Abstract
Bullous pemphigoid (BP) is an autoimmune skin disease characterized by the binding of autoantibodies to components of the hemidesmosome structure resulting in an inflammatory response and subepidermal blister formation. To investigate the role of immune orientation in the inflammatory processes associated to disease progression, blister fluid, serum and biopsy specimens were collected from thirty one consecutive BP patients. Blister fluids displayed high level of IL-6, IL-17, IL-22, IL-23, whereas TGF-β was increased in BP sera. However neither immunocytochemistry on a trans-differentiation model of IL-17-producing PBMCs nor immunohistochemistry on BP biopsy specimens could demonstrate the presence of Th17 lymphocytes. Instead innate immune cells, especially neutrophils, produced IL-17 at the skin lesional site. Of note, superpotent topical corticosteroid application quickly and dramatically reduced both IL-17 expression and clinical signs of BP. Consistently, IL-17 upregulated MMP-9 and neutrophil elastase expression, two proteases involved in blister formation, thereof further demonstrating its role in the progress of BP. Finally IL-17-induced matrix degradation originated from neutrophil activation, initiated the formation of an amplification loop of the inflammatory response that could represent the underlying phenomenon leading to the maintenance and even disease extent. Thus, our results could open new therapeutic strategies for BP patients.
INTRODUCTION
Bullous pemphigoid (BP), the most common autoimmune blistering skin disease in developed countries (Langan et al. 2008), is characterized by the binding of autoantibodies to two major proteins of the dermal-epidermal junction (DEJ), BP180 and BP230. Animal model studies showed the critical role of autoimmune complexes in triggering the inflammatory process leading to the DEJ disruption and subsequently to blister formation (Liu et al. 1993)(Hirose et al. 2011)(Oswald et al. 2012).
BP considered as a Th1/Th2 cell-mediated disease with a predominance of Th2 cells, is associated with BP180/BP230 autoantibodies production. However, autoantibodies binding cannot explain all of the diverse clinical features observed in this disease. Especially, after few weeks of treatment with either systemic or superpotent topical corticosteroid (STC), BP patients are cleared of all clinical symptoms or signs while anti-BP180 autoantibodies serum level is still high (Schmidt E 2000)(Di Zenzo et al. 2008)(Fichel et al. 2013). A dense lesional infiltration of polymorphonuclear cells (PMN) along with the production of proteases such as matrix-metalloprotease-9 (MMP-9) or neutrophil elastase (NE) were shown to be crucial in the extension of the disease (Liu et al. 1997)(Liu et al. 1998)(Liu et al. 2000a). However the mechanisms involved in the recruitment of the inflammatory cells and in the regulation of these proteases in BP are still not complete and remain to be better defined at the molecular level.
Recently compelling evidence highlighted the major contribution of Th17 cells in autoimmune diseases (Kleinewietfeld and Hafler 2013). In contrast to Th1 and Th2 cells, the recently defined Th17 lineage displays certain plasticity with respect to cytokine environment (Weaver et al. 2007)(Hoechst et al. 2011)(Lee et al. 2009). Although Treg and Th17 cells functionally antagonize each other, they both require TGF-β in their early differentiation from naive precursors (Manel et al. 2008)(Mangan et al. 2006). However and conversely to Treg, a proinflammatory cytokines network such as IL-1 or IL-6, in concert with high level of IL-23 are further required for late Th17 polarization. Besides Th17 cells, it has to be mentioned that several innate immune cell type such as innate lymphoid cells (NK and γδ T cells), neutrophils and mast cells can also produce IL-17 in inflammatory conditions (Cua and Tato 2010)(Lin et al. 2011)(Keijsers et al. 2013). In BP, some recent studies using immunohistochemical approaches have suggested the presence of Th17 cells at site of the dermal-epidermal detachment (Arakawa et al. 2011)(Fischer-Stabauer et al. 2012)(Zebrowska et al. 2013). Although Th17 cells are characterized by their ability to produce high level of IL-17 (Harrington et al. 2005)(Park et al. 2005), the mechanisms conducting to IL-17 production and its link with blister formation are still unraveled in BP.
A long-known outcome of IL-17 biological cascade is the production of inflammatory molecules such as cytokines (TNFα, IL1-β, GM-CSF, IL-6), chemokines (IL-8) and proteases (MMP-9) (Miossec and Kolls 2012), which are a hallmark of the inflammatory response associated to BP (Kasperkiewicz and Zillikens 2007). In this study, the evaluation of inflammatory cytokine expression (IL-17, IL-22, IL-6, IL-23), effector or marker of tissue damage (MMP-9 and the tri-peptide PGP), and molecules regulating the inflammatory network such as TGF-β in both serum and blister fluid samples, revealed an amplification loop originated by neutrophil-induced IL-17 that could favor the perpetuation or even the extent of the disease.
RESULTS
IL-17 and MMP-9 expression in BP
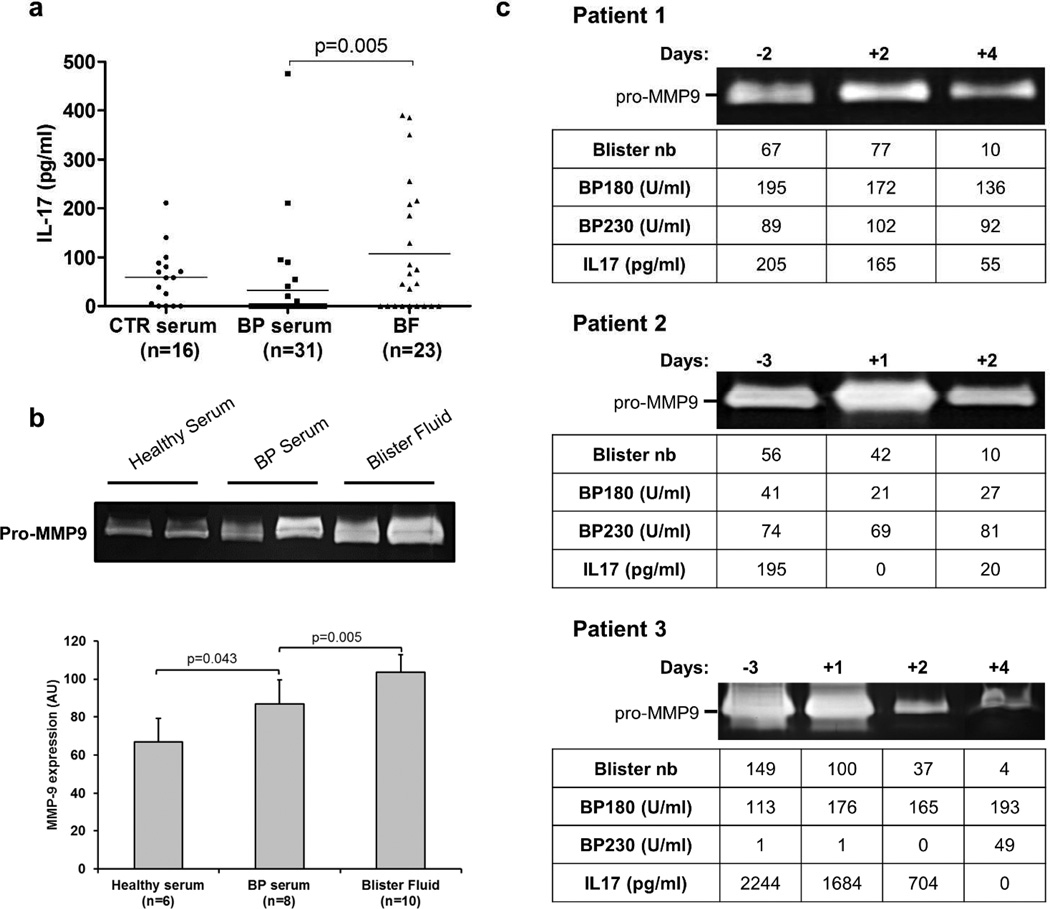
Using specific ELISA, we provided evidence for a significant IL-17 increased level in BF compared to BP serum from 31 consecutive patients (Figure 1a), whereas no variation was observed between the level of IL-17 in serum from BP patients and from age- and sex-matched controls. Concomitantly, we analyzed by gel zymography the levels of secreted MMP-9 both in blister fluids and sera obtained from BP patients at time of diagnosis. MMP-9 secretion was significantly increased in BP sera (n=8) compared to control (n=6), and was even higher in blister fluids (n=10) compared to BP sera (Figure 1b), supporting results obtained by Niimi et al (Niimi et al. 2006).
Figure 1. IL-17 levels and MMP-9 activity are positively related in blister fluid before and after topical steroid treatment in BP patients.
(a) Scatter plots of the IL-17 levels measured by ELISA in control (CTR) serum, BP serum and BF collected at time of diagnosis. The solid line represents the mean level of IL-17 in each group. (b) MMP-9 activity was measured in healthy serum, BP serum and BF by gelatin zymography and ImageJ software was used for quantification. The error bars denote the mean± SD and Mann-Whitney test was used for statistics. (c) Blister number, MMP-9 activity, IL-17, BP180 and BP230 autoantibodies levels in BF were followed before and few days after the beginning of the treatment in 3 BP patients.
In order to seek a potential relationship between IL-17 and MMP-9 as well as their involvement in the BP pathological processes, we investigated whether STC treatment could affect their expression at the lesional site. In this line, BF from three BP patients were collected before and after the early days of treatment (Joly et al. 2009). We observed a dramatic decrease of both IL-17 and MMP-9 levels after only few days of STC treatment, varying from 2 to 4 days (Figure 1c). Concomitantly, STC treatment induced a fast drop in the number of daily new blisters, whereas the level of anti-BP180/BP230 autoantibodies remained quite stable over this short period of time, emphasizing the importance of the inflammation process in BP blister occurrence.
IL-17-associated pro-inflammatory cytokines in BP
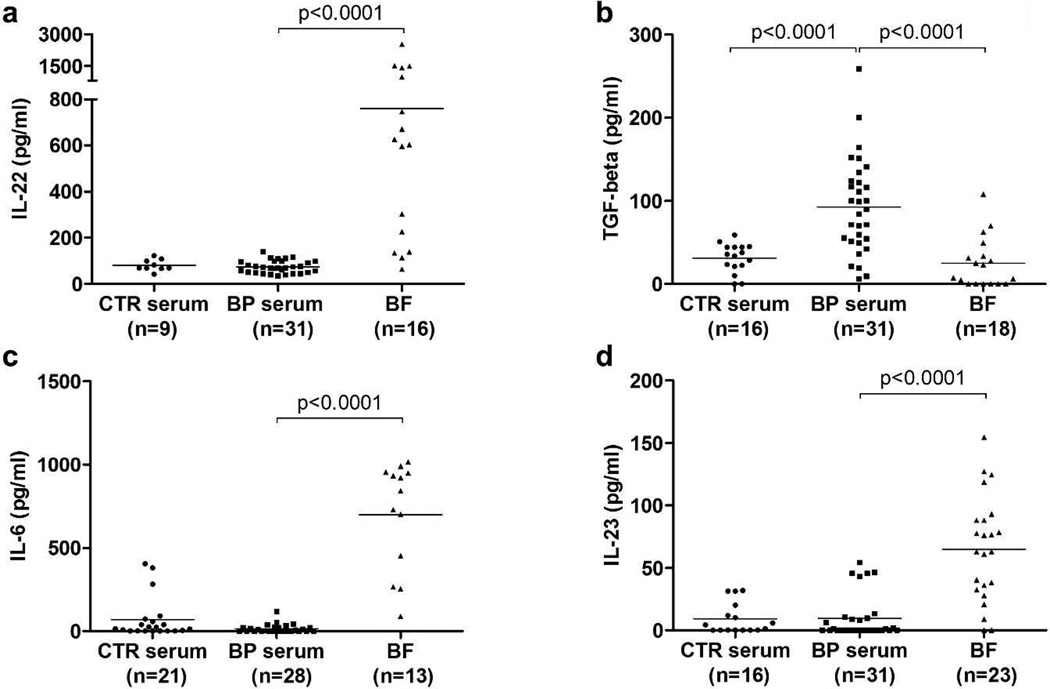
To further characterize the mechanisms associated to IL-17 expression in BP, we measured the level of TGF-β, IL-6, IL-22, IL-23, cytokines associated to the developmental plasticity of Th17 precursors in both sera and BF collected from BP patients at time of diagnosis (Figure 2). TGF-β, known to prime Th17 orientation, was significantly overexpressed in BP sera compared to BF and to serum from controls. In contrast, levels of IL-6, IL-22 and IL-23, three cytokines associated with Th17 cells terminal differentiation, were significantly higher in BF compared to BP sera, suggesting a progressive differentiation towards IL-17 producing cells, starting first in the blood and then achieved in situ nearby the site of lesion. In our series, the level of these 3 cytokines was not statistically increased in BP serum compared to controls.
Figure 2. IL-17-associated cytokine pattern in biological fluids from BP patients.
Scatter plots of the Th17-related cytokine levels IL-22 (a) and the pro-inflammatory cytokines levels TGF-β (b), IL-6 (c) and IL-23 (d) measured by ELISA in control (CTR) serum, and serum and BF from BP patients at time of diagnosis. The solid line represents the mean level of each cytokine in each group. Mann-Whitney test was used for statistics.
IL-17 expression by innate immune cells in BP
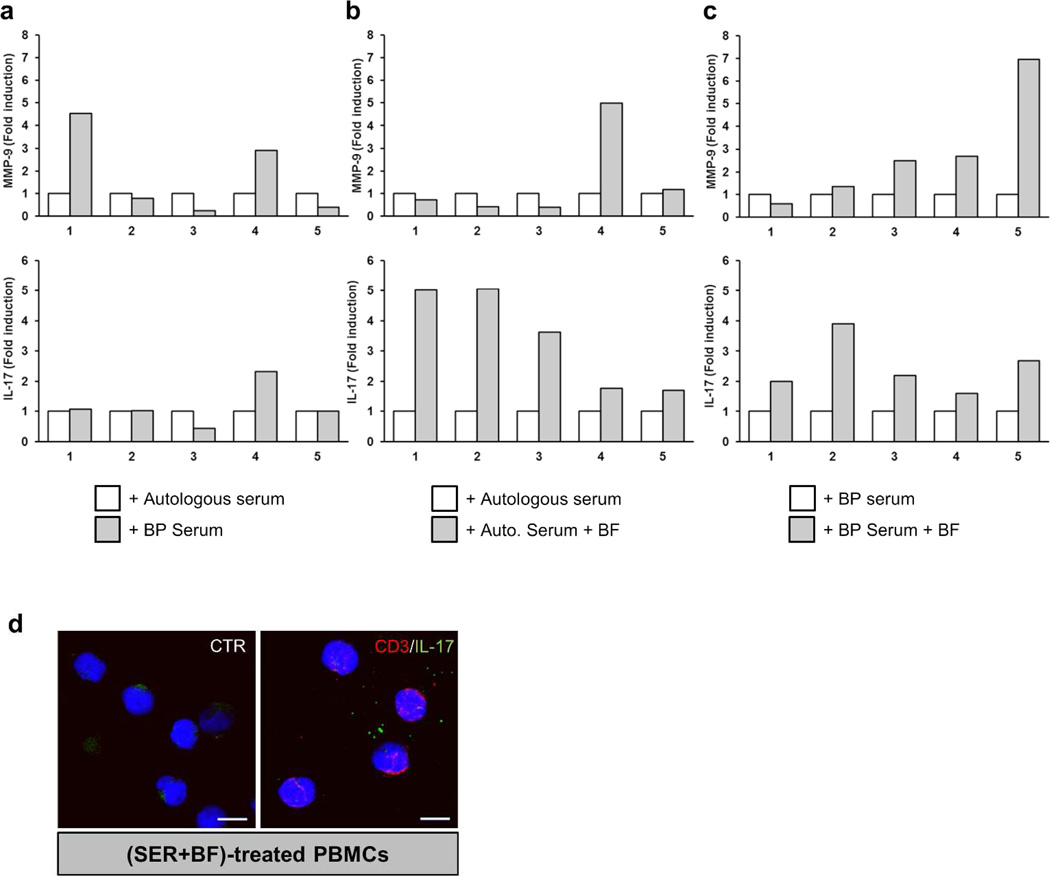
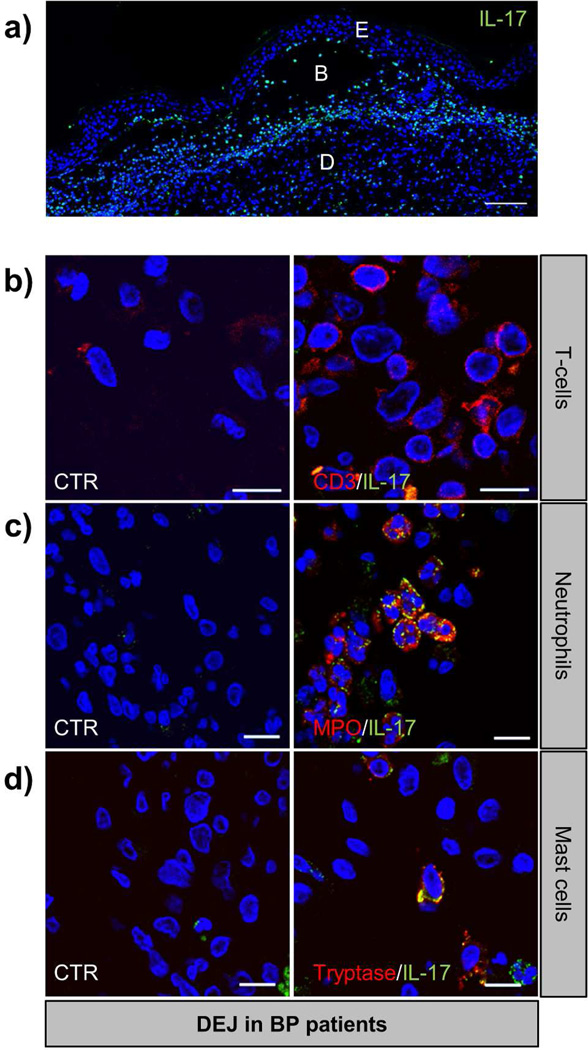
In order to verify our suspected mechanism of IL-17 production through a cell trans-differentiation process from blood to lesional skin, we established a BP-related new working model to illustrate IL-17 overexpression. To that purpose, peripheral blood mononuclear cells (PBMCs) were isolated from healthy subjects (n=5), consecutively stimulated by BP sera for 48 hours followed by an incubation with BF for an additional 48 hours. To determine the respective role of BP serum and BF in il17 and mmp9 expression, cells from the same subject stimulated with BP serum or autologous serum and combined with BF were compared to cells stimulated with either autologous or BP serum alone. mmp9 expression was analyzed concomitantly to il17 by quantitative PCR. Inter-individual variability in mmp9 and il17 expression was observed in response to BP sera alone as compared to autologous stimulation (Figure 3a). When blister fluid was consecutively added to cells initially stimulated with autologous serum, il17 but not mmp9 expression increased in all PBMCs tested (Figure 3b). Remarkably, BP serum and BF consecutive PBMC stimulation induced a substantial increase of mmp9 expression (Figure 3c). However, replacement of autologous serum by BP serum did not magnify the effects of BF on il17 expression (Figure 3b–c). Using our model, co-localization analysis by immunocytochemistry, revealed that none of the CD3+ cells expressed IL-17 (Figure 3d). Although IL-17 was highly expressed by infiltrated cells in the upper dermis of BP patient lesional skin (Figure 4a), immunohistochemistry analysis of BP biopsy specimens showed no double positive cells for CD3 and IL-17 in situ (Figure 4b). Instead, innate immune cells such as infiltrated MPO+ neutrophils and tryptase-positive mast cells strongly expressed IL-17 (Figure 4c–d).
Figure 3. Lymphocytes do not express IL-17 in a trans differentiation model.
mmp9 and il17 gene expression was analyzed by real-time qPCR in freshly and healthy isolated PBMCs (n=5). PBMCs were treated with either autologous or BP serum (a). PBMCs were consecutively treated with either autologous (b) or BP serum (c) for 48 hours and then stimulated or not with BF for an additional 48 hours. (d) Immunofluorescence double staining for IL-17 (green) and CD3 (red) on (SER+BF)-treated cells. Nuclei are stained in blue. CTR: negative control where primary antibodies were not added. Scale bar, 10 µm.
Figure 4. IL-17 is produced by neutrophils and mast cells but not lymphocytes in skin of BP patients.
Punch biopsies of skin from patients with BP were subjected to IL-17 staining alone (a) or double immunofluorescence staining for IL-17 (green) and either CD3 (b), MPO (c), or tryptase (d) in red with Hoescht counterstain (blue). These high-power fields at the dermo-epidermal junction (DEJ) illustrate the colocalization of IL-17 with MPO and tryptase (yellow) but not with CD3. CTR: negative control where primary antibodies were not added. Scale bars, 100µm (a) and 10 µm (b–d). E: epidermis, B: blister, D: dermis
IL-17 induced expression of MMP-9 and neutrophil elastase
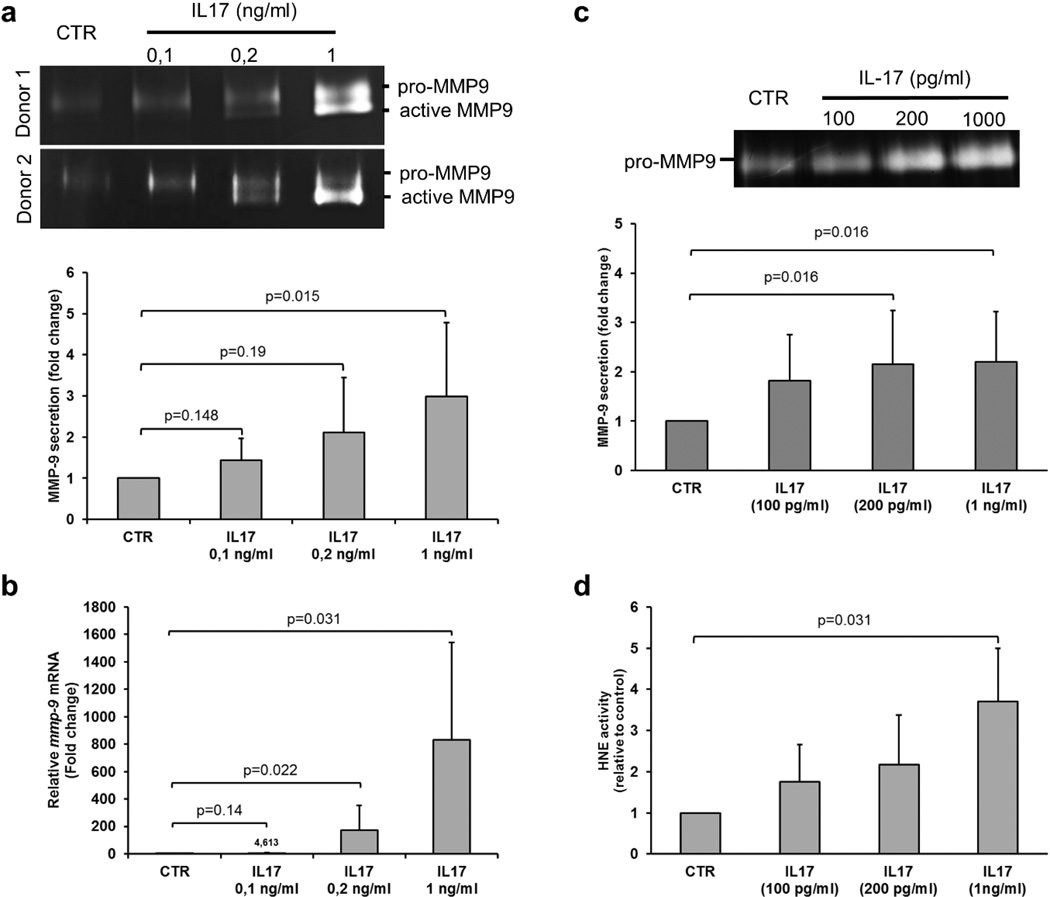
We then wondered whether IL-17 could modulate MMP-9 expression and activation from both PBMC and PMN cells. IL-17 used at a concentration equivalent to those measured in BF (0.2 ng/ml) significantly increased MMP-9 secretion from PBMC and PMN cells (Figure 5a-5c). In PBMC, MMP-9 protease, resulting from an increased mRNA expression (Figure 5b), was present under both the pro-MMP-9 and the active form, as demonstrated by the appearance of a second band on gel zymography (Figure 5a). Concurrently to MMP-9 activation in PBMC, IL-17 also significantly increased the elastase (HNE) activity released from PMN (Figure 5d).
Figure 5. IL-17 regulates proteases expression and activation in PBMC and PMN.
a) Gelatin zymography was used to detect MMP-9 gelatinolytic activity in IL-17-treated PBMCs and quantification (n=7) was done. b) mmp9 expression was measured by real-time qPCR in PBMCs stimulated with different concentrations of IL-17 (n=6). The error bars denote the mean± SEM. Freshly isolated PMNs were stimulated with different concentrations of IL-17 for one hour. (c) MMP-9 and (d) HNE activities were detected by gelatin zymography and elastase activity assay respectively. The experiments above have been repeated at least with 6 different donors and the error bars denote the mean ± SEM. Wilcoxon paired-test was used for statistics.
PGPs from BP blister fluid generate a neutrophil-mediated amplification loop
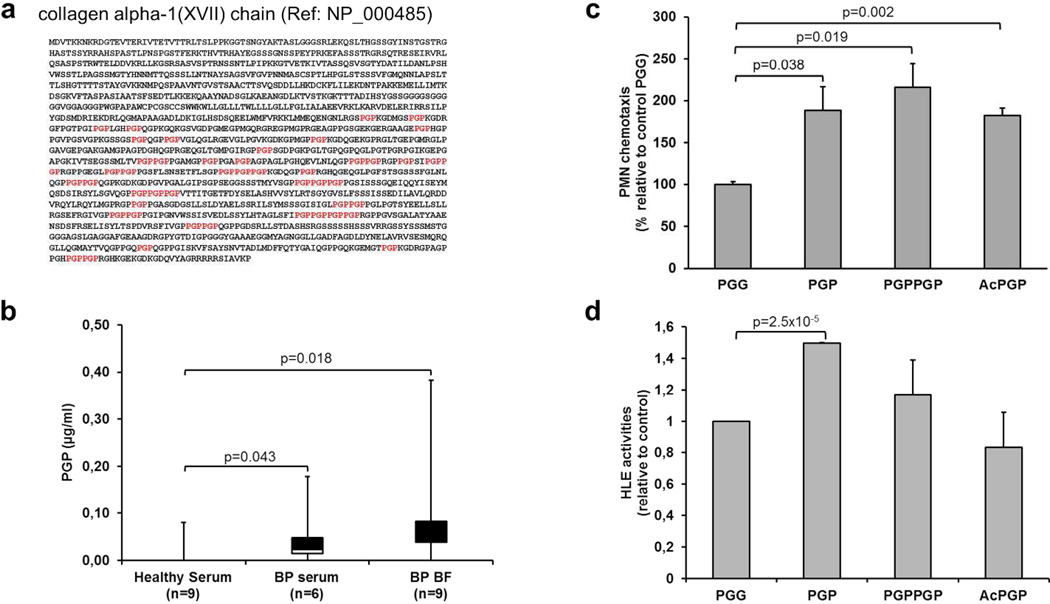
We previously showed that MMP-9 cleaved the collagenic hemidesmosomal protein BP180 into small peptides (Verraes et al. 2001). In silico analysis of human BP180 protein sequence identified, within the C-Terminal domain of BP180, 47 PGP motifs (figure 6a) able to chemoattract neutrophils (Weathington et al. 2006)(Pfister et al. 1995). Analysis by mass spectrometry revealed that PGP levels were significantly elevated in BP serum compared to serum from healthy controls, and even higher in BF showing a gradient of these peptides from the tissue lesion till the blood circulation (Figure 6b). Compared to the control peptide PGG, PGP but also PGPPGP and AcPGP peptides significantly enhanced PMN chemotaxis (figure 6c). However, only PGP peptides significantly induced HNE release from PMN (figure 6d) supporting their role in an amplifying inflammatory loop in BP.
Figure 6. PGP peptides from BP biological fluids affect PMN chemotaxis and PMN activation.
(a) PGP sequences (in red) within the C-terminal part of collagen XVII protein sequence. (b) PGP peptides detection by electrospray ionization liquid chromatography-MS/MS in serum from patients without inflammatory and/or autoimmune diseases, BP serum and BF. Data are shown as a box-and-whisker plot. The median of the distribution is indicated by the horizontal line in the box. Mann-Whitney test was used for statistics. (c) Chemotactic migration of PMN induced by PGP, PGPPGP or AcPGP peptides (10 µM) in a Boyden chamber chemotaxis assay (n=3). (d) HNE activities in PGP, PGPPGP or AcPGP-stimulated PMNs relative to control (n=3). The error bars denote the mean± SEM. Student t-test was used for statistics.
DISCUSSION
Strengthening the importance of an inflammatory response linked to MMP-9 expression in blister formation in BP (Liu et al. 1998)(Verraes et al. 2001), we here characterized an IL-17-induced MMP-9-associated inflammatory response controlled by innate immune cells. We emphasized the formation of an auto-amplification loop that could self-reinforce the chronic inflammatory state and therefore exacerbate the tissue damages observed in BP patients with severe disease.
Elevated levels of IL-17 in blister fluids support recent studies that highlighted the presence of IL-17-producing cells in BP (Arakawa et al. 2011)(Fischer-Stabauer et al. 2012)(Zebrowska et al. 2013), and suggested that BP regulation was beyond the Th1/ Th2 paradigm. Although our study confirmed the presence of TGF-β in BP serum (D’Auria et al. 1999), and of IL-6 in BF (Rhodes et al. 1999), but also described the expression of IL-23 in BF, neither immunohistochemistry on BP biopsy specimens nor immunocytochemistry approaches in our ex vivo model supported the hypothesis of an immune Th17 T-cell orientation as proposed by Arakawa and collaborators. These authors used CD4 as biological marker to characterize lymphocytes at the site of lesion. Nevertheless, CD4 is also expressed by monocyte/macrophage, dendritic cells and even by neutrophils in some conditions. Furthermore, in Arakawa’s study histological counterstain technique was used to identify IL-17 producing cells. In contrast, the use of CD3, a specific T-cell marker, associated with immunofluorescence co-localization, did not allow in our work the detection of any IL-17-producing lymphocytes. Accordingly, PBMC trans-differentiation using both serum and BF from BP patients could not illustrate the presence of IL-17 expression by lymphocyte. Hence, as CD4 is also expressed by innate immune cells such as macrophages, the results obtained in our and their studies would rather advocate for the role of innate immune cells in BP.
Our results provided compelling reasons to explore outside the Th17 cell paradigm to explain the high production of IL-17 at site of lesion in BP. Actually, IL-17 originally thought to be produced exclusively by T-cells (Fossiez et al. 1996) is also secreted by a variety of innate cells such as macrophages, neutrophils, dendritic cells (Korn et al. 2009)(Cua and Tato 2010)(Biswas et al. 2003). Although the use of our PBMC trans-differentiation model argued for a production of IL-17 by macrophages, in situ analysis demonstrated a large amount of IL-17-producing neutrophils. Besides, mast cells also expressed high level of IL-17 reinforcing the major role of innate immune cell in the inflammatory response in BP. Based on a recent report (Taylor et al. 2014), one could hypothesize that an initial IL-17 expression could subsequently stimulate neutrophils to produce even more IL-17 in an autocrine way, although such mechanism still needs to be demonstrated in BP.
Although it sounds clear that innate immune cells are involved in IL-17 production in BP, the underlying mechanisms are still unraveled. Autoantibodies form tissue-deposited immune complexes that directly activate neutrophils via their FcγR (Mayadas et al. 2014). Furthermore it was recently shown in rheumatoid arthritis that immune complex-activated neutrophils triggered the production of IL-17 (Katayama et al. 2013). It was also reported an il17 mRNA increase in purified neutrophils after administration of anti-neutrophil cytoplasmic autoantibodies (Hoshino et al. 2008). Then in BP like in other autoimmune diseases, neutrophils could produce IL-17 as a result of “sterile” injury subsequent of barrier tissue alteration following antigen processing. As above-mentioned, IL-23 was enhanced in BF from BP patients. Constitutive expression of IL-23R is a common characteristic of many IL-17-producing innate cells (Martin et al. 2009)(Yoshiga 1998), although it is not known whether neutrophils express this receptor on their surface yet. Other inflammatory processes such as leukotriene B4 application were also shown to induce neutrophil IL-17 expression (Keijsers et al. 2013), illustrating the multiplicity of pathways able to induce IL-17 production by neutrophils. Although our model study is inadequate to demonstrate the primum movens event put forward by skin sentinel cells that would lead to IL-17 production in BP, we emphasized the role of neutrophils in IL-17 production.
IL-17 enhanced the expression of PBMC- and PMN- MMP-9 under its latent and active forms and of the HNE enzyme. In BP, MMP-9 and HNE production is crucial for blister formation (Liu et al. 1998)(Liu et al. 2000a)(Verraes et al. 2001). By inactivating α1-proteinase inhibitor, MMP-9 enables HNE activity, the main enzyme responsible of BP180 degradation in BP (Liu et al. 2000b). Furthermore, IL-17 up-regulates the expression of key cytokines, chemokines and proteases involved in the recruitment of neutrophils, macrophages and lymphocytes, and drives the degradation of extracellular matrix at the site of lesion (Onishi and Gaffen 2010). Noteworthy, matrix molecule degradation also participates to BP progression. MMP-9 and neutrophil elastase cleave the extracellular domain of BP180 (Verraes et al. 2001)(Ståhle-Bäckdahl et al. 1994) (Lin et al. 2012). Release of a 12 kDa digestion product from murine BP180 displayed chemo-attractive effects on neutrophils (Lin et al. 2012). Accordingly and for the first time in BP to our knowledge, we evidenced the presence in BF of high levels of the tri-peptides PGPs, present all along the extracellular collagen-like domain of BP180. Like in other tissues (Weathington et al. 2006)(Pfister et al. 1995)(Xu et al. 2011), these tri-peptides attract and activate neutrophils in skin. PGP could therefore generate a self-propagating chronic neutrophilic inflammation in BP. Indeed, it has been shown with use of several animal models that blister formation originates from autoantibodies binding to the hemidesmosomal protein BP180 leading to PMN recruitment and activation, followed by proteases release at site of lesion. To that scheme, our results add an amplification loop in which matrix degradation product could auto-amplify the inflammatory reaction, independently of the autoimmune response. Upon IL-17 recruitment and activation, neutrophils produce even more IL-17, and release protease leading to matrix degradation and to the generation of matrix peptides that further favor neutrophil recruitment and activation at site of skin lesion. Then, the inflammatory process leading to blister formation would become independent of the immune complex formation as originally demonstrated in the progress of BP disease. According to previous models, beyond the Th1-polarized associated initiation leading to the production of pathogenic anti-BP180 antibodies (Kasperkiewicz and Zillikens 2007), the innate-associated inflammation could favor disease perpetuation or even extent this autoimmune bullous disease.
The IL-17-associated inflammatory auto-amplification loop in BP was in line with the blister content analysis before and after treatment with steroids. Indeed, STC treatment very efficiently reduced blister formation, MMP-9 and IL-17 production within the first days of treatment. Accordingly, in a BP experimental model, methylprednisolone reduced autoantibodies-induced PMN activation (Hellberg et al. 2013). Actually, patients’ early clinical signs clearing upon STC treatment were actually bound to the control of the IL-17-associated inflammatory phase of this autoimmune disease. Of note, it has been reported that activation of IL-17 receptor on B cells promotes B-cell differentiation to autoantibody-producing plasma cells (Doreau et al. 2009). Then, expression of IL-17 could link innate and adaptive immunity in autoimmune diseases such as BP, and blocking the IL-17 pro-inflammatory amplification processes could disrupt on the long term signals such as release of cryptic antigenic peptides during matrix degradation and lymphocytes B activation, feeding the inflammatory and autoimmune responses respectively. Hence, our results relative to BP are in setting with most IL-17 studies, which focused on its ability to drive inflammation in autoimmune disorders including multiple sclerosis, Crohn’s disease, rheumatoid arthritis, psoriasis and ankylosing spondylitis (Miossec and Kolls 2012). It would then be of interest to decipher whether such amplification loops of inflammation are also involved in these diseases.
In conclusion, our study documents the immune and inflammatory responses in BP, and suggests a mechanism conducting to the generation of IL-17 producing cells and overexpression of proteases. This mechanism could represent the underlying phenomenon that conducts to disease extent in BP, which has been shown to be associated with further relapse in these patients (Fichel et al. 2013). Such process is therefore of clinical importance and similarly pre-clinical studies supporting a role for IL-17 in autoinflammatory or autoimmune diseases led to current clinical trials designed to block IL-17, its inducers (i.e. IL-23, IL-6) or the IL-17 receptor (IL-17R) in autoimmunity (Miossec and Kolls 2012)(Iwakura et al. 2011). Hence, targeting IL-17 signaling cascade could be a promising therapeutic strategy in the treatment of an inflammation response associated to bullous auto-immune diseases including bullous pemphigoid.
MATERIAL AND METHODS
Study design
This prospective, monocentric, observational and translational study was conducted in the dermatology French department of Reims University Hospital, which belong to the French Referral Center for Autoimmune Bullous Diseases (Reims, Rouen, Limoges), and in the dermatology department of the Reims university of Medicine. Thirty one consecutive patients with BP admitted from 2009 to 2011 were assessed (8 men and 23 women; mean age 80.8 years, range 53–96 years). The diagnosis of BP was established on the basis of clinical and immunohistological criteria (Di Zenzo et al. 2012)(Vaillant L et al. 1998). Biological samples (serum and blister fluid) were collected at time of diagnosis and until clinical signs clearing upon steroid treatment (blister fluid). All voluntary subjects gave their written informed consent prior to inclusion in the study. Serum samples from age- and sex-matched patients without inflammatory and autoimmune diseases admitted to the trauma department of the Reims University Hospital were used as controls.
Ethics statement
The study was approved by the Ethics Committee of the University Hospital of Reims (institutional review board; 04/14/2009); patients or their relatives were informed by letter and gave their written consent, in accordance with the Helsinki Declaration.
Cell isolation and ex vivo stimulation
Peripheral Blood Mononuclear Cells (PBMCs) and PMN were obtained by density-gradient centrifugation from EDTA-treated whole blood using a density gradient medium (Granulosep, Eurobio-Abcys).
In order to mimic in vivo conditions, freshly isolated PBMCs from healthy subjects were cultured in RPMI medium with 25% BF for 48 hours, consecutively to a stimulation with either 25% autologous serum or 25% BP serum for 48 hours before to be harvested for gene expression analysis (n=10) or cytospun for immunofluorescence studies. Control PBMCs were cultured with either 25% autologous serum or BP serum alone.
PBMCs and PMN isolated from BP patients were stimulated for 1 hour respectively by IL-17, or PGG/PGP peptides (100nM) alone before to harvest cells for gene expression analysis and medium for MMP-9 secretion or HNE activity analysis.
Quantitative RT- PCR
RNA was extracted with the TriReagent (Molecular Research Center, Inc.) from freshly isolated PBMCs control or PBMC stimulated for 24h or 96h respectively with IL-17 at different concentrations (0.1, 0.2 and 1 ng/ml) or with the combination of BP serum and BF. Protocol for the quantitative RT-PCR is described in details in Supplemental Data.
Determination of cytokine levels
Cytokines (IL-17, IL-22, TGF-β, IL-23 and IL-6) and autoantibodies (BP230 and BP180) concentration were determined in BP serum and/or BF following the manufacturer’s instructions of the respective commercially available ELISA assays (R§D Systems and Clinisciences respectively).
Electrospray ionization liquid chromatography-MS/MS for PGP detection
(ESI-LC/MS/MS) for PGP detection was performed as described in (Hardison et al. 2009). PGP were measured in blister fluids and serum samples from BP patients and age- and sex-matched patients without inflammatory and autoimmune diseases.
Zymography
Gel zymography were performed on serum samples (n=8) and blister fluids (n=10) from BP patients or on PBMCs and PMNs stimulated or not with IL-17 (n=7). Serum samples from age-matched patients without inflammatory and autoimmune diseases were used as controls (n=6). MMP activities analysis was performed as described previously (Emonard and Hornebeck 1997).
HNE Activities
Human elastase activity was assessed using the N-(Methoxysuccinyl)-Ala-Ala-Pro-Val-p-4-nitroanilide (Sigma) as substrate. Levels of enzyme activity were determined from 50 µL of culture media issued from neutrophils stimulated or not IL-17. The amount of para-nitro-anilide was measured each minute for 15 minutes at 25°C by spectrometry at 405 nm (HTS 700 Plus, Perkin Elmer).
Neutrophil chemotaxis assay
Neutrophil migration assay in response to control peptide PGG or to the different chemoattractants PGP, PGPPGP and AcPGP was performed using a Boyden chamber (5 µm pore size; Millipore). The migrated neutrophils (located at the bottom side of the membrane) were counted from 3 different fields using Eclipse E400 microscope from Nikon. PGG and PGP peptides were purchased from Genescript, with purity over 95%.
Immunohistochemistry
All peri-lesional skin biopsies performed in patients with BP before introduction of any corticosteroid treatment were obtained from the Pathological Anatomy Department of Reims Hospital. Detailed procedures for analysis by immunohistochemistry or immunocytochemistry are described in the Supplemental Data.
Statistical analysis
Descriptive statistics such as means, SDs or SEMs were conducted for all quantitative measures. As the distribution of the variables we analyzed could not be assumed to be normal and some of the groups examined were small, we used nonparametric testing to compare populations in this study. Comparisons between two groups were performed using the exact Wilcoxon signed-rank test for paired data and the Mann-Whitney test for unpaired data. The results were considered significant if p values were 0.05 or less.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by research grants from the SRD (Société de Recherche Dermatologique), the CPER (Contrat Plan Etat Région) 2008, the MAISAGE-PB project supported by the regional council, and the French Department of Health’s “Projet Hospitalier de Recherche Clinique (PHRC) Interrégional” 2009. This study was also supported in part by NIH grants from the NHLBI to JEB (HL110950, HL114439 and HL07783). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart Lung and Blood Institute, National Institutes of Health, or the Food and Drug Administration. SLJ was supported by grants from the Centre des Maladies Rares at the University Hospital of Reims.
We also thank Michael Maizières for technical assistance during the study and the Plateforme IBiSA “Imagerie Cellulaire et Tissulaire” for providing microscopy equipments.
Footnotes
COMPETING INTERESTS
The authors state no conflict of interest
REFERENCES
- Arakawa M, Dainichi T, Ishii N, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Experimental Dermatology. 2011;20:1022–1024. doi: 10.1111/j.1600-0625.2011.01378.x. [DOI] [PubMed] [Google Scholar]
- Biswas P, Mantelli B, Sica A, et al. Expression of CD4 on human peripheral blood neutrophils. Blood. 2003;101:4452–4456. doi: 10.1182/blood-2002-10-3056. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- D’Auria L, Fei PC, Ameglio F. Cytokines and bullous pemphigoid. European Cytokine Network. 1999;10:123–134. [PubMed] [Google Scholar]
- Doreau A, Belot A, Bastid J, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- Emonard H, Hornebeck W. Binding of 92 kDa and 72 kDa progelatinases to insoluble elastin modulates their proteolytic activation. Biol Chem. 1997;378:265–271. doi: 10.1515/bchm.1997.378.3-4.265. [DOI] [PubMed] [Google Scholar]
- Fichel F, Barbe C, Joly P, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatology1. 2013 doi: 10.1001/jamadermatol.2013.5757. in press. [DOI] [PubMed] [Google Scholar]
- Fischer-Stabauer M, Boehner A, Eyerich S, et al. Differential in situ expression of IL-17 in skin diseases. Eur J Dermatol. 2012;22:781–784. doi: 10.1684/ejd.2012.1854. [DOI] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison MT, Galin FS, Calderon CE, et al. The Presence of a Matrix-Derived Neutrophil Chemoattractant in Bronchiolitis Obliterans Syndrome after Lung Transplantation. J Immunol. 2009;182:4423–4431. doi: 10.4049/jimmunol.0802457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hellberg L, Samavedam UKSRL, Holdorf K, et al. Methylprednisolone blocks autoantibody-induced tissue damage in experimental models of bullous pemphigoid and epidermolysis bullosa acquisita through inhibition of neutrophil activation. J Invest Dermatol. 2013;133:2390–2399. doi: 10.1038/jid.2013.91. [DOI] [PubMed] [Google Scholar]
- Hirose M, Recke A, Beckmann T, et al. Repetitive immunization breaks tolerance to type XVII collagen and leads to bullous pemphigoid in mice. J Immunol. 2011;187:1176–1183. doi: 10.4049/jimmunol.1100596. [DOI] [PubMed] [Google Scholar]
- Hoechst B, Gamrekelashvili J, Manns MP, et al. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532–6541. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Nagao T, Nagi-Miura N, et al. MPO-ANCA induces IL-17 production by activated neutrophils in vitro via classical complement pathway-dependent manner. J Autoimmun. 2008;31:79–89. doi: 10.1016/j.jaut.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H, Saijo S, et al. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Joly P, Roujeau J-C, Benichou J, et al. A comparison of two regimens of topical corticosteroids in the treatment of patients with bullous pemphigoid: a multicenter randomized study. J Invest Dermatol. 2009;129:1681–1687. doi: 10.1038/jid.2008.412. [DOI] [PubMed] [Google Scholar]
- Kasperkiewicz M, Zillikens D. The Pathophysiology of Bullous Pemphigoid. Clinic Rev Allerg Immunol. 2007;33:67–77. doi: 10.1007/s12016-007-0030-y. [DOI] [PubMed] [Google Scholar]
- Katayama M, Ohmura K, Yukawa N, et al. Neutrophils Are Essential As A Source Of Il-17 In The Effector Phase Of Arthritis. PLoS ONE. 2013;8:e62231. doi: 10.1371/journal.pone.0062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijsers RRMC, Hendriks AGM, van Erp PEJ, et al. In vivo Induction of Cutaneous Inflammation Results in Accumulation of Extracellular Trap Forming Neutrophils Expressing RORγt and IL-17. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.526. [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol. 2013 doi: 10.1016/j.smim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, et al. IL-17 and Th17 Cells. Annual Review of Immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Langan SM, Smeeth L, Hubbard R, et al. Bullous pemphigoid and pemphigus vulgaris--incidence and mortality in the UK: population based cohort study. BMJ. 2008;337:a180. doi: 10.1136/bmj.a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Mukasa R, Hatton RD, et al. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Lin AM, Rubin CJ, Khandpur R, et al. Mast Cells and Neutrophils Release IL-17 through Extracellular Trap Formation in Psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Betsuyaku T, Heimbach L, et al. Neutrophil elastase cleaves the murine hemidesmosomal protein BP180/type XVII collagen and generates degradation products that modulate experimental bullous pemphigoid. Matrix Biology. 2012;31:38–44. doi: 10.1016/j.matbio.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Diaz LA, Troy JL, et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. Journal of Clinical Investigation. 1993;92:2480–2488. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Giudice GJ, Zhou X, et al. A major role for neutrophils in experimental bullous pemphigoid. Journal of Clinical Investigation. 1997;100:1256–1263. doi: 10.1172/JCI119639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Shapiro SD, Zhou X, et al. A critical role for neutrophil elastase in experimental bullous pemphigoid. Journal of Clinical Investigation. 2000a;105:113–123. doi: 10.1172/JCI3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Shipley JM, Vu TH, et al. Gelatinase B-deficient Mice Are Resistant to Experimental Bullous Pemphigoid. J Exp Med. 1998;188:475–482. doi: 10.1084/jem.188.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou X, Shapiro SD, et al. The Serpin α1-Proteinase Inhibitor Is a Critical Substrate for Gelatinase B/MMP-9 In Vivo. Cell. 2000b;102:647–655. doi: 10.1016/s0092-8674(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nature Immunology. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, et al. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Mayadas TN, Cullere X, Lowell CA. The Multifaceted Functions of Neutrophils. Annual Review of Pathology: Mechanisms of Disease. 2014;9 doi: 10.1146/annurev-pathol-020712-164023. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- Niimi Y, Pawankar R, Kawana S. Increased expression of matrix metalloproteinase-2, matrix metalloproteinase-9 and matrix metalloproteinase-13 in lesional skin of bullous pemphigoid. Int Arch Allergy Immunol. 2006;139:104–113. doi: 10.1159/000090385. [DOI] [PubMed] [Google Scholar]
- Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald E, Sesarman A, Franzke C-W, et al. The Flavonoid Luteolin Inhibits Fcγ-Dependent Respiratory Burst in Granulocytes, but Not Skin Blistering in a New Model of Pemphigoid in Adult Mice. PLoS ONE. 2012;7:e31066. doi: 10.1371/journal.pone.0031066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister RR, Haddox JL, Sommers CI, et al. Identification and synthesis of chemotactic tripeptides from alkali-degraded whole cornea. A study of N-acetyl-proline-glycine-proline and N-methyl-proline-glycine-proline. IOVS. 1995;36:1306–1316. [PubMed] [Google Scholar]
- Rhodes LE, Hashim IA, McLaughlin PJ, et al. Blister fluid cytokines in cutaneous inflammatory bullous disorders. Acta Derm Venereol. 1999;79:288–290. doi: 10.1080/000155599750010689. [DOI] [PubMed] [Google Scholar]
- Schmidt E OK. SErum levels of autoantibodies to bp180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174–178. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M, Inoue M, Guidice GJ, et al. 92-kD gelatinase is produced by eosinophils at the site of blister formation in bullous pemphigoid and cleaves the extracellular domain of recombinant 180-kD bullous pemphigoid autoantigen. Journal of Clinical Investigation. 1994;93:2022–2030. doi: 10.1172/JCI117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Roy S, Leal SM, Jr, et al. Activation of neutrophils by autocrine IL-17A–IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat Immunol. 2014;15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant L, Bernard P, Joly P, et al. EValuation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075–1080. doi: 10.1001/archderm.134.9.1075. [DOI] [PubMed] [Google Scholar]
- Verraes S, Hornebeck W, Polette M, et al. Respective Contribution of Neutrophil Elastase and Matrix Metalloproteinase 9 in the Degradation of BP180 (Type XVII Collagen) in Human Bullous Pemphigoid. Journal of Investigative Dermatology. 2001;117:1091–1096. doi: 10.1046/j.0022-202x.2001.01521.x. [DOI] [PubMed] [Google Scholar]
- Weathington NM, van Houwelingen AH, Noerager BD, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Xu X, Jackson PL, Tanner S, et al. A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PLoS ONE. 2011;6:e15781. doi: 10.1371/journal.pone.0015781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiga Invariant NKT cells produce IL-17 through IL-23-dependent and -independent pathways with potential modulation of Th17 response in collagen-induced arthritis. International Journal of Molecular Medicine. 1998 [PubMed] [Google Scholar]
- Zebrowska A, Wagrowska-Danilewicz M, Danilewicz M, et al. IL-17 Expression in Dermatitis Herpetiformis and Bullous Pemphigoid. Mediators of Inflammation. 2013 doi: 10.1155/2013/967987. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Zenzo G, Thoma-Uszynski S, Fontao L, et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clinical Immunology. 2008;128:415–426. doi: 10.1016/j.clim.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Di Zenzo G, della Torre R, Zambruno G, et al. Bullous pemphigoid: From the clinic to the bench. Clinics in Dermatology. 2012;30:3–16. doi: 10.1016/j.clindermatol.2011.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.