Abstract
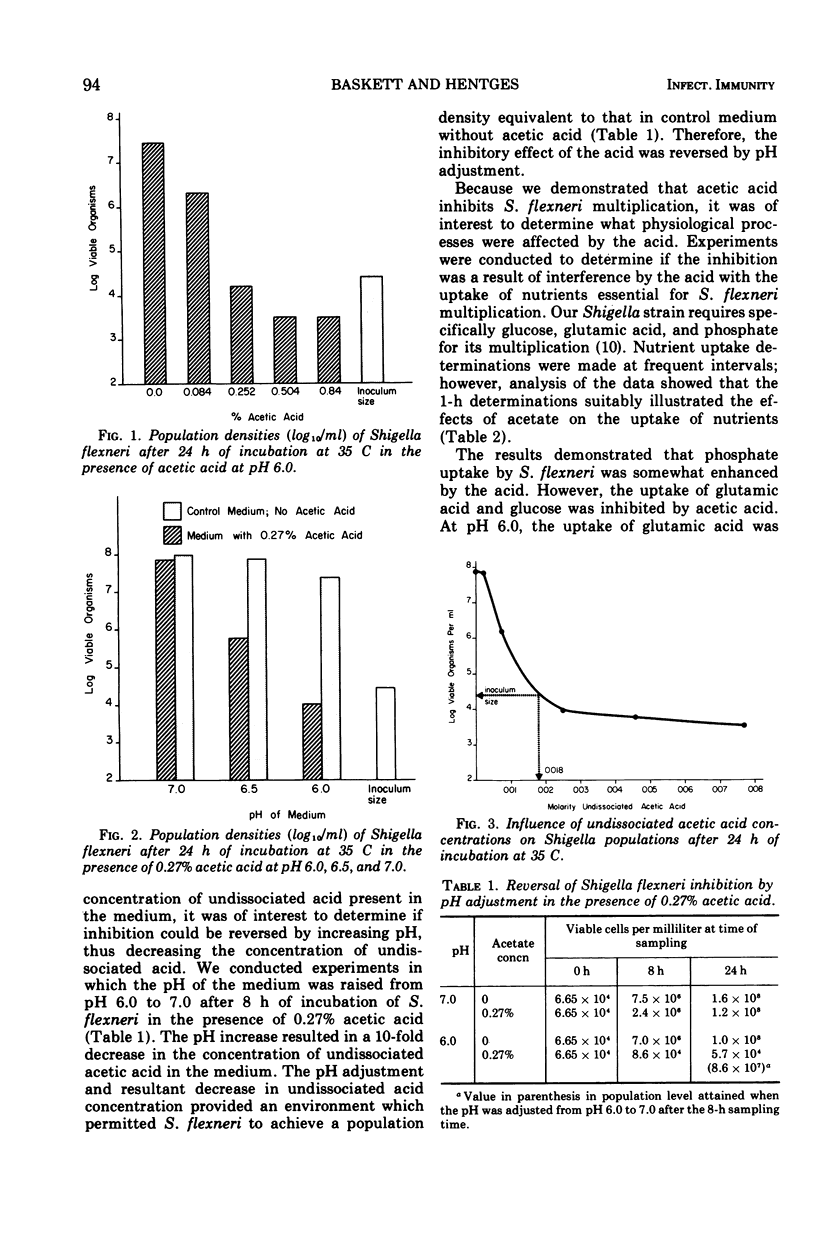
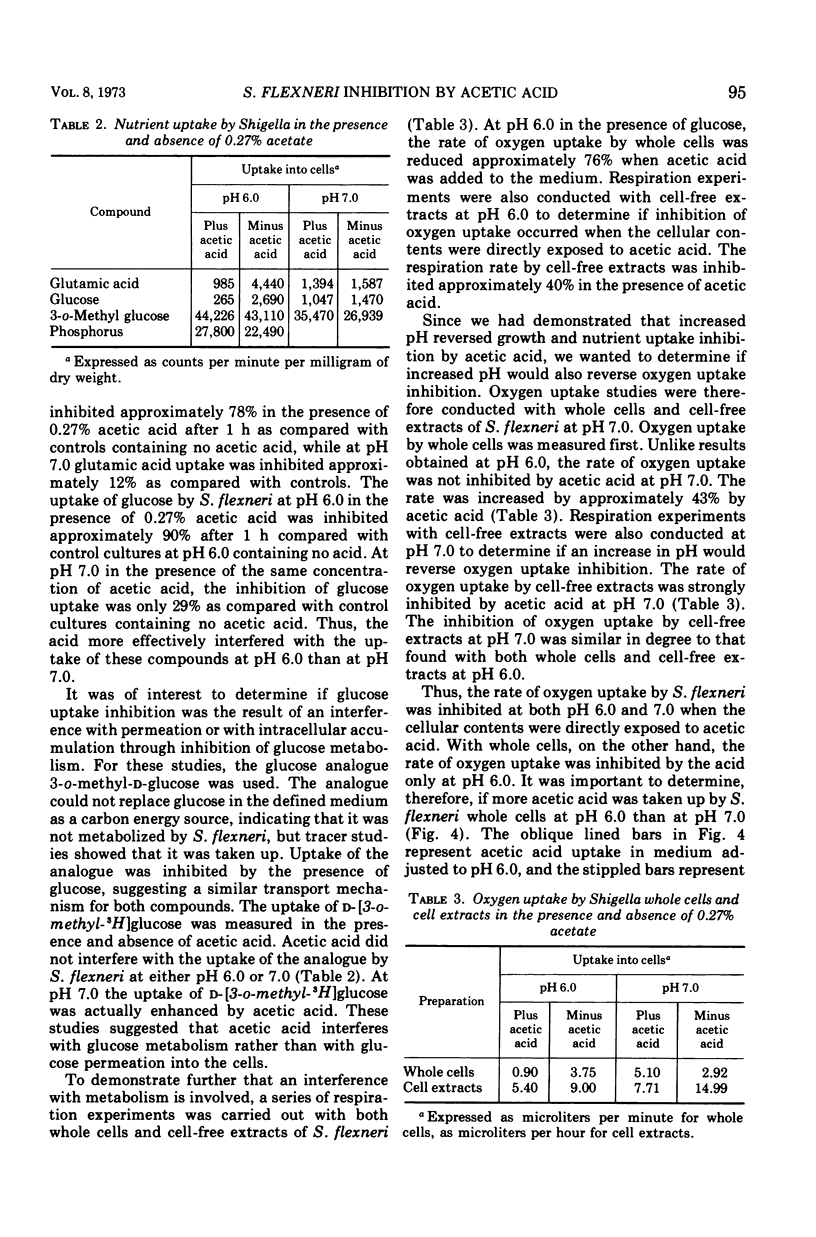
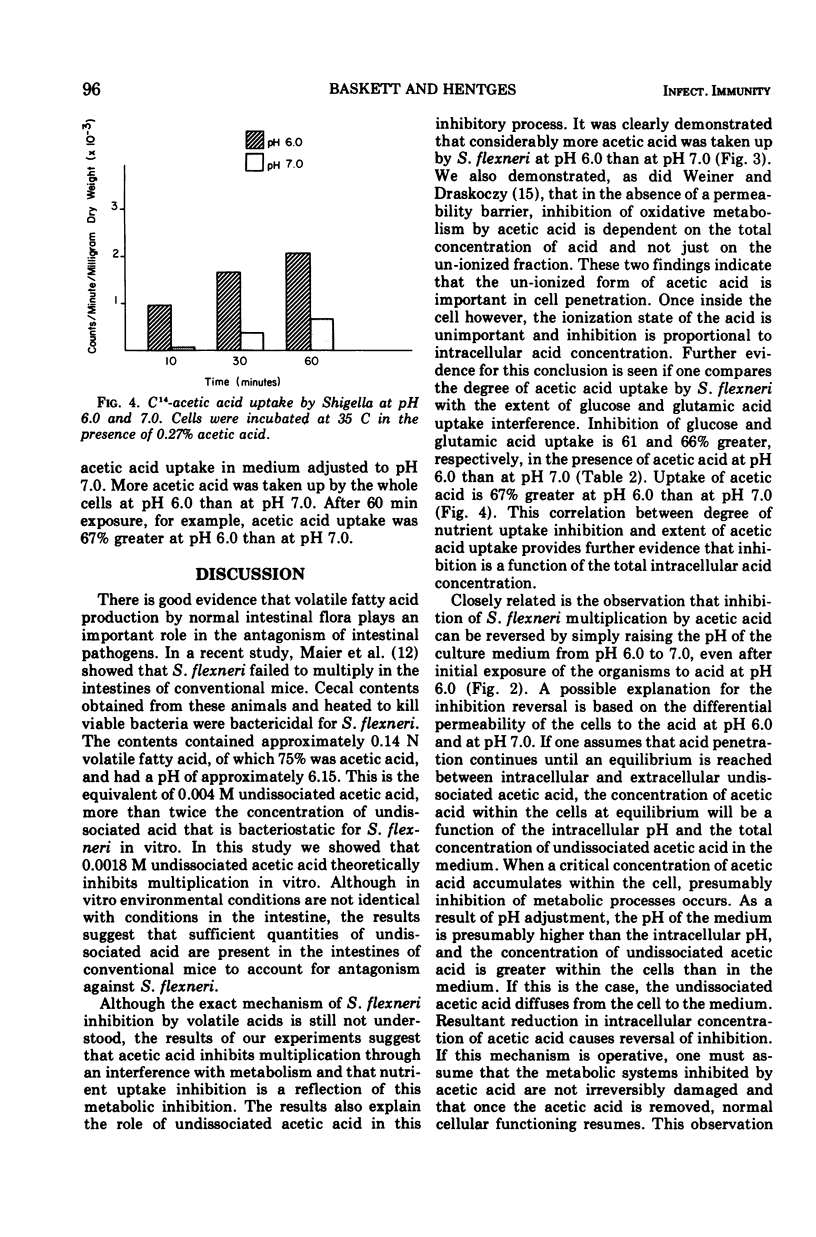
The degree of Shigella flexneri inhibition by acetic acid in a chemically defined medium was dependent upon the concentration of undissociated acetic acid in the medium. Under the conditions of the experiments, the critical concentration of undissociated acetic acid that completely inhibited S. flexneri multiplication was approximately 0.0018 M. Adjustment of the medium from pH 6.0 to 7.0 after incubation, which reduced the concentration of undissociated acid 10-fold, completely reversed inhibition, and S. flexneri attained a viable population equivalent to its population in medium without acetic acid. The effects of acetic acid on cellular processes were also studied. The acid interfered with the intracellular accumulation of glucose and glutamic acid but did not interfere with the accumulation of phosphate. The glucose analogue 3-o-methyl-D-glucose, which is taken up by S. flexneri but not metabolized, was used to determine if inhibition resulted from interference with permeation or interference with intracellular accumulation through inhibition of glucose metabolism. Acetic acid did not interfere with the uptake of the glucose analogue by S. flexneri, indicating that inhibition probably involves interference with metabolism. Further evidence for this conclusion was obtained from respiration studies with cell-free extracts in the presence and absence of acetic acid. Inhibition of oxygen uptake by acetic acid in the absence of a permeability barrier suggested a metabolic block rather than interference with permeation. The inhibition of oxygen uptake by cell-free extracts occurred at both pH 6.0 and 7.0, indicating that the degree of dissociation of the acid is unimportant regarding interference with metabolism by intracellular material. The degree of dissociation is important, however, regarding uptake of acetic acid by S. flexneri. Whole cells were more permeable to the undissociated form of acetic acid than to the dissociated form. The data indicate that acetic acid, when taken up by S. flexneri, interferes with the metabolism of glucose by the cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., MCFADDEN B. A. Use of membrane filters in the measurement of biological incorporation of radioactive isotopes. J Bacteriol. 1956 Jan;71(1):123–124. doi: 10.1128/jb.71.1.123-124.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOHNHOFF M., MILLER C. P., MARTIN W. R. RESISTANCE OF THE MOUSE'S INTESTINAL TRACT TO EXPERIMENTAL SALMONELLA INFECTION. I. FACTORS WHICH INTERFERE WITH THE INITIATION OF INFECTION BY ORAL INOCULATION. J Exp Med. 1964 Nov 1;120:805–816. doi: 10.1084/jem.120.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOHNHOFF M., MILLER C. P., MARTIN W. R. RESISTANCE OF THE MOUSE'S INTESTINAL TRACT TO EXPERIMENTAL SALMONELLA INFECTION. II. FACTORS RESPONSIBLE FOR ITS LOSS FOLLOWING STREPTOMYCIN TREATMENT. J Exp Med. 1964 Nov 1;120:817–828. doi: 10.1084/jem.120.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENTGES D. J. A simplified plating technic for bacteriologic examination of specimens of urine. Am J Clin Pathol. 1962 Sep;38:304–305. doi: 10.1093/ajcp/38.3.304. [DOI] [PubMed] [Google Scholar]
- HENTGES D. J., FULTON M. ECOLOGICAL FACTORS INFLUENCING THE RELATIONSHIPS BETWEEN KLEBSIELLA AND SHIGELLA IN MIXED CULTURE. J Bacteriol. 1964 Mar;87:527–535. doi: 10.1128/jb.87.3.527-535.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges D. J. Influence of pH on the inhibitory activity of formic and acetic acids for Shigella. J Bacteriol. 1967 Jun;93(6):2029–2030. doi: 10.1128/jb.93.6.2029-2030.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges D. J. Inhibition of Shigella flexneri by the normal intestinal flora. I. Mechanisms of inhibition by Klebsiella. J Bacteriol. 1967 Apr;93(4):1369–1373. doi: 10.1128/jb.93.4.1369-1373.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges D. J. Inhibition of Shigella flexneri by the normal intestinal flora. II. Mechanisms of inhibition by coliform organisms. J Bacteriol. 1969 Feb;97(2):513–517. doi: 10.1128/jb.97.2.513-517.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. S., Fellers C. R. Action of Acetic Acid on Food Spoilage Microörganisms. J Bacteriol. 1940 May;39(5):499–515. doi: 10.1128/jb.39.5.499-515.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B. R., Onderdonk A. B., Baskett R. C., Hentges D. J. Shigella, indigenous flora interactions in mice. Am J Clin Nutr. 1972 Dec;25(12):1433–1440. doi: 10.1093/ajcn/25.12.1433. [DOI] [PubMed] [Google Scholar]
- SAMSON F. E., KATZ A. M., HARRIS D. L. Effects of acetate and other short-chain fatty acids on yeast metabolism. Arch Biochem Biophys. 1955 Feb;54(2):406–423. doi: 10.1016/0003-9861(55)90054-0. [DOI] [PubMed] [Google Scholar]
- Sorrells K. M., Speck M. L. Inhibition of Salmonella gallinarum by culture filtrates of Leuconostoc citrovorum. J Dairy Sci. 1970 Feb;53(2):239–241. doi: 10.3168/jds.S0022-0302(70)86186-0. [DOI] [PubMed] [Google Scholar]
- WEINER N., DRASKOCZY P. The effects of organic acids on the oxidative metabolism of intact and disrupted E. coli. J Pharmacol Exp Ther. 1961 Jun;132:299–305. [PubMed] [Google Scholar]
- Wolin M. J. Volatile fatty acids and the inhibition of Escherichia coli growth by rumen fluid. Appl Microbiol. 1969 Jan;17(1):83–87. doi: 10.1128/am.17.1.83-87.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


