Abstract
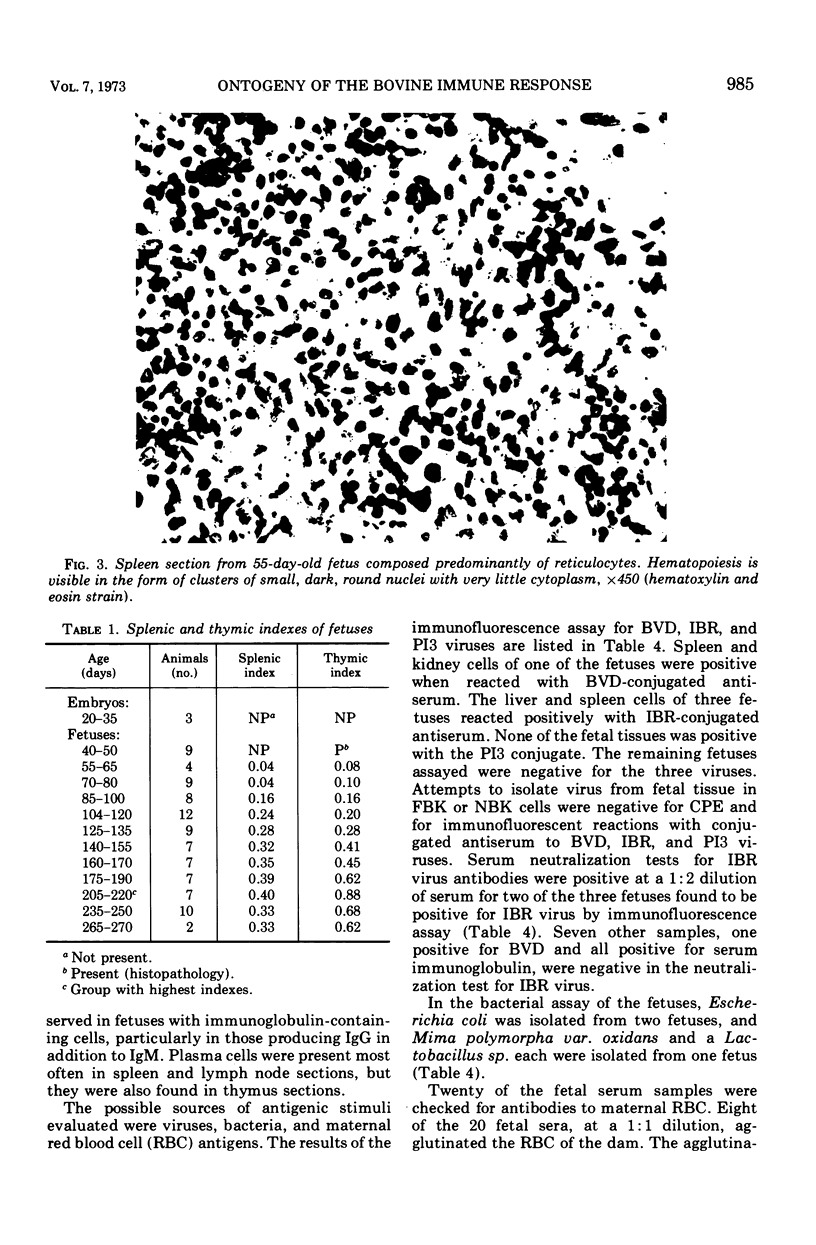
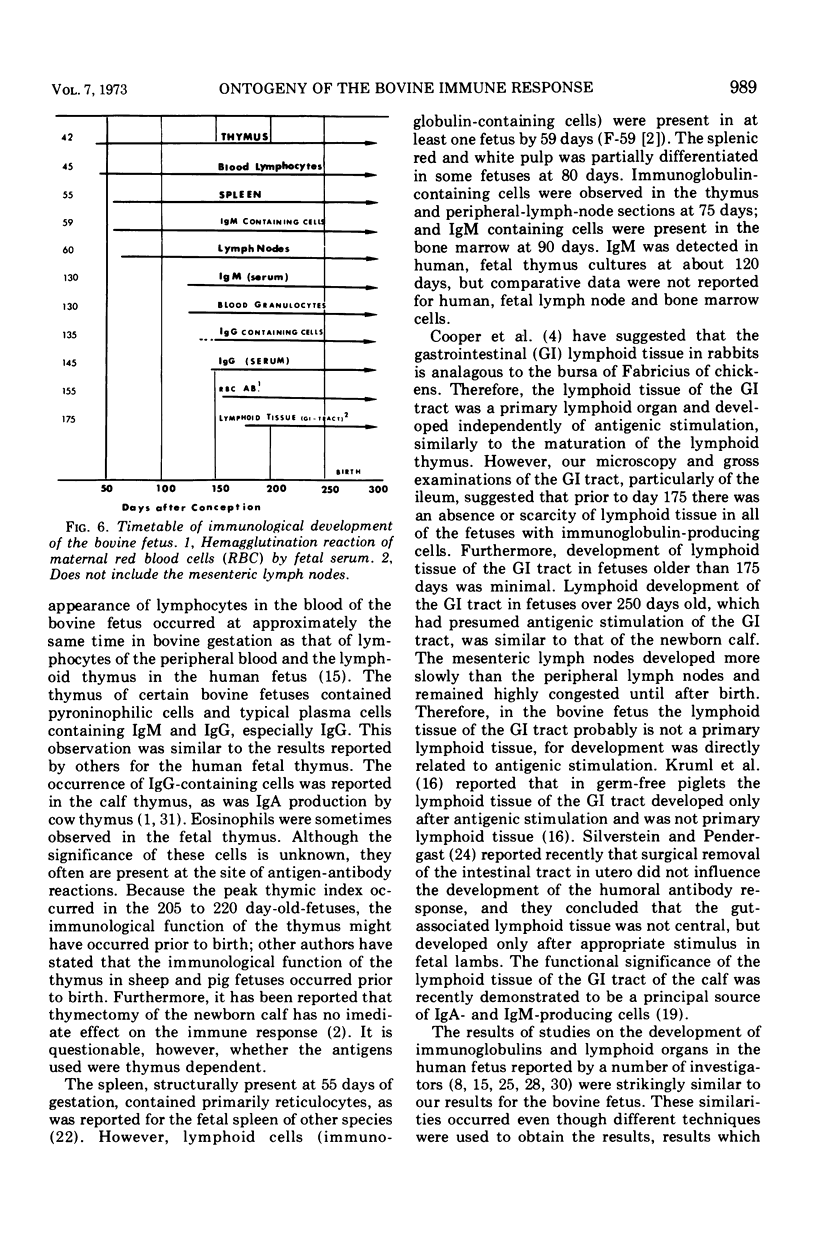
The ontogenesis of the bovine immune response was studied in three embryos (<40 days) and 106 fetuses of various ages. In the absence of overt antigenic stimulation, fetuses had lymphoid development of the thymus at 42 days of gestation, the spleen was structurally present at 55 days, and certain peripheral lymph nodes were present at 60 days. Mesenteric lymph nodes were structurally present by 100 days of gestation, and lymphoid tissue of the gastrointestinal tract, particularly the lower ileum, was observed in histologic sections of a 175-day fetus with a bacterial infection. Pyroninophilic cells, plasma cells, and germinal centers were present in lymph node sections of antigenically stimulated fetuses. Lymphoid tissue developed more rapidly in fetuses with bacteria, viral antigens, or apparent maternal red-blood-cell antigens than in the normal fetus. Thymic and splenic indices reached maximal values in the 205- to 220-day fetal age group. Immunoglobulin M (IgM)-containing cells were first observed, by immunofluorescence, in a single fetus at 59 days of gestation. Immunoglobulin G (IgG)-containing cells were observed at 145 days of gestation in one fetus with a bacterial and viral infection. IgM-containing cells were observed in 36 fetuses and IgM and IgG cells were present in seven fetuses. Spleen, lymph nodes, thymus, bone marrow, and liver of one fetus from a dam with lymphosarcoma had immunoglobulin-containing cells. Hemal lymph nodes, blood (buffy coat), Peyer patches, and heart and lung sections from fetuses with immunoglobulin-containing cells in spleen or lymph node did not have immunoglobulin-containing cells.
Antigens of the virus of bovine virus diarrhea-mucosal disease (BVD) were detected in one fetus, and antigens of infectious bovine rhinotracheitis (IBR) virus were detected in three fetuses; however, viruses were not isolated in primary bovine embryonic kidney cells. Two of the three fetuses with IBR virus antigens had neutralizing serum antibody titers to IBR virus. Bacteria including Escherichia coli, Lactobacillus sp. and Mima polymorpha var. oxidans were isolated from four fetuses. Antibodies that caused the agglutination of maternal red blood cells were present in 8 of 20 bovine fetal serum samples. The antibodies were 2-mercaptoethanol sensitive and partially heat resistant (56 C for 30 min). The ontogeny of the bovine immune response and human immune response were compared, and it was suggested that the similarities were primarily due to the two species having the same approximate gestation period of 280 days.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buttler J. E., Kiddy C. A., Maxwell C., Hylton M. B., Asofsky R. Synthesis of immunoglobulins by various tissues of the cow. J Dairy Sci. 1971 Sep;54(9):1323–1324. [PubMed] [Google Scholar]
- COONS A. H., KAPLAN M. H. Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. J Exp Med. 1950 Jan 1;91(1):1–13. doi: 10.1084/jem.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll E. J., Theilen G. H., Leighton R. L. Immunologic competence of thymectomized neonatal calves. Am J Vet Res. 1968 Jan;29(1):67–70. [PubMed] [Google Scholar]
- Cunningham A. J. The morphology of antibody-forming cells in the mouse. Aust J Exp Biol Med Sci. 1968 Apr;46(2):141–153. doi: 10.1038/icb.1968.12. [DOI] [PubMed] [Google Scholar]
- FLODIN P., KILLANDER J. Fractionation of human-serum proteins by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:402–410. doi: 10.1016/0006-3002(62)90104-x. [DOI] [PubMed] [Google Scholar]
- GOOD R. A., PAPERMASTER B. W. ONTOGENY AND PHYLOGENY OF ADAPTIVE IMMUNITY. Adv Immunol. 1964;27:1–115. doi: 10.1016/s0065-2776(08)60706-3. [DOI] [PubMed] [Google Scholar]
- Gitlin D., Biasucci A. Development of gamma G, gamma A, gamma M, beta IC-beta IA, C 1 esterase inhibitor, ceruloplasmin, transferrin, hemopexin, haptoglobin, fibrinogen, plasminogen, alpha 1-antitrypsin, orosomucoid, beta-lipoprotein, alpha 2-macroglobulin, and prealbumin in the human conceptus. J Clin Invest. 1969 Aug;48(8):1433–1446. doi: 10.1172/JCI106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans W., Schuit H. R., Klein F. An immunofluorescence procedure for the detection of intracellular immunoglobulins. Clin Exp Immunol. 1969 Apr;4(4):457–472. [PMC free article] [PubMed] [Google Scholar]
- Hiramoto R. N., Hamlin M. Photography of precipitin bands developed with fluorescent antibodies. Immunology. 1968 Jul;15(1):31–33. [PMC free article] [PubMed] [Google Scholar]
- Jacobson E. B., Thorbecke G. J. Relationship of germinal centers in lymphoid tissue to immunologic memory. 3. Proliferative response of primed cells from splenic white and red pulp following reexposure to antigen in vitro. J Immunol. 1968 Sep;101(3):515–522. [PubMed] [Google Scholar]
- Kruml J., Ludvík J., Trebichavský I., Mandel L., Kovárů F. Morphology of germ-free piglets. Folia Microbiol (Praha) 1969;14(5):441–446. doi: 10.1007/BF02872789. [DOI] [PubMed] [Google Scholar]
- Muschel L. H. Blood groups, disease, and selection. Bacteriol Rev. 1966 Jun;30(2):427–441. doi: 10.1128/br.30.2.427-441.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter P., Noakes D. E., Allen W. D. Intestinal secretion of immunoglobulins in the preruminant calf. Immunology. 1972 Sep;23(3):299–312. [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- SILVERSTEIN A. M., LUKES R. J. Fetal response to antigenic stimulus. I. Plasmacellular and lymphoid reactions in the human fetus to intrauterine infection. Lab Invest. 1962 Nov;11:918–932. [PubMed] [Google Scholar]
- Schultz R. D., Confer F., Dunne H. W. Occurrence of blood cells and serum proteins in bovine fetuses and calves. Can J Comp Med. 1971 Apr;35(2):93–98. [PMC free article] [PubMed] [Google Scholar]
- Sterzl J., Silverstein A. M. Developmental aspects of immunity. Adv Immunol. 1967;6:337–459. doi: 10.1016/s0065-2776(08)60525-8. [DOI] [PubMed] [Google Scholar]
- Yurchak A. M., Butler J. E., Tomasi T. B., Jr Fluorescent localization of immunoglobulins in the tissues of the cow. J Dairy Sci. 1971 Sep;54(9):1324–1325. [PubMed] [Google Scholar]
- van Furth R., Schuit R. E., Hijmans W. The formation of immunoglobulins by human tissues in vitro. I. The methods and their specificity. Immunology. 1966 Jul;11(1):1–11. [PMC free article] [PubMed] [Google Scholar]