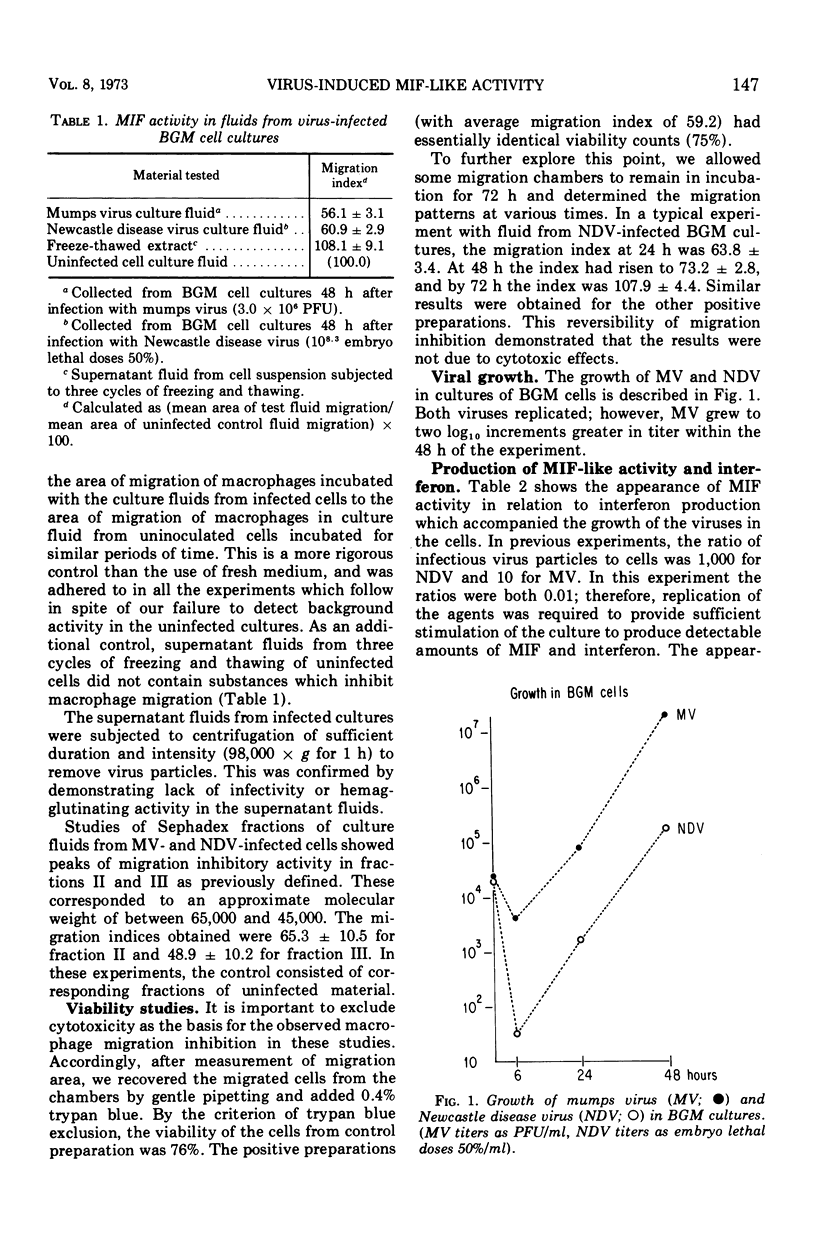
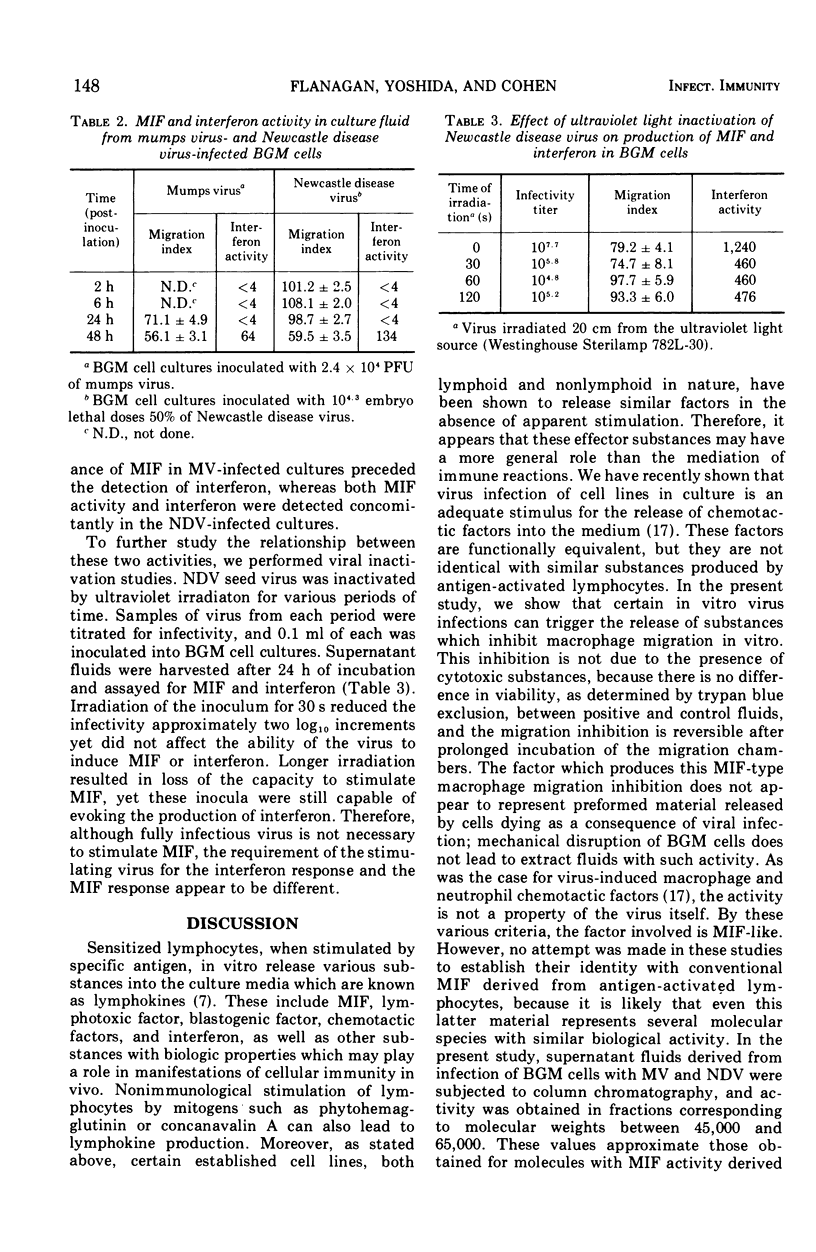
Abstract
Macrophage migration inhibitory (MIF-like) activity was demonstrated in the supernatant fluids from cultures of African green monkey kidney cells (BGM) infected with mumps virus or Newcastle disease virus. We could detect no such activity in noninfected cultures. The virus-induced activity reported here is not due to nonspecific cytotoxic material released by dead or dying cells, and it does not require cell replication for its production. Preliminary estimates of molecular weight by Sephadex G-100 chromatography revealed a broad band of activity associated with the 45,000 and 65,000 markers. These are significantly smaller than previously reported chemotactic substances from virus-infected cultures, and thus appear to represent different cell products. These MIF-like factors may be produced concomitantly with interferon. However, ultraviolet irradiation of appropriate duration abolishes the ability of viruses to induce substances with MIF-like activity while preserving the ability to induce interferon. This strongly suggests that interferon is not the agent responsible for the macrophage migration inhibition effect. The functional properties of these various cell products induced by virus infection suggest that they all may play a role in the response to virus infection in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron A. L., Olshevsky C., Cohen M. M. Characteristics of the BGM line of cells from African green monkey kidney. Brief report. Arch Gesamte Virusforsch. 1970;32(4):389–392. doi: 10.1007/BF01250067. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Gaffney J., Jimenez L. Dissociation of MIF production and cell proliferation. J Immunol. 1972 Dec;109(6):1395–1398. [PubMed] [Google Scholar]
- CARPENTER R. R. IN VITRO STUDIES OF CELLULAR HYPERSENSITIVITY. I. SPECIFIC INHIBITION OF MIGRATION OF CELLS FROM ADJUVANT-IMMUNIZED ANIMALS BY PURIFIED PROTEIN DERIVATIVE AND OTHER PROTEIN ANTIGENS. J Immunol. 1963 Dec;91:803–818. [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- Dumonde D. C., Wolstencroft R. A., Panayi G. S., Matthew M., Morley J., Howson W. T. "Lymphokines": non-antibody mediators of cellular immunity generated by lymphocyte activation. Nature. 1969 Oct 4;224(5214):38–42. doi: 10.1038/224038a0. [DOI] [PubMed] [Google Scholar]
- Flanagan T. D., Barron A. L. Plaque formation by mumps virus and inhibition by antiserum. Appl Microbiol. 1970 Feb;19(2):360–366. doi: 10.1128/am.19.2.360-366.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- Kaplan J. Staphylococcal enterotoxin B induced release of macrophage migration inhibition factor from normal lymphocytes. Cell Immunol. 1972 Feb;3(2):245–252. doi: 10.1016/0008-8749(72)90163-3. [DOI] [PubMed] [Google Scholar]
- Papageorgiou P. S., Henley W. L., Glade P. R. Production and characterization of migration inhibitory factor(s) (MIF) of established lymphoid and non-lymphoid cell lines. J Immunol. 1972 Feb;108(2):494–504. [PubMed] [Google Scholar]
- Pick E., Brostoff J., Krejci J., Turk J. L. Interaction between "sensitized lymphocytes" and antigen in vitro. II. Mitogen-induced release of skin reactive and macrophage migration inhibitory factors. Cell Immunol. 1970 May;1(1):92–109. doi: 10.1016/0008-8749(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Meyers O. L., David J. R. An in vitro assay for cellular hypersensitivity in man. J Immunol. 1970 Jan;104(1):95–102. [PubMed] [Google Scholar]
- Rocklin R. E., Remold H. G., David J. R. Characterization of human migration inhibitory factor (MIF) from antigen-stimulated lymphocytes. Cell Immunol. 1972 Nov;5(3):436–445. doi: 10.1016/0008-8749(72)90070-6. [DOI] [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubergen D. G., Feldman J. D., Pollock E. M., Lerner R. A. Production of macrophage migration inhibition factor by continuous cell lines. J Exp Med. 1972 Feb 1;135(2):255–266. doi: 10.1084/jem.135.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Cohen S., Flanagan T. D. Leukotactic factors elaborated by virus-infected tissues. J Exp Med. 1972 May 1;135(5):1095–1103. doi: 10.1084/jem.135.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Remold H. G., David J. R. The production by antigen-stimulated lymphocytes of a leukotactic factor distinct from migration inhibitory factor. Cell Immunol. 1970 Jul;1(2):162–174. doi: 10.1016/0008-8749(70)90003-1. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Janeway C. A., Jr, Paul W. E. Activity of migration inhibitory factor in the absence of antigen. J Immunol. 1972 Aug;109(2):201–206. [PubMed] [Google Scholar]
- Yoshida T., Reisfeld R. A. Two fractions with macrophage migration inhibitory activity from sensitized lymphocyte cultures. Nature. 1970 May 30;226(5248):856–857. doi: 10.1038/226856a0. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Watanuki M. [Delayed type hypersensitivity in vitro. 2. Inhibition of the antigen against migration of cells in vitro capable of transferring tuberculin hypersensitivity]. Igaku To Seibutsugaku. 1968 Oct 10;77(4):131–137. [PubMed] [Google Scholar]


