Abstract
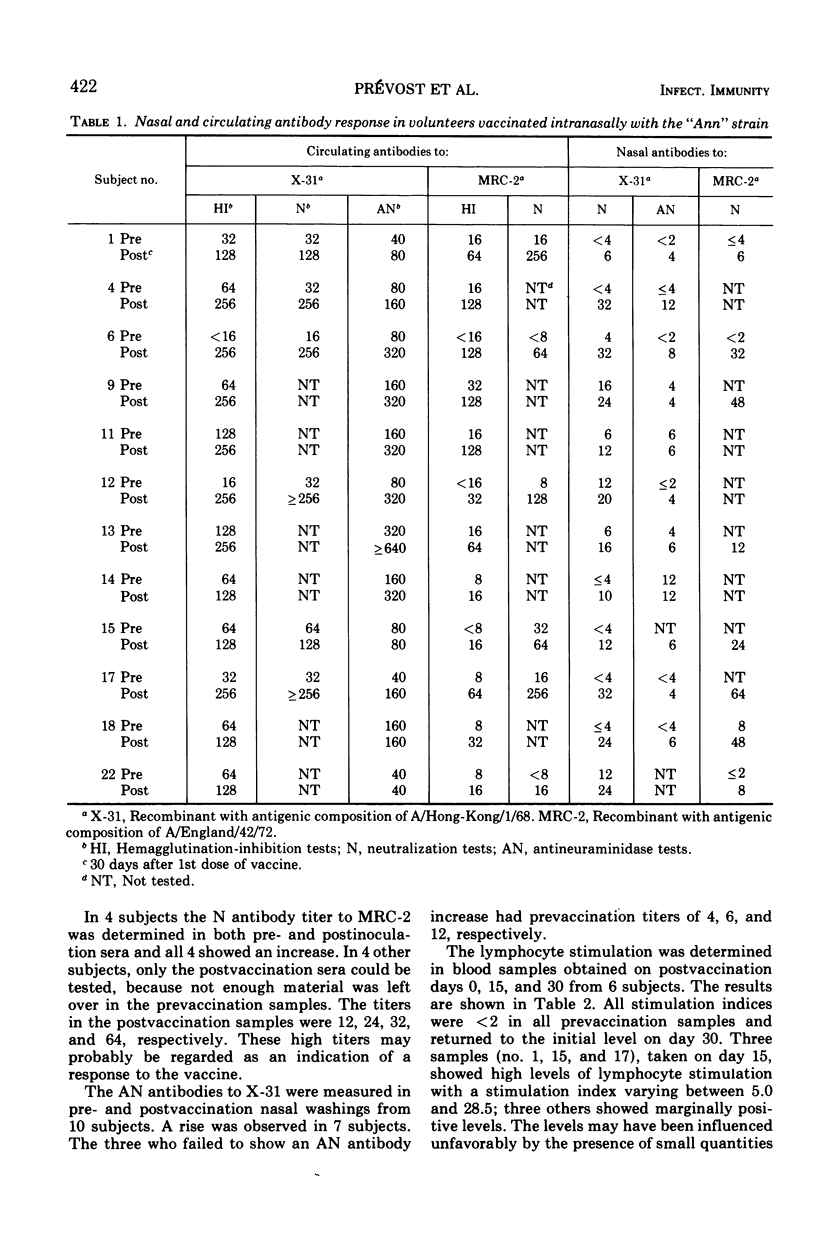
A live attenuated influenza virus (“Ann” strain) derived from A/England/878/69 was given intranasally to a group of volunteers, most of whom had already circulating antibodies against H3N2 viruses at the time of inoculation. There was a fourfold or higher increase of circulating hemagglutination-inhibiting antibodies in those volunteers who had relatively low initial titers. The response was lower in those with initially higher serum titers. The pattern of the serum neutralizing antibody response was very similar. The geometric means of the antineuraminidase antibodies were 67 and 118 pre- and postvaccination, respectively. All subjects showed a rise in local neutralizing antibodies in their nasal secretions with geometric means of 4 and 17 pre- and postvaccination, respectively. The levels of local antineuraminidase antibodies also rose in most subjects. In addition to the response to the homologous virus type, the antibody formation to the recent A/England/42/72 was measured in the sera and nasal secretions of some subjects. There was a clearcut response in most of the sera and in all of the secretions examined. The stimulation of circulating lymphocytes was measured in 6 volunteers. All volunteers showed a temporary stimulation. The stimulation index ranged between 2.5 and 28.5.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare A. S., Bynoe M. L. Attenuation of human influenza A viruses. Br Med J. 1969 Oct 25;4(5677):198–201. doi: 10.1136/bmj.4.5677.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare A. S., Hall T. S. Recombinant influenza-A viruses as live vaccines for man. Report to the Medical Research Council's Committee on Influenza and other Respiratory Virus Vaccines. Lancet. 1971 Dec 11;2(7737):1271–1273. doi: 10.1016/s0140-6736(71)90597-6. [DOI] [PubMed] [Google Scholar]
- Beare A. S., Hobson D., Reed S. E., Tyrrell D. A. A comparison of live and killed influenza-virus vaccines. Report to the Medical Research Council's Committee on Influenza and other Respiratory Virus Vaccines. Lancet. 1968 Aug 24;2(7565):418–422. doi: 10.1016/s0140-6736(68)90463-7. [DOI] [PubMed] [Google Scholar]
- Beare A. S. Laboratory characteristics of attenuated influenza viruses. Bull World Health Organ. 1969;41(3):595–598. [PMC free article] [PubMed] [Google Scholar]
- Caron G. A., Sarkany I., Williams H. S., Todd A. P., Gell H. M. Radioactive method for the measurement of lymphocyte transformation in vitrol. Lancet. 1965 Dec 18;2(7425):1266–1268. doi: 10.1016/s0140-6736(65)92282-8. [DOI] [PubMed] [Google Scholar]
- FAZEKAS DE ST GROTH S., WHITE D. O. An improved assay for the infectivity of in influenza viruses. J Hyg (Lond) 1958 Mar;56(1):151–162. doi: 10.1017/s0022172400037621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone D. S., Hamilton-Smith S., Schild G. C., Buckland R., Chinn S., Tyrrell D. A. Antibody responses and resistance to challenge in volunteers vaccinated with live attenuated, detergent split and oil adjuvant A2-Hong Kong-68 (H 3 N 2 ) influenza vaccines. A report to the Medical Research Council Committee on Influenza and other Respiratory Virus Vaccines. J Hyg (Lond) 1972 Sep;70(3):531–543. doi: 10.1017/s0022172400063117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frösner G. G., Gerth H. J. Antigenunterschiede zwischen pferdeseruminhibitorempfindlichen und unempfindlichen Influenza A 2-Substämmen aus dem gleichen Isolat. Arch Gesamte Virusforsch. 1972;36(3):317–323. [PubMed] [Google Scholar]
- Kilbourne E. D. Future influenza vaccines and the use of genetic recombinants. Bull World Health Organ. 1969;41(3):643–645. [PMC free article] [PubMed] [Google Scholar]
- Maassab H. F., Francis T., Jr, Davenport F. M., Hennessy A. V., Minuse E., Anderson G. Laboratory and clinical characteristics of attenuated strains of influenza virus. Bull World Health Organ. 1969;41(3):589–594. [PMC free article] [PubMed] [Google Scholar]
- Mills J., Van Kirk J., Hill D. A., Chanock R. M. Evaluation of influenza virus mutants for possible use in a live virus vaccine. Bull World Health Organ. 1969;41(3):599–606. [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., Oxford J. S., Shore S. L., McLaren C., Stuart-Harris C. Immunity to influenza in ferrets. I. Response to live and killed virus. Br J Exp Pathol. 1972 Apr;53(2):153–167. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. L., Farber P. A., Notkins A. L. In vitro stimulation of sensitized lymphocytes by herpes simplex virus and vaccinia virus. Proc Natl Acad Sci U S A. 1972 Mar;69(3):756–760. doi: 10.1073/pnas.69.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCE L. Kaolin treatment of sera for removal of nonspecific inhibitors to Asian strains of influenza virus. Proc Soc Exp Biol Med. 1960 Feb;103:425–427. doi: 10.3181/00379727-103-25545. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Spencer C. S., Johnson J. E., 3rd Respiratory and systemic cellular and humoral immune responses to influenza virus vaccine administered parenterally or by nose drops. Cell Immunol. 1972 Feb;3(2):294–300. doi: 10.1016/0008-8749(72)90168-2. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Wigley FM Small P. A., Jr Specificity of respiratory secretion antibody against influenza virus. J Immunol. 1970 Dec;105(6):1477–1483. [PubMed] [Google Scholar]


