt: 9 m | pt: 6% | 1. Yes 2. Yes 3. Yes 4. Yes |
| Brown 1997 31 USA | Out-patients in substance use hospital programme (age range 27–58 years) | Clinic | • DSM-III-R alcohol dependence • BDI ≥ 10 | 1. CBT-D + TAU 2. Placebo control + TAU | 1. 8 × 45 m 2. 8 × 45 m | 1. 19 2. 16 (71%) | BDI MHAM-D POMS | 1. % of days abstinent 2. Drinks per drinking day 3. % totally abstinent 4. % drinking heavily | pt: 3 m fu: 6 m | pt: 3 fu: 8.6 | 1. No 2. n.i. 3. No 4. No |
| Brown 2006 24 USA | Out-patient veterans in double diagnosis programme (age range 31–68) | Clinic | • DSM-IV alcohol, cannabis and/or stimulant dependence • DSM-IV MDD | 1. iCBT/MI (gr) + TAU 2. TSF (gr) + TAU | 1. 36 × 1 hour 2. 36 × 1 hour | 1. 48 2. 42 (92%) | HAM-D-21 | 1. % of days abstinent | pt: 6 m fu: 9, 12 m | pt: 26.7 | 1. Yes 2. No 3. No 4. No |
| Brown 2011 78 USA | Out-patients aged 18–65 years in substance use programme | Clinic | • DSM-IV alcohol dependence • BDI ≥ 15 | 1. CBT-D + TAU 2. Placebo control + TAU | 1. 8 × 45 m 2. 8 × 45 m | 1. 83 2. 83 (67%) | BDI HAM-D | 1. % of days abstinent 2. Drinks per drinking day | pt: 1.5 m fu: 3, 6, 12 m | pt: 14 fu 3: 7 fu 6: 5 fu 12: 7 | 1. Yes 2. Yes 3. Yes 4. Yes |
| Hides 2011 58 Australia | Out-patients aged 16–25 in substance use service | Clinic | • K10 ≥ 17 • Weekly AOD use above recommended level in past month | 1. ICBT/MI + TAU 2. TAU | 1. 12 sessions | 1. 60 2. 28 (63%) | CES-D HAM-D-17 K 10 | 1. Days of use per month 2. Mean drinks p.m. 3. Mean drinks per drinking day | pt: 3 m fu: 6 m | pt: 24 fu: 24 | 1. No 2. No 3. Yes 4. No |
| Hunter 2012 59 USA | Out-patients aged 18+ in substance use programme | Clinic | • BDI >13 • One or more substance use disorders (AUDIT-C or DAST) | 1. ICBT/MI (gr) + TAU 2. TAU | 1. 18 × 2 hours | 1. 47 2. 26 (52%) | BDI-II | 1. Drinks per drinking day | pt: 3 m fu: 6 m | pt: 12.3 fu: 5.5 | 1. Yes 2. Yes 3. Yes 4. Yes |
| Kay-Lambkin 2009 56 Australia | Adults ≥17 | Referrals alcohol/other health-care settings; TV and print media | • BDI-II ≥ 17 • DSM-IV life-time MDD • Current problematic AOD use above recommended level | 1. ICBT/MI + BI 2. ICBT/MI-c + BI 3. BI | 1. 10 × 1 hour 2. 10 × 1 hour 3. 1 session | 1. 21 2. 22 3. 24 (46%) | BDI-II | 1. Mean no. of alcohol use occasions | pt: 3 m fu: 6, 12 m | pt: 15.5 fu 6: 18.6 fu 12: 15.5 | 1. Yes 2. Yes 3. Yes 4. Yes |
| Kay-Lambkin 2011 79 Australia | Adults ≥16 | Referrals alcohol/other health-care settings; TV and print media | • BDI-II ≥ 17 • Alcohol or cannabis use at harmful levels last month | 1. ICBT/MI (face-to-face)a 2. ICBT/MI-ca 3. PCT | 1. 10 × 1 hour 2. 10 × 1 hour 3. 10 × 1 hour | 1. 185a 2. 89 (57%) | BDI-II | 1. Mean drinks per day 2. % abstinent 3. % with ≥50% reduction 4. % drinking above harmful threshold | pt: 3 m | pt: 40.5 | 1. Yes 2. n.i. 3. Yes 4. Yes |
| Lydecker 2010 60 USA | Out-patient veterans in double diagnosis programme | Clinic | • DSM-IV alcohol, cannabis/stimulant dependence • DSM-IV life-time MDD • Recent substance use HDRS >20 | 1. ICBT/MI (gr) + pharma. 2. TSF (gr) + pharma. | 1. 36 × 1 hour 2. 36 × 1 hour | 1. 107 2. 99 (92%) | HAM-D-21 | 1. % of days substance-use–abstinent | pt: 6 m fu:15 m | pt:19.4 fu: 34.5 | 1. Yes 2. n.i. 3. No 4. Yes |
| Watkins 2011 61 USA | In-patients aged +18 in substance use programme | Clinic | • PHQ-8 ≥ 5 • PHQ-8 ≥ 5 • BDI-II ≥ 17 | 1. ICBT/MI (gr) + TAU 2. TAU | 1. 16 × 2 hours | 1. 140 2. 159 (52%) | BDI-II | 1. Alcohol use days as % of days available for use | pt: 3 m fu: 6 m | pt:13 fu:14.4 | 1. No 2. No 3. No 4. Yes |
Methodological qualities: 1, adequate sequence generation; 2, allocation to conditions by an independent party; 3, blinding of outcome assessors or use of self-report outcomes only; 4, intention-to-treat analyses.
In the meta-analysis the CBT/MI face-to-face and computerized experimental conditions are combined and compared to the PCT control condition, as this was the way the data were presented and amenable.
Adverts = advertisements; alc = alcohol; alcpo = alcohol population; AOD = alcohol and other drugs; AUDIT = Alcohol Use Disorder Identification Test; BDI = Beck Depression Inventory; BI = brief intervention; BSP = brief supportive psychotherapy; C = control condition; CBT = cognitive-behavioural therapy; CBT-D = cognitive-behavioural treatment for depression; CES-D = Center for Epidemiologic Studies Depression Scale; CO = completers-only analysis; DAST = Drugs Abuse Screening Test; DSM = Diagnostic Statistical Manual of Mental Disorders; depr = depression; f = female; fu = follow-up; GAF = Global Assessment of Functioning; gr = group sessions; HADS = Hamilton Anxiety and Depression Scale; HAM-D = Hamilton Rating Scale for Depression; ICBT = integrated cognitive–behavioural therapy; ICBT/MI-c = integrated cognitive–behavioural therapy-computerized ‘integrated’, focusing on both alcohol and depression; IPT-DD = interpersonal psychotherapy adapted for dysthymic disorder; ITT = intention-to-treat analysis; K10 = Kessler Psychological Distress Scale; m = male; max. = maximum; MCMI = Millon Clinical Multiaxial Inventory; MDD = major depressive disorder; MHAM-D = Modified Hamilton Depression Rating Scale; MI = motivational interviewing; MMPI = Minnesota Multiphasic Personality Inventory; n.i. = no information; no. = number; PHQ = Patient Health Questionnaire; pharma. = pharmacotherapy; PCT = person-centred therapy; POMS = Profile of Mood States; pt = post-treatment; p.w. = per week; SCL-90 = Symptom Checklist; SET = self-examination therapy; s-r = self-reports; TAU = treatment as usual; TSF = 12-Step Facilitation Therapy.
For studies with more than one post-treatment assessment, we used the earliest to ensure maximum consistency in follow-up durations. Our initial selection from the first search was based on information derived from titles, abstracts and keywords; if these yielded insufficient information to assess inclusion criteria, the full paper was retrieved. All papers were assessed independently on inclusion and exclusion criteria and quality by two independent raters (H.R. and J.d.W.). Any disagreement was resolved by discussion and consensus. Authors were approached (8, H.R.) when relevant data were missing.
Quality assessment
We assessed the validity of the included studies using four criteria from the Risk of Bias Assessment tool, developed by the Cochrane Collaboration 36. The tool (which can be applied to both randomized and non-randomized studies) verifies study attributes that are possible sources of bias, including adequate generation of the allocation sequence, concealment of the allocation to the different conditions, preclusion of knowledge of the allocated interventions (blinding of assessors) and handling of incomplete outcome data. We rated incomplete data handling as positive if intention-to-treat analyses were conducted (see Table 1).
Study characteristics
We coded characteristics of the analysed studies as described in Table 1.
Meta-analyses
We calculated mean effect sizes (Hedges's g) for each comparison, using the computer program Comprehensive Meta-Analysis (CMA, version 2.2.021 37). Cohen's d is the standardized difference between the two means divided by the pooled standard deviation at post-test. Hedges's g is a variation of Cohen's d that corrects for potential bias due to small sample sizes 38. Effect sizes of approximately 0.8 can be considered large, 0.5 moderate and 0.2 small 39. Because several studies had small samples, we corrected the effect sizes for bias using the procedures suggested by Hedges & Olkin 38.
We calculated separate effect sizes for depression and for alcohol consumption (the primary outcome measures in the analysed studies). If means and standard deviations were not reported, we contacted the study authors to obtain these and/or used other statistics to calculate the effect sizes according to the procedures implemented in our meta-analysis software. Where possible, data from intention-to-treat analyses were used; completers-only data were used if the former were unavailable. If more than one depression or alcohol outcome measure was reported in a single study, we averaged the effect sizes from those measures to produce a single summary effect size for use in the meta-analysis, adjusting those calculations statistically to account for variance introduced by the multiple measures 40. Figures 2 and 3 show the studies for which this was the case; outcome was then indicated with ‘combined’.
Figure 2.
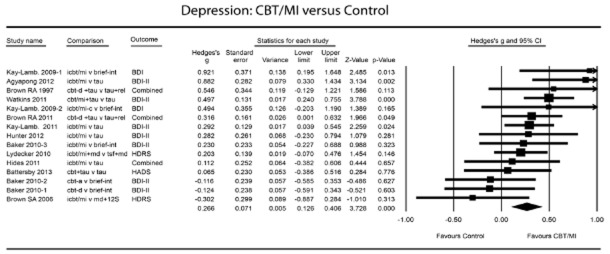
Depression: cognitive-behavioural therapy/motivational interviewing (CBT/MI) versus control
As we expected considerable heterogeneity among the studies, we calculated the mean effect sizes using a random-effects model. This assumes that the included studies were drawn from ‘populations’ of studies that differ systematically from one another (heterogeneity). It thus assumes that the effect sizes resulting from included studies differ not only because of the random error within studies (as in the fixed-effects model), but also because of true variation in effect size from one study to the next. We calculated the Q-statistic, which assesses the presence versus the absence of heterogeneity, but report only whether or not it was significant. As a test of homogeneity of effect sizes, we also calculated the I2-statistic, which quantifies the heterogeneity in percentages. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity, with 25% as low, 50% as moderate and 75% as high 41.
We also calculated numbers needed to treat (NNTs), using the formulae provided by Kraemer & Kupfer 42. NNT estimates the number of patients that need to be treated in order to have a beneficial impact on one person.
Subgroup analyses were conducted according to the mixed-effects model, whereby studies within subgroups are pooled with the random-effects model and tests for significant differences between subgroups are conducted with the fixed-effects model. We also used meta-regression analyses to identify any associations between the effects on depression and those on alcohol outcomes.
To detect possible publication bias, we examined the funnel plots visually for the primary outcome measures for symmetry. A funnel plot is a scatterplot of treatment effect against a measure of study size. A symmetrical inverted funnel shape indicates low publication bias, whereas an asymmetrical funnel indicates potential publication bias which may jeopardize the results and conclusion of the meta-analysis conducted. We conducted Egger's linear regression test 43 of the intercept to quantify the bias captured by the funnel plot and test whether or not it was significant. The Duval & Tweedie 44 trim-and-fill analysis was performed to further verify an unbiased estimate of the pooled effect size. This method enables an estimation of the number of missing studies that might exist in a meta-analysis and the effect that these studies might have had on its outcome.
Power calculations
We calculated beforehand how many would be needed to ensure sufficient statistical power to identify relevant effects. This was important because we sought studies based on treatment-to-treatment comparisons, so that small effect sizes were to be expected. The power calculation was conducted according to the procedures described by Borenstein and colleagues 40. We hoped to find enough studies to enable identification of a small effect size of d = 0.30 based on the random-effects model. The power calculations indicated that this would require at least 15 studies with a mean sample size of 40 (20 participants per condition). Conservatively, that assumes a medium level of between-study variance (τ2), a statistical power of 0.80 and a significance level of α < 0.05. Alternatively, we would need 10 studies with 60 participants each to detect an effect size of d = 0.30.
Results
Selection and inclusion of studies
Figure 1 shows a flow-chart describing the study selection procedure. Twelve studies (15 comparisons) were included in the meta-analysis. In reporting the results we followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist 45.
Characteristics of included studies: CBT/MI plus TAU versus control conditions
Table 1 summarizes selected study characteristics. They assessed a total of 1721 patients (1026 in experimental and 695 in control conditions) and thus provided sufficient statistical power (see Power calculations section). Three studies assessed the presence of depression and alcohol use disorders with diagnostic interviews. In the remaining studies, patients scored above a cut-off point on a self-report depression or alcohol scale. Nine studies applied a randomized controlled study design, three a controlled design only. Ten of the 15 CBT/MI conditions consisted of combined treatment strategies focusing on both alcohol and depression, four applied depression-focused CBT only and one applied CBT for alcohol only. Most CBT/MI procedures were added to TAU. TAU included psychosocial counselling and/or medication treatment. All the studies used validated outcome assessment instruments (for assessment of symptoms of depression: Beck Depression Inventory (BDI) and BDI-II 46, Hospital Anxiety and Depression Scale (HADS) 47, Hamilton Rating Scale for Depression (HAM-D) 48, Center for Epidemiologic Studies—Depression (CES-D) 49, Symptom Checklist-90 Revised, Depression (SCL-90-R-D) 50, Profile of Mood States (POMS) 51,52, Kessler Psychological Distress Scale (K10) 51 or Children's Depression Rating Scale (CDRS) 53). Alcohol use was measured by various consumption outcomes such as quantity and abstinence measures, as assessed using the alcohol time-line follow-back (TLFB 54 and Alcohol Use Disorders Identification Test (AUDIT) questionnaires 55).
Quality of included studies
The quality of the studies varied (see Table 1). Nine reported adequate sequence generation; three were non-randomized. Seven reported allocation to conditions by an independent party. Eight reported blinding of outcome assessors or used only self-report outcomes. Eight conducted intention-to-treat analyses. Dropout rates varied from 3 to 40%. Six studies met all four pre-defined quality criteria.
Effects of CBT/MI versus control groups: depression
Figure 2 and Table 2 show that the effects of CBT/MI on decrease of depression symptoms over controls were small but significant at post-test (g = 0.27, 95% CI = 0.13–0.41, P < 0.001, random-effects model; NNT = 6.58). Heterogeneity was low and non-significant (I2 = 37.51). Between-study variance (τ2) was small (0.005), resulting in considerable statistical power. A post-hoc power calculation showed that our set of studies had sufficient statistical power (1.00) on the basis of the random-effects model (based on the low level of between-study variance, τ2 = 0.003, and a significance level of 0.05).
Table 2.
Effects of adjunct cognitive-behavioural therapy/motivational interviewing (CBT/MI) on decrease in symptoms of depression in comparison with treatment-as-usual control groups, and subgroup analyses of associations between effect sizes and study characteristics (Hedges's ga)
| CBT/MI versus TAU | Subgroup | n comp | g | 95% CI | I2b | Pc | NNT |
|---|---|---|---|---|---|---|---|
| All studies | 15 | 0.27 | 0.13 to 0.41*** | 37.51 | 6.58 | ||
| One effect size per study (lowest excluded) | 13 | 0.27 | 0.14 to 0.41*** | 27.42 | 6.58 | ||
| One effect size per study (highest excluded) | 13 | 0.26 | 0.09 to 0.44** | 46.31 | 6.85 | ||
| Subgroup analyses | |||||||
| Type of control | TAU | 9 | 0.30 | 0.11 to 0.47*** | 39.34 | 0.624 | 5.95 |
| Brief treatment | 6 | 0.22 | −0.03 to 0.47 | 41.48 | 8.06 | ||
| Randomization | Yes | 12 | 0.23 | 0.07 to 0.39** | 38.80 | 0.197 | 7.69 |
| No | 3 | 0.43 | 0.21 to 0.64*** | 0 | 4.20 | ||
| Analyses | ITT | 12 | 0.26 | 0.09 to 0.43** | 48.36 | 0.803 | 6.85 |
| CO | 3 | 0.30 | 0.05 to 0.54* | 0 | 5.95 | ||
| Recruitment | Community | 6 | 0.22 | −0.01 to .47 | 41.48 | 0.624 | 8.06 |
| Clinic | 9 | 0.30 | 0.12 to 0.47*** | 39.34 | 5.95 | ||
| Population | Alcohol | 7 | 0.23 | −0.01 to 0.47* | 46.91 | 0.640 | 7.69 |
| Substance use (incl. alcohol) | 8 | 0.30 | 0.13 to 0.47*** | 33.08 | 5.95 | ||
| Age | (Young) adults ≥16 years | 7 | 0.20 | −0.01 to 0.41 | 31.07 | 0.446 | 8.93 |
| Adult ≥18 years | 8 | 0.31 | 0.12 to 0.56*** | 44.14 | 5.75 | ||
| Diagnosis of both conditions | Yes | 3 | 0.26 | −0.29 to 0.81 | 76.65 | 0.965 | 6.85 |
| No | 12 | 0.27 | 0.14 to 0.41*** | 19.82 | 6.58 | ||
| Focus of treatment | Integrated | 10 | 0.27 | 0.10 to 0.45** | 32.56 | 0.828 | 6.58 |
| Single (depression or alcohol) | 5 | 0.24 | −0.03 to 0.51 | 55.43 | 7.46 | ||
| Patient status | In-patient | 1 | 0.50 | 0.24 to 0.75*** | 0 | 0.082 | 3.62 |
| Out-patient | 14 | 0.23 | 0.09 to 0.38*** | 31.69 | 7.69 | ||
| Individual/group | Individual | 10 | 0.30 | 0.11 to 0.49** | 38.75 | 0.597 | 5.95 |
| Group | 5 | 0.22 | −0.02 to 0.45 | 47.97 | 8.06 | ||
| Digital versus face-to-face | Internet | 2 | 0.73 | 0.30 to 1.16*** | 0 | 0.030 | 2.54 |
| Face-to-face | 13 | 0.23 | 0.09 to 0.36*** | 30 | 7.69 | ||
CI = confidence interval; n comp = number of comparisons; NNT = number needed to treat; CO = completers-only analysis; ITT = intention-to-treat analysis; TAU = treatment as usual.
According to the random-effects model.
The P-values in this column indicate whether the Q-statistic is significant (I2-statistics do not include a test of significance).
The P-values in this column indicate whether the difference between the effect sizes in the subgroups is significant.
*P ≤ 0.05; **P < 0.01; ***P ≤ 0.001.
Two studies 30,56 compared groups receiving different types of CBT/MI with a single control group, so that multiple comparisons from these studies were included in the same analysis. The fact that these were not independent of one another could have reduced artificially the heterogeneity of the analysed studies, thereby affecting the pooled effect size. We therefore conducted sensitivity analyses that included only one effect size per study: one meta-analysis incorporating the largest effect size only and a second incorporating the smallest only. As Table 2 shows, these had little influence on the pooled effect size, nor did they produce differences in heterogeneity.
Effects of CBT/MI versus control groups: alcohol
The overall mean effect size indicating the post-test difference between CBT/MI and control groups concerning a decrease in alcohol consumption was small (g = 0.17, 95% CI = 0.07–0.28, P < 0.001; random-effects model; NNT = 10.42). Results are shown in Fig. 3 and Table 3. Heterogeneity was very low (I2 = 0.15). A post-hoc power calculation showed that our set of studies had sufficient statistical power (0.87) on the basis of the random-effects model (based on the low level of between-study variance, τ2 = 0.003, and a significance level of 0.05). There were two studies that compared groups receiving different types of CBT/MI with a single control group 30,56. Sensitivity analyses showed little influence of these on the pooled effect size or differences in heterogeneity (Table 3).
Figure 3.
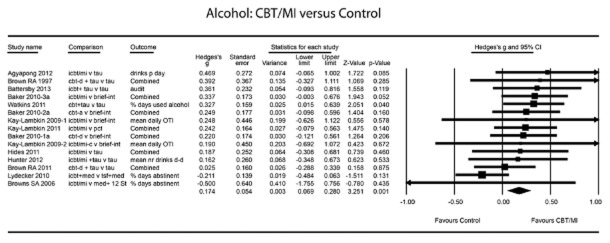
Alcohol: cognitive-behavioural therapy/motivational interviewing (CBT/MI) versus control
Table 3.
Effects of adjunct cognitive-behavioural therapy/motivational interviewing (CBT/MI) on decrease in alcohol consumption in comparison with treatment-as-usual control groups, and subgroup analyses of associations between effect sizes and study characteristics (Hedges's ga)
| CBT/MI versus TAU | Subgroup | n comp | g | 95% CI | I2b | Pc | NNT |
|---|---|---|---|---|---|---|---|
| All studies | 15 | 0.17 | 0.07 to 0.28** | 0.15 | 10.42 | ||
| One effect size per study (lowest excluded) | 13 | 0.18 | 0.05 to 0.31** | 13.94 | 9.80 | ||
| One effect size per study (highest excluded) | 13 | 0.16 | 0.04 to 0.28** | 7.75 | 11.11 | ||
| Subgroup analyses | |||||||
| Type of control | TAU | 9 | 0.11 | −0.04 to 0.32 | 33.13 | 0.354 | 16.13 |
| Other treatment | 6 | 0.26 | 0.10 to 0.42** | 0 | 6.85 | ||
| Randomization | Yes | 12 | 0.15 | 0.03 to 0.28* | 12.59 | 0.310 | 11.90 |
| No | 3 | 0.30 | 0.05 to 0.55* | 0 | 5.95 | ||
| Analyses | ITT | 12 | 0.20 | 0.07 to 0.32** | 13.65 | 0.546 | 8.93 |
| CO | 3 | 0.11 | −0.04 to 0.36 | 0 | 16.13 | ||
| Recruitment | Community | 6 | 0.26 | 0.10 to 0.42** | 0 | 0.354 | 6.85 |
| Clinic | 9 | 0.14 | −0.04 to 0.32 | 33.13 | 12.82 | ||
| Population | Alcohol | 7 | 0.24 | 0.10 to 0.39*** | 0 | 0.270 | 7.46 |
| Substance use | 8 | 0.11 | −0.07 to 0.29 | 21.73 | 16.13 | ||
| Diagnoses of both conditions | Yes | 3 | 0.00 | −0.54 to 0.54 | 62.75 | 0.394 | |
| No | 12 | 0.24 | 0.12 to 0.35*** | 0 | 7.46 | ||
| Age | (young)-adult i ≥16 years | 7 | 0.25 | 0.10 to 0.40*** | 0 | 0.411 | 7.14 |
| Adult | 8 | 0.14 | −0.06 to 0.35 | 41.05 | 12.82 | ||
| Focus of treatment | Integrated | 10 | 0.15 | 0.00 to 0.33 | 21.57 | 0.699 | 11.90 |
| Single (depression or alcohol) | 5 | 0.21 | 0.05 to 0.37** | 0 | 8.47 | ||
| Patient status | In-patient | 1 | 0.33 | 0.01 to 0.64* | 0 | 0.310 | 5.43 |
| Out-patient | 14 | 0.15 | 0.04 to 0.27** | 0 | 11.90 | ||
| Individual/group | Individual | 10 | 0.23 | 0.10 to 0.39*** | 0 | 0.454 | 7.69 |
| Group | 5 | 0.11 | −0.19 to 0.40 | 56.23 | 16.13 | ||
| Digital versus face-to-face | Internet | 2 | 0.39 | −0.06 to 0.85 | 0 | 0.346 | 4.59 |
| Face-to-face | 13 | 0.16 | 0.05 to 0.28** | 6.26 | 11.11 | ||
CI = confidence interval; n comp = number of comparisons; NNT = number needed to treat; CO = completers-only analysis; ITT = intention-to-treat analysis; TAU = treatment as usual.
According to the random-effects model.
The P-values in this column indicate whether the Q-statistic is significant (I2-statistics do not include a test of significance).
The P-values in this column indicate whether the difference between the effect sizes in the subgroups is significant.
*P ≤ 0.05; **P < 0.01; ***P ≤ 0.001.
Subgroup analyses
No significant differences emerged for decrease of depression symptoms or alcohol consumption in association with any of the subgroup analyses we conducted (see Tables 2 and 3). The comparison between digital and face-to-face CBT/MI for depression was, however, significant (P = 0.030) in favour of digital CBT/MI (g = 0.73, CI = 0.30–1.16 and g = 0.23, CI = 0.09–0.36, respectively).
Meta-regression
A higher number of sessions was associated negatively and significantly with the effect size for alcohol outcome (β = −0.016, 95% CI = −0.027 to −0.005, P = 0.004); for depression, the association was non-significant. A higher effect size for alcohol outcome was associated significantly with a higher effect size for depression (β = 0.511, 95% CI = −0.04 to 0.99, P = 0.003); the reverse relationship was not significant.
Follow-up assessments
For seven studies (eight comparisons 24,56–61), we could assess the impact of CBT/MI on depressive symptoms at a follow-up measurement 6–12 months post-treatment. A similar small effect size (g = 0.26, 95% CI = −0.01 to 0.54; random-effects model) was found, but with only a trend towards significance and with a high level of heterogeneity (P = 0.063, I2 = 65.433). For the impact of CBT/MI on decrease of alcohol consumption 61, a significant effect was maintained and increased at follow-up (g = 0.31, 95% CI = 0.16–0.47, P < 0.001; random-effects model; nil heterogeneity; eight studies, nine comparisons 24,29,31,56,57,59,60).
Publication bias
Inspection of the funnel plot and performance of the trim-and-fill procedure indicated no publication bias for the studies in terms of depression effect sizes; bias for alcohol was low. After adjustment for missing studies, the effect size for alcohol outcome diminished from g = 0.17 to g = 0.14 (95% CI = 0.04–0.25; trimmed studies = 3), and Egger's test did not indicate an asymmetrical funnel plot (P > 0.10).
Discussion
CBT/MI as an adjunct to treatment as usual (or as an alternative to it) appears effective for treating (young) adult patients with comorbid MDD and AUD (clinical or subclinical). Effect sizes were small but significant and comparable to those found by Hobbs and colleagues 14 (a review that included only two studies on alcohol, depression and psychotherapy). We had expected small effect sizes, given that our meta-analysis involved treatment-to-treatment comparisons. These effect sizes and corresponding NNTs are lower than those found in the meta-analysis of Nunes & Levin 26 for the antidepressant treatment of comorbid MDD and substance use dependency. This meta-analysis showed NNTs of 4.72 (depression) and 7.14 (alcohol), while our study found NNTs of 6.58 (depression) and 10.42 (alcohol).
Follow-up assessments up to 12 months post-treatment showed that the effect size for depression was maintained, with a trend towards significance (P = 0.063). A similar enduring CBT effect for patients with depression only has been found in a recent meta-analysis 62. The beneficial effect of CBT/MI on alcohol outcomes in our study even strengthened over time (from g = 0.18 to g = 0.32, P < 0.001). This apparent delayed impact of CBT in reducing alcohol use has been labelled by Carroll and colleagues as a ‘sleeper effect’ 63. It may be explained by the cognitive and relapse skills that patients learn during treatment and can still apply afterwards 64,65.
In our study, the impact on depression appears to have been achieved earlier than the effect on alcohol use. At least two points may arise from that finding. First, the alcohol outcome may result from good CBT/MI depression response per se. Secondly, reduced alcohol consumption during treatment may have also been a factor, in view of the positive association between reduced alcohol consumption and improved mood found in our analysis. In our study, however, a higher effect size for depression outcome did not correspond significantly with a higher effect size for alcohol outcome, as was the case in Nunes & Levin's meta-analysis of antidepressant medication. This may also suggest an alcohol sleeper effect in our study. Such results must be interpreted with caution, however, as assessing moderators from study-level data is subject to various difficulties, such as limited power for moderator analyses 66. Meta-analysis using individual patient-level data may overcome some of these problems (see e.g. 21).
Another possible explanation for our lack of association between depression and alcohol outcomes is that there may not have been enough variation in effect sizes to detect such associations (see Limitations). That could also be one reason for the lack of significant differences between any of the subgroups we evaluated (in combination with the small numbers of studies in some subgroup comparisons). The comparison between digital and face-to-face treatments was, however, significant. Digitally delivered treatments such as those over the internet have been proved clinically and are cost-effective for clinical and subclinical AUD 20,67,68 and depression 69,70. Given that most comorbid patients receive out-patient treatment 71, easily accessible treatment facilities such as those delivered partly via the internet or smartphone are worth exploring in more detail.
We did not find a significant difference between integrated and single-focus CBT/MI. Hence, we cannot argue the superiority of the former over the latter, as carried out by some studies that recommend integrated psychotherapeutic treatment for MDD and AUD 13,32.
Interestingly, we found that a higher number of CBT/MI sessions was associated significantly and negatively with alcohol outcome (P < 0.001) and non-significantly with depression outcome. This may suggest a lack of superior effect of intensive CBT/MI over briefer alcohol treatments. A similar lack of superiority was reported in the meta-analysis by Moyer and colleagues for problem drinkers and in the Project March results 22,72. This suggests that brief alcohol interventions could be explored as first-step treatments for comorbid alcohol problems, to be followed by more intensive components for patients who do not respond adequately. Another question yet to be answered within this context relates to the minimal required number of brief treatment sessions in order to obtain treatment effectiveness.
Study limitations
We assessed overall outcomes in terms of depressive symptoms and alcohol consumption. The effect sizes for alcohol use were based on varying measures, and there were not enough studies to look separately at distinctions such as abstinence or percentage of heavy drinking days. Such diversity of measures is a common problem in alcohol studies, as noted by Sobell and colleagues 73; and, as Hobbs and colleagues 14 have pointed out, that may have resulted in less variability in outcome than would have been found in studies with fewer or single measures. To minimize this potential bias, we followed guidelines in meta-analytical strategy manuals 40.
We included both randomized and non-randomized controlled studies in our analyses, but found no difference between those two designs in terms of effect sizes for alcohol or depression. Such a difference might have been expected, as randomization often yields lower clinical outcomes 74.
Generalization of our study results requires caution, as all but one of the included studies involved out-patients only.
Clinical considerations
Combined CBT/MI treatment of comorbid clinical and subclinical MDD and AUD, both for clinical and community samples, shows promising results. The observed effects were small, but they could still imply a major health impact in view of the high prevalence of comorbidity of these disorders, the related high burden of disease and the preference by many patients of CBT/MI over antidepressants 75.
Conclusion
Further research is needed in terms of large-scale randomized controlled trials that could strengthen the findings of this meta-analysis. Future studies should focus on the feasibility of brief and digital interventions, single-focus versus integrated CBT/MI, clear descriptions of what treatment-as-usual involves, possible predefined moderators and mediators of treatment outcomes, long-term follow-ups, alcohol outcome measures such as reliable clinical change and cost-effectiveness. Our meta-analysis may provide a basis for such efforts.
Acknowledgments
We like to thank the authors for the provision of additional information on their studies and Mr Michael Dallas for the English language corrections.
Declaration of interests
None.
References
- 1.Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101:76–88. doi: 10.1111/j.1360-0443.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 2.Boschloo L, Vogelzangs N, Smit JH, Van den Brink W, Veltman DJ, Beekman AT, et al. Comorbidity and risk indicators for alcohol use disorders among persons with anxiety and/or depressive disorders: findings from the Netherlands Study of Depression and Anxiety (NESDA) J Affect Disord. 2011;131:233–242. doi: 10.1016/j.jad.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clin Psychol Rev. 2000;20:173–189. doi: 10.1016/s0272-7358(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 4.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 5.Jane-Llopis E, Matytsina I. Mental health and alcohol, drugs and tobacco: a review of the comorbidity between mental disorders and the use of alcohol, tobacco and illicit drugs. Drug Alcohol Rev. 2006;25:515–536. doi: 10.1080/09595230600944461. [DOI] [PubMed] [Google Scholar]
- 6.Gadermann AM, Alonso J, Vilagut G, Zaslavsky AM, Kessler RC. Comorbidity and disease burden in the National Comorbidity Survey Replication (NCS-R) Depress Anxiety. 2012;29:797–806. doi: 10.1002/da.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco C, Alegria AA, Liu SM, Secades-Villa R, Sugaya L, Davies C, et al. Differences among major depressive disorder with and without co-occurring substance use disorders and substance-induced depressive disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2012;73:865–873. doi: 10.4088/JCP.10m06673. [DOI] [PubMed] [Google Scholar]
- 8.Chisholm D, Diehr P, Knapp M, Patrick D, Treglia M, Simon G. Depression status, medical comorbidity and resource costs. Evidence from an international study of major depression in primary care (LIDO) Br J Psychiatry. 2003;183:121–131. doi: 10.1192/bjp.183.2.121. [DOI] [PubMed] [Google Scholar]
- 9.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 10.Nunes EV, Levin FR. Treatment of co-occurring depression and substance dependence: using meta-analysis to guide clinical recommendations. Psychiatr Ann. 2008;38:nihpa128505. doi: 10.3928/00485713-20081101-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nunes EV, Hennessy G, Slezer J. Depression in patients with substance use disorders. In: Nunes EV, Selzer J, Levounis P, Davies CA, editors. Substance Dependence and Co-Occurring Psychiatric Disorders. Kingston, NJ: Civic Research Institute; 2010. pp. 1.1–1.36. [Google Scholar]
- 12.Pettinati HM, O'Brien CP, Dundon WD. Current status of co-occurring mood and substance use disorders: a new therapeutic target. Am J Psychiatry. 2013;170:23–30. doi: 10.1176/appi.ajp.2012.12010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesse M. Integrated psychological treatment for substance use and co-morbid anxiety or depression vs. treatment for substance use alone. A systematic review of the published literature. BMC Psychiatry. 2009;9:6. doi: 10.1186/1471-244X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobbs JD, Kushner MG, Lee SS, Reardon SM, Maurer EW. Meta-analysis of supplemental treatment for depressive and anxiety disorders in patients being treated for alcohol dependence. Am J Addict. 2011;20:319–329. doi: 10.1111/j.1521-0391.2011.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuijpers P, van Straten A, Andersson G, van Oppen P. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol. 2008;76:909–922. doi: 10.1037/a0013075. [DOI] [PubMed] [Google Scholar]
- 16.Project Match Research Group. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- 17.Vasilaki EI, Hosier SG, Cox WM. The efficacy of motivational interviewing as a brief intervention for excessive drinking: a meta-analytic review. Alcohol Alcohol. 2006;41:328–335. doi: 10.1093/alcalc/agl016. [DOI] [PubMed] [Google Scholar]
- 18.Cuijpers P, Smit F, van Straten A. Psychological treatments of subthreshold depression: a meta-analytic review. Acta Psychiatr Scand. 2007;115:434–441. doi: 10.1111/j.1600-0447.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders [DSM-IV-TR tm] 4th edn. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 20.Riper H, Spek V, Boon B, Conijn B, Kramer J, Martin-Abello K, et al. Effectiveness of E-self-help interventions for curbing adult problem drinking: a meta-analysis. J Med Internet Res. 2011;13:e42. doi: 10.2196/jmir.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bower P, Kontopantelis E, Sutton A, Kendrick T, Richards DA, Gilbody S, et al. Influence of initial severity of depression on effectiveness of low intensity interventions: meta-analysis of individual patient data. BMJ. 2013;346:f540. doi: 10.1136/bmj.f540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97:279–292. doi: 10.1046/j.1360-0443.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York: Guilford Press; 2002. [Google Scholar]
- 24.Brown SA, Glasner-Edwards SV, Tate SR, McQuaid JR, Chalekian J, Granholm E. Integrated cognitive behavioral therapy versus twelve-step facilitation therapy for substance-dependent adults with depressive disorders. J Psychoact Drugs. 2006;38:449–460. doi: 10.1080/02791072.2006.10400584. [DOI] [PubMed] [Google Scholar]
- 25.Cuijpers P, Munoz RF, Clarke GN, Lewinsohn PM. Psychoeducational treatment and prevention of depression: the ‘Coping with Depression’ course thirty years later. Clin Psychol Rev. 2009;29:449–458. doi: 10.1016/j.cpr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- 27.Roy-Byrne PP, Pages KP, Russo JE, Jaffe C, Blume AW, Kingsley E, et al. Nefazodone treatment of major depression in alcohol-dependent patients: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2000;20:129–136. doi: 10.1097/00004714-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Altamura AC, Mauri MC, Girardi T, Panetta B. Alcoholism and depression: a placebo controlled study with viloxazine. Int J Clin Pharmacol Res. 1990;10:293–298. [PubMed] [Google Scholar]
- 29.Hides L, Samet S, Lubman DI. Cognitive behaviour therapy (CBT) for the treatment of co-occurring depression and substance use: current evidence and directions for future research. Drug Alcohol Rev. 2010;29:508–517. doi: 10.1111/j.1465-3362.2010.00207.x. [DOI] [PubMed] [Google Scholar]
- 30.Baker AL, Kavanagh DJ, Kay-Lambkin FJ, Hunt SA, Lewin TJ, Carr VJ, et al. Randomized controlled trial of cognitive–behavioural therapy for coexisting depression and alcohol problems: short-term outcome. Addiction. 2010;105:87–99. doi: 10.1111/j.1360-0443.2009.02757.x. [DOI] [PubMed] [Google Scholar]
- 31.Brown RA, Evans DM, Miller IW, Burgess ES, Mueller TI. Cognitive–behavioral treatment for depression in alcoholism. J Consult Clin Psychol. 1997;65:715–726. doi: 10.1037//0022-006x.65.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker AL, Thornton LK, Hiles S, Hides L, Lubman DI. Psychological interventions for alcohol misuse among people with co-occurring depression or anxiety disorders: a systematic review. J Affect Disord. 2012;139:217–229. doi: 10.1016/j.jad.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Randall CL, Thomas S, Thevos AK. Concurrent alcoholism and social anxiety disorder: a first step toward developing effective treatments. Alcohol Clin Exp Res. 2001;25:210–220. [PubMed] [Google Scholar]
- 34.Markowitz JC, Kocsis JH, Christos P, Bleiberg K, Carlin A. Pilot study of interpersonal psychotherapy versus supportive psychotherapy for dysthymic patients with secondary alcohol abuse or dependence. J Nerv Ment Dis. 2008;196:468–474. doi: 10.1097/NMD.0b013e31817738f1. [DOI] [PubMed] [Google Scholar]
- 35.Cuijpers P, van Straten A, Warmerdam L, Andersson G. Psychological treatment of depression: a meta-analytic database of randomized studies. BMC Psychiatry. 2008;8:36. doi: 10.1186/1471-244X-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. version 5.1.0, updated March 2011. Available at: http://www.cochrane-handbook.org (accessed 21 August 2012) (Archived at http://www.webcitation.org/6MB98uB0f on 27 December 2013)
- 37.2010. Comprehensive meta-analysis [computer program], version 2.2.021. Englewood, NJ, USA.
- 38.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 39.Cohen J. Statistical Power Analyses for the Behavioral Sciences, revised edn. New York: Academic Press; 1997. [Google Scholar]
- 40.Borenstein MHL, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, UK: Wiley; 2009. [Google Scholar]
- 41.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59:990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 45.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd edn. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 47.Hawthorne G, Biddle D, Goulopoulos J. User Manual: The ACPMH Assessment and Evaluation Protocols. 3rd edn. Melbourne: Australian Centre for Posttraumatic Mental Health; 2004. [Google Scholar]
- 48.Miller IW, Bishop S, Norman WH, Maddever H. The Modified Hamilton Rating Scale for Depression: reliability and validity. Psychiatry Res. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- 49.Radloff LS. The CES-D Scale. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 50.Derogatis L. SCL-90-R Administration, Scoring and Procedures Manual. Towson, MD: Derogatis; 1983. [Google Scholar]
- 51.Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003;60:184–189. doi: 10.1001/archpsyc.60.2.184. [DOI] [PubMed] [Google Scholar]
- 52.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 53.Mason BJ, Kocsis JH, Leon AC, Thompson S. Measurement of severity and treatment response in dysthymia. Psychiatr Ann. 1993;23:625–631. [Google Scholar]
- 54.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 55.Saunders JB, Aasland OG, Babor TF, Defuentes-Merillas L, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 56.Kay-Lambkin FJ, Baker AL, Lewin TJ, Carr VJ. Computer-based psychological treatment for comorbid depression and problematic alcohol and/or cannabis use: a randomized controlled trial of clinical efficacy. Addiction. 2009;104:378–388. doi: 10.1111/j.1360-0443.2008.02444.x. [DOI] [PubMed] [Google Scholar]
- 57.Agyapong VI, McLoughlin DM, Farren CK. Six-months outcomes of a randomised trial of supportive text messaging for depression and comorbid alcohol use disorder. J Affect Disord. 2013;151:100–104. doi: 10.1016/j.jad.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 58.Hides LM, Elkins KS, Scaffidi A, Cotton SM, Carroll S, Lubman DI. Does the addition of integrated cognitive behaviour therapy and motivational interviewing improve the outcomes of standard care for young people with comorbid depression and substance misuse? Med J Aust. 2011;195:S31–37. doi: 10.5694/j.1326-5377.2011.tb03263.x. [DOI] [PubMed] [Google Scholar]
- 59.Hunter SB, Watkins KE, Hepner KA, Paddock SM, Ewing BA, Osilla KC, et al. Treating depression and substance use: a randomized controlled trial. J Subst Abuse Treat. 2012;43:137–151. doi: 10.1016/j.jsat.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lydecker KP, Tate SR, Cummins KM, McQuaid J, Granholm E, Brown SA. Clinical outcomes of an integrated treatment for depression and substance use disorders. Psychol Addict Behav. 2010;24:453–465. doi: 10.1037/a0019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watkins KE, Hunter SB, Hepner KA, Paddock SM, de la Cruz E, Zhou AJ, et al. An effectiveness trial of group cognitive behavioral therapy for patients with persistent depressive symptoms in substance abuse treatment. Arch Gen Psychiatry. 2011;68:577–584. doi: 10.1001/archgenpsychiatry.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cuijpers P, Hollon SD, van Straten A, Bockting C, Berking M, Andersson G. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3:e002542. doi: 10.1136/bmjopen-2012-002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- 64.Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, et al. A comparison of contingency management and cognitive–behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 65.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 66.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 67.Riper H, Kramer J, Conijn B, Smit F, Schippers G, Cuijpers P. Translating effective web-based self-help for problem drinking into the real world. Alcohol Clin Exp Res. 2009;33:1401–1408. doi: 10.1111/j.1530-0277.2009.00970.x. [DOI] [PubMed] [Google Scholar]
- 68.Smit F, Lokkerbol J, Riper H, Majo MC, Boon B, Blankers M. Modeling the cost-effectiveness of health care systems for alcohol use disorders: how implementation of eHealth interventions improves cost-effectiveness. J Med Internet Res. 2011;13:e56. doi: 10.2196/jmir.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richards D, Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32:329–342. doi: 10.1016/j.cpr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Warmerdam L, Smit F, van Straten A, Riper H, Cuijpers P. Cost–utility and cost–effectiveness of internet-based treatment for adults with depressive symptoms: randomized trial. J Med Internet Res. 2010;12:e53. doi: 10.2196/jmir.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Substance Abuse and Mental Health Services Administration (SAMHSA) 2006. National Survey of Substance Abuse Treatment Services (N-SSATS). Washington, DC: SAMHSA.
- 72.Matching alcoholism treatments to client heterogeneity: treatment main effects and matching effects on drinking during treatment. Project MATCH Research Group. J Stud Alcohol. 1998;59:631–639. doi: 10.15288/jsa.1998.59.631. [DOI] [PubMed] [Google Scholar]
- 73.Sobell LC, Sobell MB, Connors GJ, Agrawal S. Assessing drinking outcomes in alcohol treatment efficacy studies: selecting a yardstick of success. Alcohol Clin Exp Res. 2003;27:1661–1666. doi: 10.1097/01.ALC.0000091227.26627.75. [DOI] [PubMed] [Google Scholar]
- 74.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 75.Hansen HV, Kessing LV. Adherence to antidepressant treatment. Expert Rev Neurother. 2007;7:57–62. doi: 10.1586/14737175.7.1.57. [DOI] [PubMed] [Google Scholar]
- 76.Agyapong VI, Ahern S, McLoughlin DM, Farren CK. Supportive text messaging for depression and comorbid alcohol use disorder: single-blind randomised trial. J Affect Disord. 2012;141:168–176. doi: 10.1016/j.jad.2012.02.040. [DOI] [PubMed] [Google Scholar]
- 77.Battersby MW, Beattie J, Pols RG, Smith DP, Condon J, Blunden S. A randomised controlled trial of the Flinders Program of chronic condition management in Vietnam veterans with co-morbid alcohol misuse, and psychiatric and medical conditions. Aust NZ J Psychiatry. 2013;47:451–462. doi: 10.1177/0004867412471977. [DOI] [PubMed] [Google Scholar]
- 78.Brown RA, Ramsey SE, Kahler CW, Palm KM, Monti PM, Abrams D, et al. A randomized controlled trial of cognitive-behavioral treatment for depression versus relaxation training for alcohol-dependent individuals with elevated depressive symptoms. J Stud Alcohol Drugs. 2011;72:286–296. doi: 10.15288/jsad.2011.72.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kay-Lambkin FJ, Baker AL, Kelly B, Lewin TJ. Clinician-assisted computerised versus therapist-delivered treatment for depressive and addictive disorders: a randomised controlled trial. Med J Aust. 2011;195:S44–50. doi: 10.5694/j.1326-5377.2011.tb03265.x. [DOI] [PubMed] [Google Scholar]


