Abstract
Reactive oxygen species play a central role in the pathophysiology of the age-related decrease in male fertility. It has been reported that the total protein of DJ-1 was decreased in a proteomic analysis of seminal plasma from asthenozoospermia patients and a DJ-1 protein acts as a sensor of cellular redox homeostasis. Therefore, we evaluated the age-related changes in the ratio of the oxidized/reduced forms of the DJ-1 protein in the epididymis. In addition, the protective effects of S-allyl cysteine (SAC), a potent antioxidant, were evaluated against sperm dysfunction. Male rats aged 15–75 weeks were used to assess age-associated sperm function and oxidative stress. Sperm count increased until 25 weeks, but then decreased at 50 and 75 weeks. The rate of sperm movement at 75 weeks was decreased to approximately 60% of the rate observed at 25 weeks. Expression of DJ-1 decreased, but oxidized-DJ-1 increased with age. In addition, 4-hydroxy-2-nonenal modified proteins in the epididymis increased until 50 weeks of age. The total number and DNA synthetic potential of the sperm increased until 25 weeks, and then decreased. In rats 75 weeks of age, SAC (0.45% diet) attenuated the decrease in the number, motility, and DNA synthesis of sperm and inhibited the oxidized proteins. These results suggest that SAC ameliorates the quality of sperm subjected to age-associated oxidative stress.
Keywords: sperm function, aging, S-allyl cysteine, oxidative stress, oxidized DJ-1
Introduction
Male infertility is caused by abnormal semen. Some possible reasons for abnormal semen are stress, eating habits, and lifestyle choice.(1) In many mammals including humans, mature sperm is present at low temperatures, in a low-oxygen environment in the testicles and epididymis, but will be exposed to oxidative stress during the fertilization process. Flagellar swimming ability will depend on the ATP supply from the mitochondria in the sperm, but the susceptibility of sperm to oxidative stress is higher than that of any other cell. We have reported previously that leakage of reactive oxygen species (ROS) from the sperm mitochondria precedes sperm dysfunction.(2)
There is currently interest in using seminal ROS levels as a marker of male infertility.(3) A positive correlation between excessive levels of ROS and abnormal sperm concentrations, motility, and morphology has been previously demonstrated.(4) Human sperm exposed to oxidative stress, which is mediated by lipid peroxidation, and leads to apoptosis and infertility.(5) It therefore appears that age-related male infertility may be associated with oxidative stress. Antioxidants such as vitamin C, vitamin E, and carotenoids have been shown to decrease the oxidative damage to spermatozoa.(6)
S-allyl cysteine (SAC) is a potent antioxidant agent and a water-soluble compound that is less toxic than other antioxidants that are found in aged garlic(7) and is easily absorbed in the gastrointestinal tract. SAC can be rapidly detected in several tissues (the kidney, liver, lung, and brain) and its bioavailability in rats reaches 98%.(8) N-acetyl-SAC has been identified as a metabolite of SAC in the urine of dogs and humans. This suggests that SAC could be metabolized by N-acetyltransferase. Furthermore, Steiner and Li(9) have reported that SAC and its metabolite(s) are candidates for compliance markers in clinical studies involving aged garlic extracts. SAC levels in blood reached levels of several hundred ppb (10−6 M order) in humans who had consumed 2.4 g/day of aged garlic extracts.(9) It has been suggested that SAC may have prophylactic properties at a clinical level. However, further investigation is required to elucidate the precise protective mechanisms exerted by this antioxidant in aging and toxicity paradigms.
In contrast, DJ-1, which was originally identified as an oncogene, is a ubiquitous redox-responsive cytoprotective protein with diverse functions.(10) Wang et al.(11) have reported the presence of 101 differentially expressed proteins in the seminal plasma of asthenozoospermia (AS) patients. The level of DJ-1 was significantly lower in the samples from the AS patients than in samples from healthy donors. This data, combined with the observation that levels of ROS are about three-fold higher in the AS patient samples, suggests that the redox status of DJ-1 is a candidate biomarker for sperm dysfunction. DJ-1 has three cysteines at amino acid positions 46, 53, and 106. C106 is the first to be oxidized by the addition of SO3H or SO2H, followed by C46 and C53 after a dose of H2O2 was added to cultured cells.(12) C106 is required for DJ-1 to exert its activity against oxidative stress.(10,13) C106 is both functionally essential and subject to oxidation to cysteine-sulfinate and cysteine-sulfonate. Consequently, it has been proposed that oxidative modification of C106 allows DJ-1 to act as a sensor of cellular redox homeostasis and to participate in cytoprotective signaling pathways in the cell.(13) Therefore, this study focused on the regulation of motility and changes in the oxidized-DJ-1/DJ-1 ratio as a biomarker for sperm dysfunction. In addition, the protective effects of SAC on age-related sperm dysfunction were studied.
Materials and Methods
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM), penicillin, and streptomycin were obtained from Gibco (Paisley, UK). Superoxide dismutase (SOD; 3,000 U/mg) was obtained from Calbiochem (Nottingham, UK). The 8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4-(2H,3H) dione (L-012) and other reagents were obtained from Wako Pure Chem. Ind., Ltd. (Osaka, Japan). SAC was synthesized internally; a purity of 99.8% and the structure were confirmed by NMR.
Animals
Fischer rats (8 weeks of age) were purchased from Charles River (Tokyo, Japan). The rats were kept in a 12/12 h light-dark cycle with free access to standard rat chow (CE-2; Clea, Tokyo, Japan) and tap water. Starting at 10 weeks of age, all the rats had free access to standard laboratory chow (control group) or to an SAC-containing diet (0.45% w/w in the diet), which markedly improved pulmonary fibrosis and type 2 diabetes in our other models.(14,15) Animals were cared for according to the specifications outlined in the Guiding Principles for the Care and Use of Laboratory Animals–approved by the Authorities of the Local Committee on Experimental Animal Research.
The control animals were killed when they were 15, 25, 50, and 75 weeks of age under anesthesia with urethane (5 g/kg; i.p.). The SAC-treated animals were killed at 75 weeks of age under anesthesia. Blood was collected using heparinized syringes, and the epididymis and testes were dissected. The right epididymis from each rat was used to prepare the sperm solutions. The epididymal fat was also removed and the epididymis was then placed on a paper towel to remove any liquid before being weighed. For the sperm sampling, the cauda epididymis was cut with surgical scissors on the side of the corpus epididymis, where the vas deferens attaches to the epididymis.
Sperm concentration and motility
To determine the sperm count in the cauda epididymis, the excised cauda epididymis was punctured in 1 ml of TYH medium (Mitsubishi Chemical Medience Co. Tokyo, Japan) using an 18G needle. Next, a portion of the sperm suspension was sequentially diluted with TYH medium (to 1:20 and 1:100) and incubated at 37°C for 5 min in a CO2 incubator. A 10-µl volume of each individual sperm solution (1:100) was then immediately transferred to a slide chamber for measurements. We determined the sperm motility index (SMI), straight-line velocity, curvilinear velocity, and amplitude and frequency of the sperm head trajectory at 37°C using a computer-aided sperm motility analysis system (SMAS; Kaga Solnet, Tokyo, Japan)(16) on a thermo plate (Tokai hit, Shizuoka, Japan) under an optical microscope. The supernatant sperm solution described above (1:100) was diluted four-fold with 5% saline, and a 10-µl aliquot of the sperm/saline mixture was introduced to each counting chamber, and analyzed using a hemocytometer.
Western blotting for HNE-J2, Cu/Zn-SOD, Mn-SOD, DJ-1, and oxidized DJ-1
Epididymis tissues were homogenized and sonicated in 0.3 ml of ice-cold 10 mM Tris-HCl (pH 7.4) containing 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 5 mM ethylenediaminetetraacetic acid (EDTA), 25 mM β-glycerophosphate, 1 mM Na3VO4, 50 mM NaF, 0.2 mM tosyl phenylalanyl chloromethyl ketone (TPCK), 0.1 mM N-α-tosyl-l-lysine chloromethyl ketone (TLCK), and a protease inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany). The homogenates were centrifuged at 12,000 g for 20 min. Each of the collected supernatants (20 µg) were analyzed by immunoblotting using 12.5% gels for HNE-J2, Cu/Zn-SOD, Mn-SOD, DJ-1, and oxidized DJ-1. It has been previously shown that 4-hydroxy-2-nonenal (HNE)-modified proteins, and its protein adducts, are good markers for lipid peroxidation induced by ROS.(17) Anti-HNE-J2 (1:10,000, Japan Institute of the Control of Aging, Shizuoka, Japan), anti-Cu/Zn-SOD (1:25,000, Novus Biologicals, Littleton, CO), Mn-SOD (1:10,000, Abcam Japan, Tokyo), anti-DJ-1 (1:2,000, Epitomics, Inc., Burlingame, CA), and anti-oxidized DJ-1 (C106) (1:1,000, RayBiotech, Inc., Norcross, GA) antibodies were diluted with Can Get Signal solution (Toyobo, Osaka, Japan). All the secondary antibodies were diluted with Can Get Signal solution (1:20,000).
Evaluation of sperm DNA synthesis
The thymidine analogue 5'-bromo-2'-deoxyuridine (BrdU) is incorporated into dividing cells during DNA synthesis during the S phase of the cell cycle.(18) BrdU replaces thymidine during DNA replication, and once it is incorporated into the new DNA, it will remain in place and be passed down to the cell progeny upon division. Thirty minutes after BrdU (50 mg/kg) was intravenously injected into the tail vein, the rats were killed as described above. BrdU immunohistochemistry was used to identify the S-phase cells throughout the thick tissue sections fixed with a standard formaldehyde-based fixative. BrdU was detected in the tissue using specific primary antibodies (Chemicon, Temecula, CA).
Western blotting for c-kit in the testis
The c-kit receptor (a tyrosine kinase family member) is a proto-oncogene that is important in germ cell migration and maturation that has been found on the acrosomal region of mature sperm. Given that phosphorylation at Tyr-721 is important for interaction with PIK3R1, which is the regulatory subunit of phosphatidylinositol 3-kinase, testis and sperm from mature male mice were examined for phosphorylated c-kit receptor (Phospho-Tyr721; Signalway Antibody, College Park, MD) utilizing Western blot analysis techniques. The protein from the same testis was also utilized for the Western blot analysis described above.(19)
Statistical Analysis
We performed statistical analyses using the Microsoft Excel 2010 add-in software package (ver. 6.0; Esumi, Tokyo, Japan). Differences were considered to be statistically significant if p<0.05. The effects of SAC at 75 weeks were analyzed using Mann-Whitney’s U test (n = 6).
Results
Changes in body and testis weight
Fig. 1A shows that body weight increased with age and that the weight of the testis increased up to 50 weeks of age. At 75 weeks of age, the testis weight was less than that at 50 weeks. SAC did not affect the ratio of the testis weight/body weight compared to the control group at 75 weeks of age (Fig. 1B).
Fig. 1.
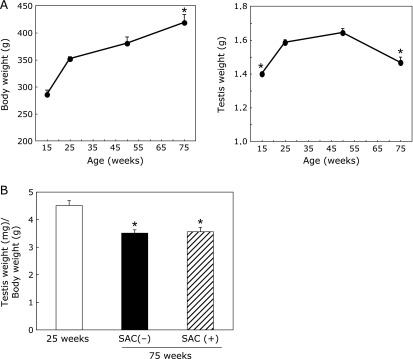
Changes in body and testis weight (A) and the ratio of testis weight (mg)/body weight (g) (B). (A) Animals were fed with control chow and drinking water ad libitum until the indicated age. Values are the mean ± SEM (n = 4–5). (B) Some animals were fed a SAC-containing diet (0.45% of the standard laboratory chow) from 10 to 75 weeks of age. Values are the mean ± SEM (n = 4–5). *p<0.01 vs 25 weeks of age.
Sperm concentration and seminal parameters
The sperm concentration decreased gradually in rats over 25 weeks of age (Table 1). The total sperm motility was reduced significantly in rats over 50 weeks of age (Fig. 2). SAC significantly attenuated the decrease in the sperm concentration and motility at 75 weeks (Fig. 2B). Age did not significantly affect any of the other parameters (i.e., the straight-line velocity, curvilinear velocity, and amplitude and frequency of the sperm head trajectory). A morphological analysis also revealed no significant changes in the sperm structure (results not shown).
Table 1.
Changes in age-dependent sperm numbers in the epididymis
| Age (weeks) | Sperm numbers (×107)/epididymis |
|---|---|
| 15 | 9.0 ± 0.1* |
| 25 | 12.1 ± 0.8 |
| 50 | 10.0 ± 0.8 |
| 75 SAC (−) | 5.9 ± 0.7** |
| 75 SAC (+) | 6.7 ± 0.4**,# |
Values are the mean ± SEM (n = 4–5). *p<0.05, **p<0.01 vs 25 weeks of age. #p<0.05 vs 75 weeks of control.
Fig. 2.
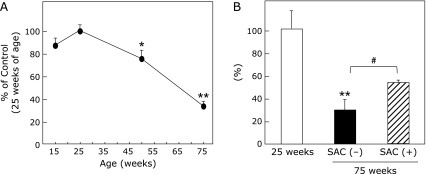
Time course (A) of total sperm motility and effects of SAC at 75 weeks (B). A portion of the sperm suspension was diluted with TYH medium and incubated at 37°C for 5 min in a CO2 incubator. A 10-µl aliquot of the sperm solution (1:100) was then immediately transferred to a slide chamber for a computer-aided sperm motility analysis on a thermo plate under an optical microscope. Total sperm motility (A) was calculated as the percentage of motile sperm in the total sperm concentration. Values are the mean ± SEM as a percentage of the motility at 25 weeks of age, which was set equivalent to 100% (n = 4–5). *p<0.05, **p<0.01 compared to 25 weeks of age. (B) Total sperm motility at 75 weeks. #p<0.05.
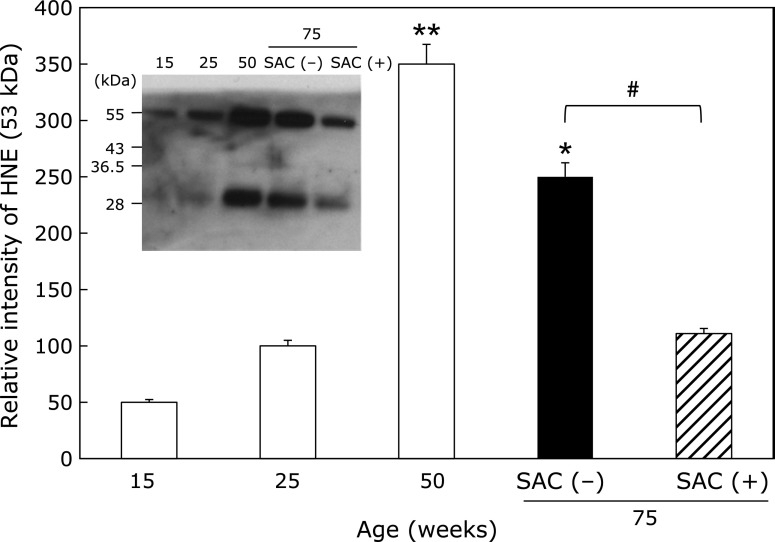
HNE-modified proteins
We found that the generation of ROS per sperm cell gradually increased in an age-dependent manner (Supplemental Fig. 1*). In consistent with the results, HNE-modified proteins (29 and 53 kDa) were detected in the epididymis. The expression level of the HNE-modified protein at 53 kDa increased with age until 50 weeks of age (Fig. 3). However, at 75 weeks of age, the 53 kDa HNE-modified protein was reduced compared to the amount detected at 50 weeks in the control group. SAC treatment further reduced the level of the HNE-modified protein. The same trends were observed for the 29-kDa protein as the 53-kDa protein (data not shown).
Fig. 3.
Effects of SAC on lipid peroxidationin the epididymis. The collected supernatants (20 µg) were analyzed by immunoblotting using an anti-HNE-J2 antibody. Values are the mean ± SEM (n = 4–5). *p<0.05, **p<0.01 compared to 25 weeks of age. #p<0.05.
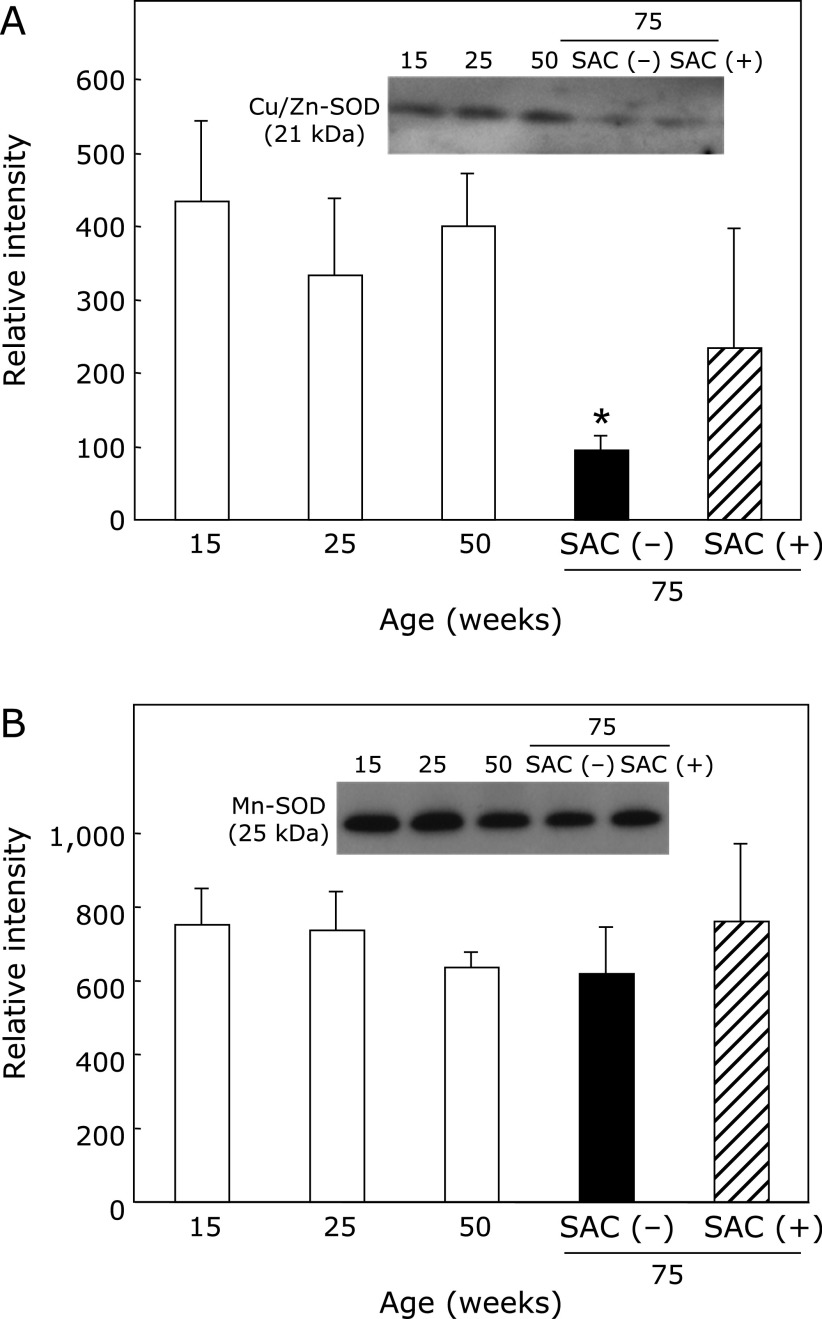
Expression of Cu/Zn-SOD, Mn-SOD, and DJ-1
Cu/Zn-SOD was significantly decreased at 75 weeks of age, but aging had almost no effect on the level of Mn-SOD (Fig. 4, Supplemental Fig. 2*). SAC treatment significantly attenuated the decrease in Cu/Zn-SOD at 75 weeks of age.
Fig. 4.
Effects of SAC on Cu/Zn-SOD and Mn-SOD in the epididymis. The collected supernatants were analyzed by immunoblotting. (A) Cu/Zn-SOD and (B) Mn-SOD. Values are the mean ± SEM (n = 4–5). *p<0.05 vs 25 weeks of age.
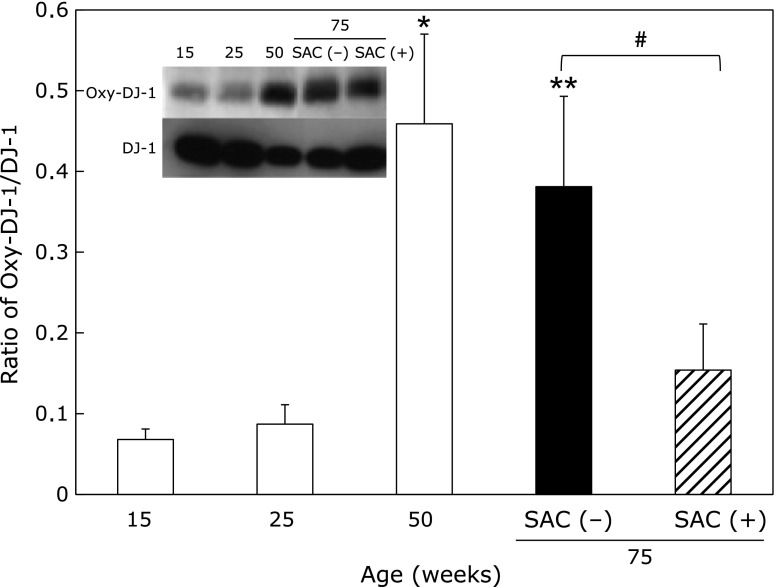
DJ-1, an antioxidative protein, was decreased with age. In contrast, the oxidized form of DJ-1 and the oxidized-DJ-1/DJ-1 ratio were increased with age. SAC significantly inhibited the increase in the ratio at 75 weeks of age (Fig. 5).
Fig. 5.
Effects of SAC on the oxidized DJ-1/DJ-1 ratio in the epididymis. The collected supernatants were analyzed using an anti-DJ-1 antibody (A-loading protein/lane: 0.2 µg) and an anti-oxidized DJ-1 antibody (A-loading protein/lane: 2.5 µg) using 12.5% gels. Values are the mean ± SEM (n = 4–5). *p<0.05, **p<0.01 vs 25 weeks of age. #p<0.01.
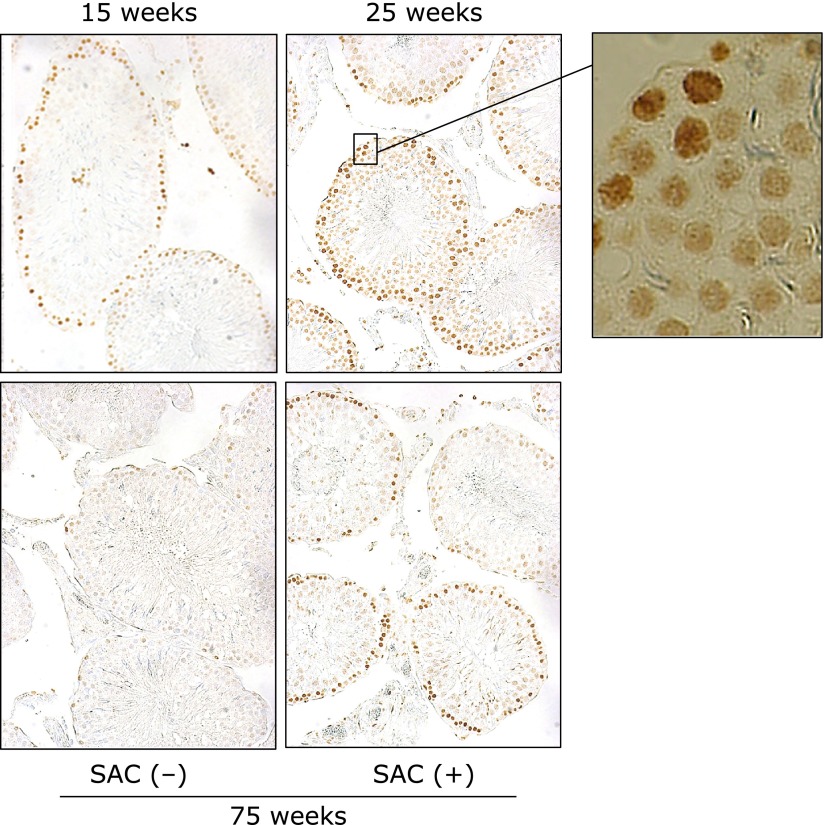
Spermatogenesis in the testis
BrdU incorporation analysis, which measures DNA synthesis, indicated that continuous spermatogenesis was maintained for at least 50 weeks in the control testis (data not shown). BrdU positive cells (brown) were detected in the spermatogonia. At 75 weeks of age, spermatogenesis was markedly reduced, but it was ameliorated by treatment with SAC (Fig. 6).
Fig. 6.
Effects of SAC on DNA synthesis in the testis. Rats were killed 30 min after BrdU (50 mg/kg) was intravenously injected into the tail vein. Representative microscope photographs (×100) are shown. The far right panel is a higher magnification of the area marked by a square in the 25 weeks image. Positive cells were detected in the spermatogonia.
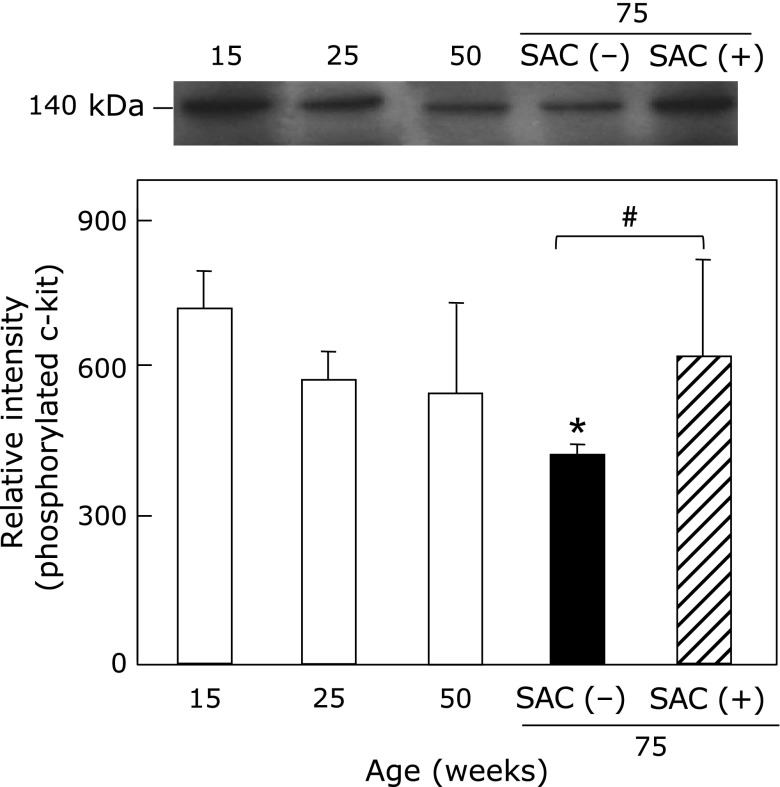
Fig. 7 shows the effects of SAC on phosphorylated tyrosine 721 c-kit in the testis. C-kit phosphorylation on serine/threonine or tyrosine residues is an important regulatory mechanism in signal transduction during spermatogenesis, oogenesis, and fertilization.(20) Phosphorylated c-kit was significantly decreased at 75 weeks in control animals, but SAC treatment maintained the levels of c-kit phosphorylation (Supplemental Fig. 2*).
Fig. 7.
Effects of SAC on phosphorylated tyrosine 721 c-kit in the testis. The collected supernatants of the testis were analyzed for anti-c-kit (phosphorylated tyrosine 721). Values are the mean ± SEM (n = 4–5). *p<0.05 vs 25 weeks of age. #p<0.05.
Discussion
Our data indicate that SAC treatment prevents age-related sperm dysfunction and oxidative-stress effects. Previous studies by our group indicated that SAC improved acute and chronic liver injuries,(21,22) lung injuries,(14) and diabetes mellitus.(15) There have also been numerous reports that SAC inhibits oxidative damage, which has been implicated in aging and a variety of diseases.(23) However, this study provides the first report of SAC associated protective effects on sperm function during aging.
SAC treatment prevented the decrease in total sperm motility and the increase of oxidation-modified proteins such as HNE and oxidized DJ-1 that occur with age. These results suggest that SAC improved the ability of sperm to respond to oxidative stress which happen in the testes of older males. In particular, it has been reported that DJ-1 is localized to the surface of the posterior part of the head and the anterior part of the midpiece in ejaculated spermatozoa.(24) DJ-1 was also present on the sperm flagella, suggesting it has two putative roles in fertilization: binding to the egg and flagella movement.(24) In addition, DJ-1 serves as a scavenger for ROS through oxidation of its cysteine residues. (25) It has previously been shown that DJ-1 is related to male infertility,(11) however, this study is the first to show that DJ-1 decreases and oxidized DJ-1 increases in the epididymis with age. At age 50 weeks, oxidized DJ-1/DJ-1 ratio significantly increased, however, sperm motility and sperm number moderately decreased. Therefore, the ratio could be as an earlier biomarker for sperm dysfunction. Moreover, the detection of oxidized DJ-1/DJ-1 ratio before the expression of sperm dysfunction may lead to develop a strategy for preventing the functional deterioration.
In humans, sperm ATP content is correlated to mobility.(26) Similar results have suggested that sperm motility is largely dependent on the total energy output from the mitochondrial compartment.(27,28) We found that the generation of ROS per sperm cell gradually increased in an age-dependent manner (Supplemental Fig. 1*). In this study, we did not evaluate the effects of SAC on ROS generation from sperm cells, but the HNE levels and oxidized DJ-1 results are evidence that oxidative stress is reduced in SAC-treated rats. These results consistent with our preliminary data using by electron spin resonance showed that SAC effectively scavenged OH radical and ROO radical. The identities of the HNE-modified proteins (29 and 53 kDa) were not determined. However, it has been reported that an estrogen receptor (29 kDa) is abundant on the sperm surface.(29) Sperm-associated P450 aromatase (~53 kDa), which synthesizes estrogen, also plays a role in the regulation of epididymal function that is proportional to the number of sperm being transported.(30) The estrogen receptor and P450 aromatase are candidates for the 29 or 53 kDa proteins, respectively but this should be further examined.
DJ-1 is a multifunctional oxidative stress response protein that defends cells against reactive oxygen species and mitochondrial damage.(13) The gene for DJ-1is related to an autosomal recessive form of early-onset Parkinson’s Disease.(31) Thus, DJ-1 is a multifunctional protein that plays essential roles in tissues with higher order biological functions such as the testis and brain.(32)
SAC, S-ethyl cysteine, and S-propyl cysteine in d-galactose (DG) treatment have neuroprotective effects in mice.(33,34) Based on these findings, these compounds could be potent agents against the progression of neurodegenerative disorders, such as Alzheimer’s disease, via their anti-amyloid beta, antiglycative, and antioxidative effects respectively. DJ-1 and Cu/Zn-SOD are both tightly connected to the Nrf2 protein, which is a transcription factor and master regulator for the expression of many antioxidant/detoxification genes.(35) In addition to antioxidant properties, SAC has been reported to protect against several agents that induce neurotoxicity in the rat striatum through the involvement of Nrf2 activation and modulation of signaling kinase cascades.(36–38) In the epididymis, SAC may increase Cu/Zn-SOD and DJ-1 via the Nrf2 pathway. Our data show that SAC inhibited DJ-1 oxidation and increase in HNE levels in the epididymis, and therefore, SAC might exert significant protective effects against other diseases related to the oxidative stress as well as aging-related dysfunction. Further studies in humans will be necessary to support the clinical use of SAC as a drug to prevent the oxidative damage that has been observed in chronic degenerative disorders.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research to Y. M. (No. 00362989) from the Japan Society for the Promotion of Science. We would like to thank Ms. Atsuko Tominaga of Osaka City University Medical School and Mr. Takahiro Kawagishi, Ms. Madoka Yasui, and Ryuhei Yamamoto of Doshisha University for their technical assistance. S. T., H. I., and Y. M. designed and conducted the research; Y. N. and T. Y. provided the essential materials (SMAS, etc.); S. T., H. I., T. T., and Y. M. analyzed the data; S. T. and Y. M. wrote the manuscript; Y. M. had primary responsibility for final content. All authors read and approved the final manuscript.
Conflict of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Hirsh A. Male subfertility. Bmj. 2003;327:669–672. doi: 10.1136/bmj.327.7416.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minamiyama Y, Ichikawa H, Takemura S, Kusunoki H, Naito Y, Yoshikawa T. Generation of reactive oxygen species in sperms of rats as an earlier marker for evaluating the toxicity of endocrine-disrupting chemicals. Free Radic Res. 2010;44:1398–1406. doi: 10.3109/10715762.2010.510523. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Sharma RK, Nallella KP, Thomas AJ, Jr, Alvarez JG, Sikka SC. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86:878–885. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Ikemoto I, Loughlin KR. Levels of reactive oxygen species before and after sperm preparation: comparison of swim-up and L4 filtration. Arch Androl. 1994;32:169–174. doi: 10.3109/01485019408987783. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura BN, Lawson G, Chan JY, et al. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radical Biol Med. 2010;49:1368–1379. doi: 10.1016/j.freeradbiomed.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal A, Prabakaran SA. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005;43:963–974. [PubMed] [Google Scholar]
- 7.Colín-González AL, Santana RA, Silva-Islas CA, Chánez-Cárdenas ME, Santamaría A, Maldonado PD. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid Med Cell Longev. 2012;2012:907162. doi: 10.1155/2012/907162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagae S, Ushijima M, Hatono S, et al. Pharmacokinetics of the garlic compound S-allylcysteine. Planta Med. 1994;60:214–217. doi: 10.1055/s-2006-959461. [DOI] [PubMed] [Google Scholar]
- 9.Steiner M, Li W. Aged garlic extract, a modulator of cardiovascular risk factors: a dose-finding study on the effects of AGE on platelet functions. J Nutr. 2001;131:980S–984S. doi: 10.1093/jn/131.3.980S. [DOI] [PubMed] [Google Scholar]
- 10.Kahle PJ, Waak J, Gasser T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Wang J, Zhang HR, et al. Proteomic analysis of seminal plasma from asthenozoospermia patients reveals proteins that affect oxidative stress responses and semen quality. Asian J Androl. 2009;11:484–491. doi: 10.1038/aja.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinumi T, Kimata J, Taira T, Ariga H, Niki E. Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;317:722–728. doi: 10.1016/j.bbrc.2004.03.110. [DOI] [PubMed] [Google Scholar]
- 13.Wilson MA. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal. 2011;15:111–122. doi: 10.1089/ars.2010.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizuguchi S, Takemura S, Minamiyama Y, et al. S-allyl cysteine attenuated CCl4-induced oxidative stress and pulmonary fibrosis in rats. Biofactors. 2006;26:81–92. doi: 10.1002/biof.5520260108. [DOI] [PubMed] [Google Scholar]
- 15.Takemura S, Minamiyama Y, Kodai S, et al. S-Allyl cysteine improves nonalcoholic fatty liver disease in type 2 diabetes Otsuka Long-Evans Tokushima Fatty rats via regulation of hepatic lipogenesis and glucose metabolism. J Clin Biochem Nutr. 2013;53:94–101. doi: 10.3164/jcbn.13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isobe T. New method to estimate the possibility of natural pregnancy using computer-assisted sperm analysis. Syst Biol Reprod Med. 2012;58:339–347. doi: 10.3109/19396368.2012.700759. [DOI] [PubMed] [Google Scholar]
- 17.Signorini C, De Felice C, Durand T, et al. Isoprostanes and 4-hydroxy-2-nonenal: markers or mediators of disease? Focus on Rett syndrome as a model of autism spectrum disorder. Oxid Med Cell Longev. 2013;2013:343824. doi: 10.1155/2013/343824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: a new reagent for detection of DNA replication. Science. 1982;218:474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- 19.Meldrum DR, Gambone JC, Morris MA, Esposito K, Giugliano D, Ignarro LJ. Lifestyle and metabolic approaches to maximizing erectile and vascular health. Int J Impot Res. 2012;24:61–68. doi: 10.1038/ijir.2011.51. [DOI] [PubMed] [Google Scholar]
- 20.Kierszenbaum AL. Tyrosine protein kinases and spermatogenesis: truncation matters. Mol Reprod Dev. 2006;73:399–403. doi: 10.1002/mrd.20456. [DOI] [PubMed] [Google Scholar]
- 21.Kodai S, Takemura S, Minamiyama Y, et al. S-allyl cysteine prevents CCl(4)-induced acute liver injury in rats. Free Radic Res. 2007;41:489–497. doi: 10.1080/10715760601118361. [DOI] [PubMed] [Google Scholar]
- 22.Shinkawa H, Takemura S, Minamiyama Y, et al. S-allylcysteine is effective as a chemopreventive agent against porcine serum-induced hepatic fibrosis in rats. Osaka City Med J. 2009;55:61–69. [PubMed] [Google Scholar]
- 23.Ray B, Chauhan NB, Lahiri DK. The ”aged garlic extract:” (AGE) and one of its active ingredients S-allyl-L-cysteine (SAC) as potential preventive and therapeutic agents for Alzheimer’s disease (AD) Curr Med Chem. 2011;18:3306–3313. doi: 10.2174/092986711796504664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida K, Sato Y, Yoshiike M, Nozawa S, Ariga H, Iwamoto T. Immunocytochemical localization of DJ-1 in human male reproductive tissue. Mol Reprod Dev. 2003;66:391–397. doi: 10.1002/mrd.10360. [DOI] [PubMed] [Google Scholar]
- 25.Meulener MC, Xu K, Thomson L, Ischiropoulos H, Bonini NM. Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. Proc Natl Acad Sci U S A. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Froman DP, Feltmann AJ. Sperm mobility: a quantitative trait of the domestic fowl (Gallus domesticus) Biol Reprod. 1998;58:379–384. doi: 10.1095/biolreprod58.2.379. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz-Pesini E, Diez C, Lapeña AC, et al. Correlation of sperm motility with mitochondrial enzymatic activities. Clin Chem. 1998;44:1616–1620. [PubMed] [Google Scholar]
- 28.Ruiz-Pesini E, Diez-Sánchez C, López-Pérez MJ, Enríquez JA. The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr Top Dev Biol. 2007;77:3–19. doi: 10.1016/S0070-2153(06)77001-6. [DOI] [PubMed] [Google Scholar]
- 29.Luconi M, Muratori M, Forti G, Baldi E. Identification and characterization of a novel functional estrogen receptor on human sperm membrane that interferes with progesterone effects. J Clin Endocrinol Metab. 1999;84:1670–1678. doi: 10.1210/jcem.84.5.5670. [DOI] [PubMed] [Google Scholar]
- 30.Hess RA, Bunick D, Bahr JM. Sperm, a source of estrogen. Environ Health Perspect. 1995;103 (Suppl 7):59–62. doi: 10.1289/ehp.95103s759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang ZQ, Zhou HY, Chen SD. The role of DJ-1 in the pathogenesis of Parkinson’s disease. Neurosci Bull. 2006;22:232–234. [PubMed] [Google Scholar]
- 32.Honbou K, Suzuki NN, Horiuchi M, et al. The crystal structure of DJ-1, a protein related to male fertility and Parkinson’s disease. J Biol Chem. 2003;278:31380–31384. doi: 10.1074/jbc.M305878200. [DOI] [PubMed] [Google Scholar]
- 33.Luo C, Li Y, Wang H, Feng Z, Long J, Liu J. Mitochondrial accumulation under oxidative stress is due to defects in autophagy. J Cell Biochem. 2013;114:212–219. doi: 10.1002/jcb.24356. [DOI] [PubMed] [Google Scholar]
- 34.Tsai SJ, Chiu CP, Yang HT, Yin MC. s-Allyl cysteine, s-ethyl cysteine, and s-propyl cysteine alleviate β-amyloid, glycative, and oxidative injury in brain of mice treated by D-galactose. J Agric Food Chem. 2011;59:6319–6326. doi: 10.1021/jf201160a. [DOI] [PubMed] [Google Scholar]
- 35.Milani P, Ambrosi G, Gammoh O, Blandini F, Cereda C. SOD1 and DJ-1 converge at Nrf2 pathway: a clue for antioxidant therapeutic potential in neurodegeneration. Oxid Med Cell Longev. 2013;2013:836760. doi: 10.1155/2013/836760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colín-González AL, Luna-López A, Königsberg M, Ali SF, Pedraza-Chaverri J, Santamaría A. Early modulation of the transcription factor Nrf2 in rodent striatal slices by quinolinic acid, a toxic metabolite of the kynurenine pathway. Neuroscience. 2014;260:130–139. doi: 10.1016/j.neuroscience.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 37.Garcia E, Santana-Martínez R, Silva-Islas CA, et al. S-allyl cysteine protects against MPTP-induced striatal and nigral oxidative neurotoxicity in mice: participation of Nrf2. Free Radic Res. 2014;48:159–167. doi: 10.3109/10715762.2013.857019. [DOI] [PubMed] [Google Scholar]
- 38.Tobón-Velasco JC, Vázquez-Victorio G, Macías-Silva M, et al. S-allyl cysteine protects against 6-hydroxydopamine-induced neurotoxicity in the rat striatum: involvement of Nrf2 transcription factor activation and modulation of signaling kinase cascades. Free Radic Biol Med. 2012;53:1024–1040. doi: 10.1016/j.freeradbiomed.2012.06.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.