Since Breast Imaging Reporting and Data System (BI-RADS) category 3 lesions have a low malignancy rate (0.8%; 95% confidence interval: 0.3%, 1.7%) (six of 745 lesions) and since only one (0.1%) lesion had suspicious changes at short-interval follow-up and only one (0.1%) lesion had a suspicious change at 1-year follow-up, both of which showed node-negative invasive ductal carcinoma, a recommendation of yearly follow-up for BI-RADS category 3 lesions may be appropriate.
Purpose
To prospectively validate predefined breast ultrasonographic (US) Breast Imaging Reporting and Data System (BI-RADS) category 3 criteria in a multicenter setting in an elevated-risk population.
Materials and Methods
The American College of Radiology Imaging Network 6666 database was reviewed for prospectively defined BI-RADS category 3 lesions. Patient characteristics, lesion US features at initial detection, and work-up recommendations were analyzed with descriptive statistics. Exact 95% confidence intervals (CIs) were given, where appropriate. Lesion reference standard was biopsy or a minimum of 1-year follow-up. In addition, malignancy rate for lesions that had at least 2 years of follow-up data or that had biopsy data was calculated.
Results
Of 2662 participants, 519 (19.5%) had 745 BI-RADS category 3 lesions (25.5% of 2916 US lesions other than simple cysts), with a median size of 7 mm (range, 2–135 mm). The number of new BI-RADS category 3 lesions decreased with year 2–3 screening, but the percentage of new BI-RADS category 3 lesions was stable at 26.4% (506 of 1920 lesions), 23.6% (142 of 601 lesions), and 24.6% (97 of 395 lesions), respectively. Of 745 BI-RADS category 3 lesions, 124 (16.6%) were ultimately sampled for biopsy. Six malignancies (0.8% of BI-RADS category 3 lesions; 95% confidence interval [CI]: 0.3%, 1.7%) occurred in five (1.0%) of 519 participants: Five malignancies were invasive (median size, 10 mm; size range, 2–18 mm), and one was node positive. When the analysis is limited to lesions with at least 2-year follow-up or biopsy, the malignancy rate among BI-RADS category 3 lesions is 0.9% (95% CI: 0.3%, 2.0%). Three malignant BI-RADS category 3 lesions were sampled for biopsy because of a suspicious change at follow-up (two N0 lesions, one each at 6- and 12-month follow-up; one N1 lesion at 24-month follow-up), one was sampled for biopsy because of an upgrade after additional mammography (NX), one was found at mastectomy for another cancer (N0), and one was found at prophylactic contralateral mastectomy in the same patient (NX).
Conclusion
As BI-RADS category 3 lesions have a low malignancy rate (0.8%; 95% CI: 0.3%, 1.7%) and only 0.1% of the cancers had suspicious changes at 6-month follow-up and only one (17%; 95% CI: 0.4%, 64%) of six malignancies were node positive at detection (24-month follow-up), a recommendation of 1-year diagnostic follow-up may be appropriate for BI-RADS category 3 lesions detected at screening US.
©RSNA, 2013
Introduction
The role of ultrasonography (US) in the characterization of breast lesions has expanded from cystic versus solid distinction to detection of lesions in both diagnostic (1–3) and screening (4–11) settings, with detailed classification of lesions (12–14). The Breast Imaging and Reporting Data System (BI-RADS) US lexicon (12,13) provides standard terminology with which to describe lesion features that are then used to determine management on the basis of stratified risk of malignancy. As with mammography, specific lesions, such as circumscribed oval masses (including those with two or three gentle lobulations), have been proposed to be probably benign (BI-RADS category 3) on baseline US images (14). BI-RADS category 3 lesions have a malignancy rate of less than 2% at mammography (15–17) and in most diagnostic US series (18–22); however, rates of 2.6%–8.0% have been observed (23,24). BI-RADS category 3 lesions are common at work-up of screening mammographic abnormalities, occurring in 6% of examinations (16,25–27), and despite their low malignancy rate, they require additional follow-up or even biopsy that results in substantial patient anxiety (28). Little has been written about the prevalence or outcomes of BI-RADS category 3 lesions detected at screening US (29).
The American College of Radiology Imaging Network (ACRIN) 6666 protocol was performed to evaluate the addition of US screening in a population with dense breasts and elevated risk of breast cancer (4,30). Supplemental US increased the cancer detection rate with each annual screening beyond that of mammography alone; it increased the detection rate by 5.3 cancers per 1000 women in the 1st year and by 3.7 cancers per 1000 women per year in both the 2nd year and the 3rd year. However the increase in cancer detection rate resulted in an increase in the number of false-positive findings. The addition of US screening prompted 207 (7.8%) of 2659 women to undergo biopsy and another 284 (10.7%) of 2659 women to undergo short-term follow-up in year 1; 242 (5.0%) of 4814 women underwent biopsy prompted by US, and 180 (3.7%) of 4814 women underwent short-term follow-up in years 2 and 3 (30). The purpose of this analysis was to prospectively evaluate predefined BI-RADS category 3 criteria in the ACRIN 6666 multicenter setting.
Materials and Methods
In the ACRIN 6666 trial, researchers evaluated annual physician-performed whole-breast screening US added to independently performed mammography (4,30). There were three annual rounds of screening, with clinical follow-up in year 4. Patients were women with dense breasts and at least one more risk factor (4). The study used the standardized US technique, documentation, and interpretive criteria (31). Physician investigators completed standardized training in the BI-RADS US lexicon (12) and were required to successfully complete interpretive skills tasks with mammography and US (32). The presence of simple cysts was recorded by breast. Mass shape, margins, echogenicity, and other BI-RADS features were prospectively recorded for lesions other than simple cysts. Special cases were prospectively defined as (a) complicated cysts, (b) clustered microcysts, (c) calcifications without a mass, (d) intraductal mass, (e) lymph node, and (f) postsurgical scar. We also prospectively recorded if the finding was multiple bilateral circumscribed masses and detailed features of the largest such mass.
US lesions classified as BI-RADS category 3 could not have suspicious features. Interpretive criteria were detailed prospectively (31). Lesions considered probably benign at US could not be palpable or have any suspicious features. These included the following when identified at baseline screening: (a) oval masses parallel to the skin and hypoechoic to fat, with circumscribed borders and no posterior features or minimal posterior enhancement, including multiple bilateral masses with these features if seen only at US; (b) hyperechoic masses with central hypoechoic to anechoic components suggesting fat necrosis; (c) hypoechoic oval masses with homogeneous low-level internal echoes that otherwise met the criteria for simple cysts (circumscribed acoustic enhancement); (d) microlobulated or oval masses composed entirely of clustered microcysts with or without layering microcalcifications; (e) probably artifactual posterior shadowing at the interface of fat lobules without any associated mass, which changes appearance on changing the angle of insonation; and (f) architectural distortion thought to be due to postsurgical scarring, which could be classified as probably benign or benign at the discretion of the investigator. According to our protocol, such lesions were to be followed up for 6, 12, and 24 months; however, actual recommendations were recorded separately from assessments. Biopsy was to be performed for any abnormal interval change at any follow-up point; an abnormal interval change was defined as any suspicious change, an increase in volume of more than 20%, or both (31,33). If a lesion decreased by more than 20% in volume or resolved at any follow-up examination, further follow-up was not required (31). We have previously reported on cystic breast lesions, including simple cysts, complicated cysts, and complex cystic and solid masses (34), as well as on results for multiple bilateral circumscribed masses seen at US (35); the latter was not restricted to BI-RADS category 3 lesions. In this analysis, we reviewed lesions initially classified as BI-RADS category 3 at screening US and detailed the occurrence, eventual work-up, and effect of follow-up timing on detection of malignancies in participants with BI-RADS category 3 lesions; this analysis was not limited to cystic lesions.
Study Participants
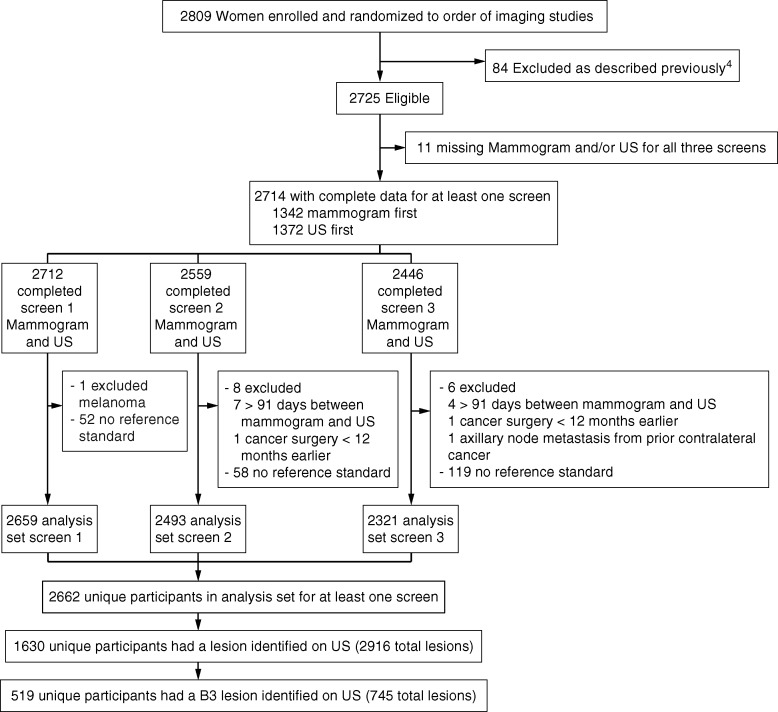
We recruited 2809 women from 21 sites between April 2004 and February 2006, of whom 2725 were eligible (4). The database was reviewed for lesions assessed as BI-RADS category 3 at first description at any of the three annual screening US examinations. All BI-RADS category 3 US lesions were included in the analysis. All participants with at least one BI-RADS category 3 lesion were included in the analysis, including those with a more severe BI-RADS assessment elsewhere in either breast. Patient characteristics and lesion US features were reviewed. Work-up recommendations were summarized. If biopsy was recommended for a BI-RADS category 3 lesion, the reason was recorded from predetermined categories (31). A reference standard of at least 11-month follow-up or biopsy has been described previously (4). Of 2659 (year 1), 2493 (year 2), and 2321 (year 3) analyzable participants (30), 2662 unique participants had at least one analyzable US screening examination, for a total of 7473 screening US examinations.
Statistical Analysis
Analysis was performed at the lesion level. A lesion was counted only once, even if it was recorded more than once across 3 years of screening. We present results for lesions assessed as BI-RADS category 3 at the first description of the lesion. Lesion descriptions are based on the initial features when the lesion was first reported. For malignant lesions, the attributed feature description from the year when the cancer was diagnosed was noted. Treatment mastectomy was counted as biopsy, but prophylactic mastectomy was not. In addition, we had calculated the malignancy rate for the lesions that had either a minimum 2-year follow-up or biopsy. The χ2 test was used to compare independent proportions, and the two-sample Student t test was used to compare means of the continuous variables. The 95% confidence intervals (CIs) were calculated as exact CIs. All P values were two-sided, and the significance level was set at .05. All analyses were performed by using statistical software (SAS, version 9.2; SAS Software, Cary, NC).
Results
Occurrence of BI-RADS Category 3 Lesions
Of 2662 participants, 1630 (61.2%; median age, 55 years; range, 25–86 years) had 2916 US lesions (Fig 1). A total of 519 unique participants (19.5% of the 2662 women screened and 31.8% of the 1630 women with US lesions) had 745 BI-RADS category 3 lesions (median size, 7 mm; size range, 2–135 mm). The number of all newly detected US lesions declined over the 3 years of screening; there were 1920 lesions in 358 (13.5%) of 2659 women at year 1, 601 lesions in 121 (4.9%) of 2493 women at year 2, and 395 lesions in 86 (3.7%) of 2321 women at year 3. Forty-three (8.3%) of 519 women had new lesions at multiple time points, and 476 (91.7%) of 519 women had a new BI-RADS category 3 lesion at only one time point. The percentage of newly reported BI-RADS category 3 lesions assessed as BI-RADS category 3 was stable, however, with 506 (26.4%) of 1920 new US lesions assessed as BI-RADS category 3 in year 1, 142 (23.6%) of 601 new US lesions assessed as BI-RADS category 3 in year 2, and 97 (24.6%) of 395 new US lesions assessed as BI-RADS category 3 in year 3.
Figure 1:
Flowchart of BI-RADS category 3 (B3) lesions.
Characteristics
Patient characteristics are presented in Table 1. Of the 519 participants with at least one BI-RADS category 3 lesion and the 1111 participants with lesions other than BI-RADS category 3 lesions, those with BI-RADS category 3 lesions were younger on average (54.1 years vs 55.9 years, P < .001) and were more likely to be premenopausal (29.3% [152 of 519 patients] vs 19.1% [212 of 1111 patients], P < .001), to be of Hispanic or Latino ethnicity (13.5% [70 of 519 participants] vs 6.8% [76 of 1111 participants], P < .001), or to have a lifetime risk of 25% or higher based on the Gail or Claus model (23.1% [120 of 519 patients] vs 14.4% [160 of 1111 patients], P < .001). A smaller percentage of women with BI-RADS category 3 lesions had a personal history of breast cancer (48.0% [249 of 519 patients]) than did women without a personal history of breast cancer (62.3% [692 of 1111 patients]) (P < .001).
Table 1.
Participant Characteristics
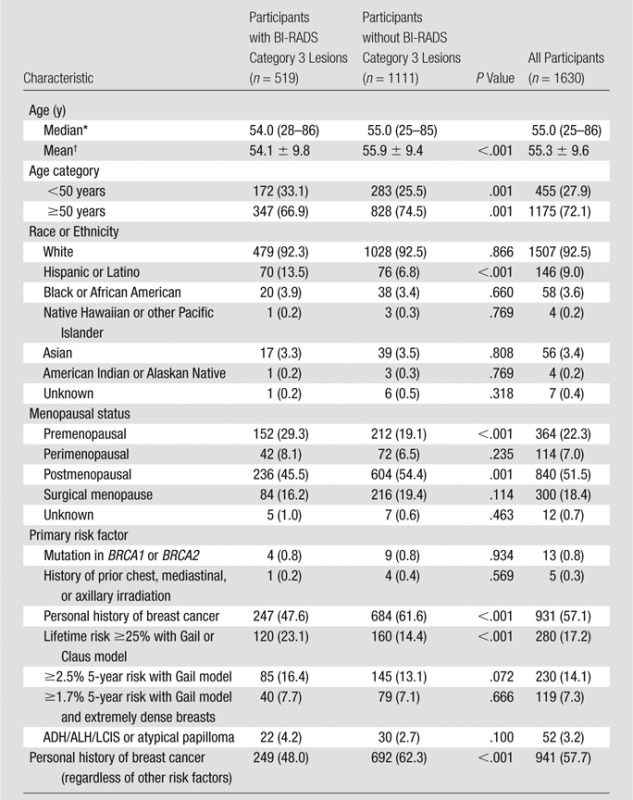
Note.—Unless otherwise indicated, data are number of patients, and data in parentheses are percentages. ADH = atypical ductal hyperplasia, ALH = atypical lobular hyperplasia, LCIS = lobular carcinoma in-situ.
Data in parentheses are the range.
Data are mean ± standard deviation.
Lesion Characterization
The frequency of special cases, calcifications, and lesions palpable in retrospect during US is listed in Table 2. Of 745 BI-RADS category 3 lesions, 306 (41.1%) were lesions with special features. There were 183 complicated cysts (one cancer, 0.5%), 33 clustered microcysts (one cancer, 3%), 14 multiple bilateral circumscribed benign-appearing masses (no cancers), 33 postsurgical scars (one cancer, 3%), 10 lymph nodes (no cancers), and 33 others (no cancers). Six (0.8%) of 745 BI-RADS category 3 lesions were malignant, and none of the malignancies were palpable at the time of US screening. The remaining 439 lesions not described as special cases were oval or had two or three gentle lobulations (n = 377, three [0.8%] cancers), with the three malignancies included among 361 circumscribed oval (0.8% malignancy rate), round (n = 36, no cancers), or irregular (n = 26, no cancers) masses. Table 3 lists the lesion features of BI-RADS category 3 lesions with no special features and the observed rates of malignancy. Overall, six (0.8%) of 745 BI-RADS category 3 lesions were malignant, and none of the malignancies were palpable at the time of US screening.
Table 2.
Frequency and Malignancy Rate among 745 BI-RADS Category 3 Lesions Identified at Screening US by Special Case Type, Calcification, and Palpability
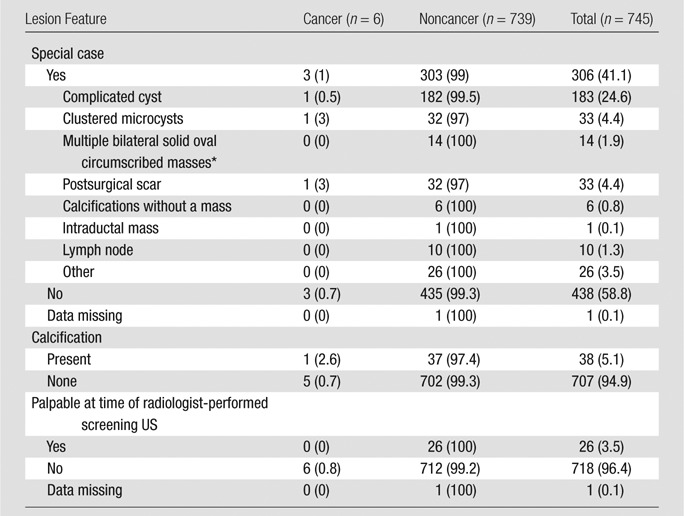
Note.—Data are number of lesions, and data in parentheses are percentages.
Berg et al (35) described a subset of 2172 evaluable women with two breasts enrolled in ACRIN 6666. In these women, 41 lesions were classified as multiple bilateral circumscribed masses and probably benign (none were malignant): (a) eleven lesions had multiple, bilateral, solid oval circumscribed masses (three more such lesions taken from women with only one breast at the time of the study have been included herein), (b) one lesion was described as clustered microcysts, (c) 17 were described as complicated cysts, (d) seven appear in Table 3 as oval and circumscribed masses, and (e) five appear in Table 3 as two or three gentle lobulations and circumscribed masses.
Table 3.
Frequency and Malignancy Rate of 439 BI-RADS Category 3 Lesions Other than Special Cases
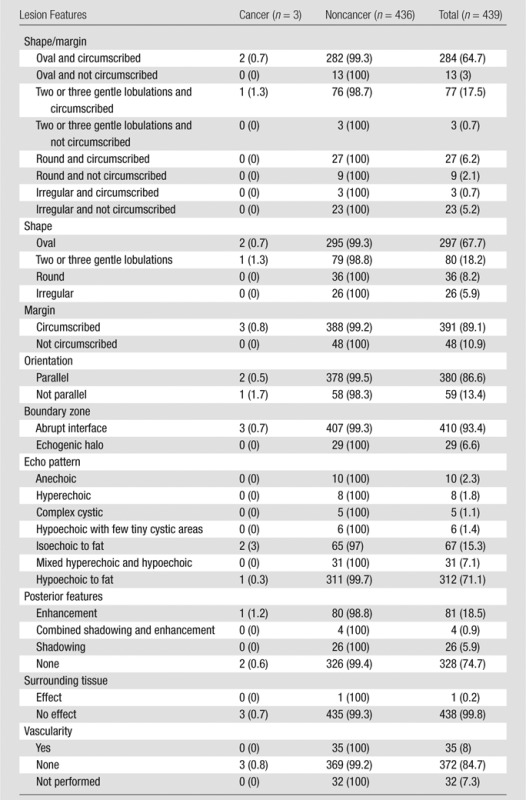
Note.—Data are number of lesions, and data in parentheses are percentages.
Of 519 participants with BI-RADS category 3 lesions, 349 (67.2%) also had cysts (simple or complicated). The cancer rate in participants with cysts (three of 349 patients [0.9%]) was not significantly different (P = .74) from that in participants without cysts (two of 170 patients [1.2%]).
Work-up Recommendations
Initial work-up recommendations based on initial US-only results for 745 BI-RADS category 3 lesions were 1-year follow-up (n = 23 [3.1%]), 6-month follow-up (n = 524 [70.3%]), additional imaging (n = 125 [16.8%]), or biopsy (n = 73 [9.8%]) (Table E1 [online]).
After integrated interpretation with mammography, 21 (2.8%) BI-RADS category 3 lesions (including one malignancy) were recommended for additional imaging, 561 (75.3%) were recommended for short-term follow-up, 72 (9.7%) were recommended for biopsy, 66 (8.8%) were recommended for 1-year follow-up, and 25 (3.4%) were missing data. After integrated interpretation with mammography, five (0.7%) of 672 BI-RADS category 3 lesions were upgraded to biopsy (no malignancies) and five (6.8%) of 73 were downgraded from biopsy (no malignancies).
Of the 745 BI-RADS category 3 lesions, 561 were recommended for short-interval follow-up after interpretation with mammography. Of these, 426 (420 US, six mammography) (75.9%) completed follow-up; 338 of these were recommended to return to routine mammography, 78 were recommended for additional 6-month follow-up (coinciding with the annual examination), and 10 were recommended for biopsy. Of the 10 lesions recommended for biopsy, biopsy was performed in seven, and none of the lesions were malignant. Biopsy was performed in all nine BI-RADS category 3 lesions recommended for biopsy after 12 months of follow-up, and one was malignant.
Of the 648 BI-RADS category 3 lesions that were first seen either in year 1 or year 2 of follow-up, by and including the annual examination, 251 (38.7%) lesions were no longer present, four (0.6%) had changed to BI-RADS category 1 lesions, 275 (42.4%) had changed to BI-RADS category 2 lesions, 110 (17.0%) remained BI-RADS category 3 lesions, seven (1.1%) had changed to BI-RADS category 4a lesions, and one (0.2%) had changed to a BI-RADS category 4b lesion.
Biopsy and Malignancy Rates
Of 745 BI-RADS category 3 lesions, 124 (16.6%) were ultimately sampled for biopsy (including one treatment mastectomy), revealing five malignancies (4.0% of such biopsies). One additional cancer was detected at prophylactic mastectomy, for an overall malignancy rate of 0.8% (95% CI: 0.3%, 1.7%) (six of 745 lesions). When we limit our analysis to a more conservative reference standard that requires at least 2-year follow-up or biopsy, the malignancy rate among BI-RADS category 3 lesions would be 0.9% (95% CI: 0.3%, 2.0%) (six of 636 lesions).
Of the six malignancies, two were diagnosed at initial detection (one more was excised during prophylactic mastectomy at the initial time point), and one malignancy was diagnosed at each of the following time points: 6, 12, and 24 months after initial US detection. Table 4 shows the biopsy rate, positive biopsy rate, and cancer rate for all 2916 US lesions. The malignancy rate of BI-RADS category 3 lesions (0.8%) was significantly lower than that of BI-RADS category 4a lesions (11 [2.8%] of 400 lesions, P = .01). If we exclude prophylactic mastectomy results, pathologic analysis of biopsied BI-RADS category 3 lesions revealed 37 (31.1%) benign cystic lesions, 28 (22.6%) lesions with fibrosis or a fibrocystic change, 25 (20.2%) fibroadenomas, 14 (11.3%) cases of benign breast tissue, three (2.4%) cases of fat necrosis, five (4.0%) malignancies, four (3.2%) sclerosing adenoses, four (3.2%) benign papillary lesions, and four (3.2%) findings that could only be classified as other. Of the 124 lesions that were sampled for biopsy, 64 had a reason provided. These reasons were as follows: participant preference (n = 23 [35.9%]), patient risk factors (n = 25 [39.0%]), increasing volume (n = 3 [4.7%]), interval suspicious change (n = 1 [1.6%]), and investigator uncertainty (n = 12 [18.8%]).
Table 4.
Biopsy Rate, Positive Biopsy Rate, and Cancer Rate in 2916 US Screening-detected Lesions across 7473 Examinations in 2662 Participants
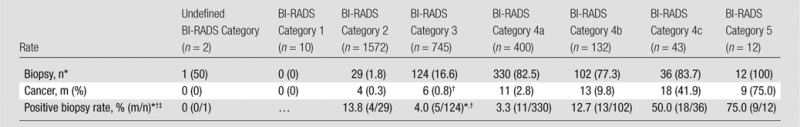
Note.—Unless otherwise indicated, data are number of lesions, and data in parentheses are percentages.
Mastectomies (but not prophylactic mastectomy) are included in the biopsy counts.
One cancer was diagnosed with prophylactic mastectomy (not included in positive biopsy rate), and one cancer was diagnosed with mastectomy for a different lesion; thus a total of six malignancies were diagnosed.
Data are percentages, and data in parentheses are those used to calculate the percentages.
Malignancies Classified as BI-RADS Category 3
The six malignant BI-RADS category 3 lesions occurred in five (1.0%) of 519 participants with BI-RADS category 3 lesions. Of the six malignancies, five (83%) were invasive, with a median size of 10 mm (range, 2–18 mm), and one node was positive at surgery (Table 5). Images of the BI-RADS category 3 lesions subsequently found to be malignant are presented in Figures 2–4 and Figures E1 and E2 (online). One time 0 lesion (Fig 2) that was classified as a clustered microcyst had suspicious changes. It appeared solid with distortion at 12-month screening, and it may not have even been the same lesion, with biopsy showing an 18-mm invasive lobular carcinoma. One patient with a separate BI-RADS category 4c lesion that was classified as an invasive ductal carcinoma (IDC) at biopsy elected to undergo bilateral mastectomy and also had bilateral BI-RADS category 3 lesions that were classified as (a) contralateral DCIS (NX) and (b) ipsilateral IDC and DCIS (N0), respectively (Fig 4).
Table 5.
Details of the Six Malignant BI-RADS Category 3 Lesions
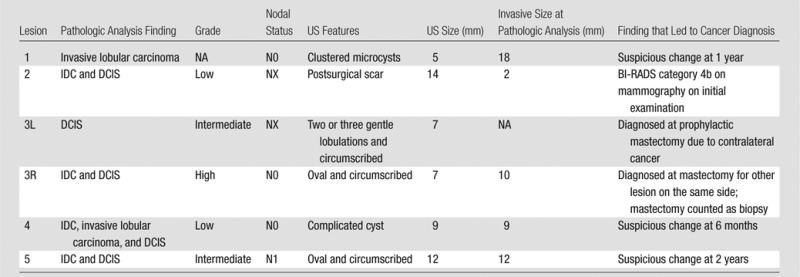
Note.—DCIS = ductal carcinoma in situ, 3L = left breast contralateral to cancer seen at mammography, 3R = right breast in the same patient as 3L, ipsilateral to another cancer seen at mammography, NA = not applicable.
Figure 2a:
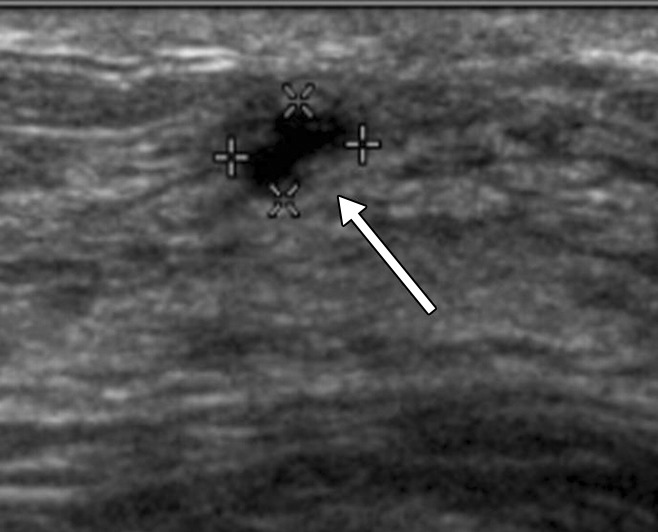
(a, b) Sonograms show a BI-RADS category 3 lesion (arrow) in a woman aged 43 years at 1-year follow-up. (a) This lesion was described as 5-mm clustered microcysts at 1-year follow-up. (b) At 2-year follow-up, the lesion had suspicious changes and was reclassified as a BI-RADS category 4a solid lesion. The lesion was not detected with mammography in either year. At biopsy, the lesion was determined to be an 18-mm invasive lobular cancer (N0).
Figure 4a:
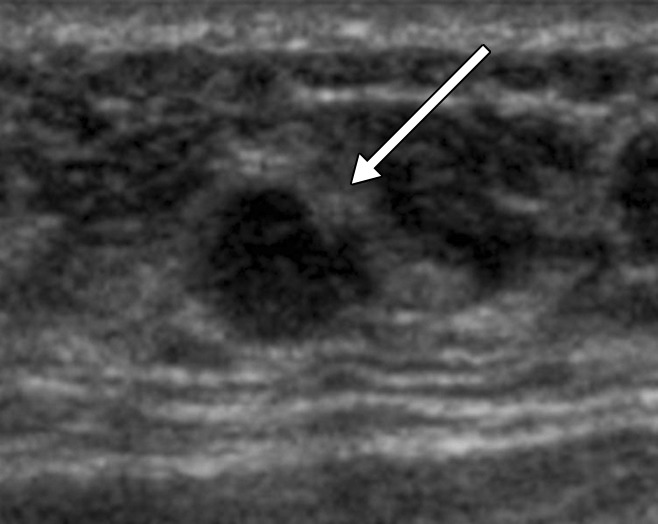
(a, b) US images in a 46-year-old woman with a BI-RADS category 4C lesion in her right breast that was classified as grade 3 invasive ductal cancer at pathologic analysis. At screening US, she also had a 7-mm right breast lesion (arrow in b) and a 7-mm left breast lesion (arrow in a), both of which were classified as BI-RADS category 3; these lesions were not detected at mammography. She elected to undergo bilateral mastectomy (prophylactic on the left side). On the basis of lesion locations, the lesion in the left breast (a) was thought to correlate with DCIS (NX), whereas the lesion in the right breast (b) was thought to correlate with a 10-mm IDC with associated DCIS (N0).
Figure 2b:
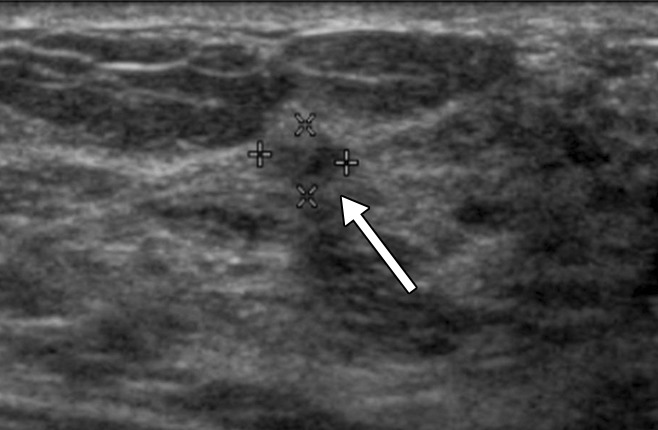
(a, b) Sonograms show a BI-RADS category 3 lesion (arrow) in a woman aged 43 years at 1-year follow-up. (a) This lesion was described as 5-mm clustered microcysts at 1-year follow-up. (b) At 2-year follow-up, the lesion had suspicious changes and was reclassified as a BI-RADS category 4a solid lesion. The lesion was not detected with mammography in either year. At biopsy, the lesion was determined to be an 18-mm invasive lobular cancer (N0).
Figure 3a:
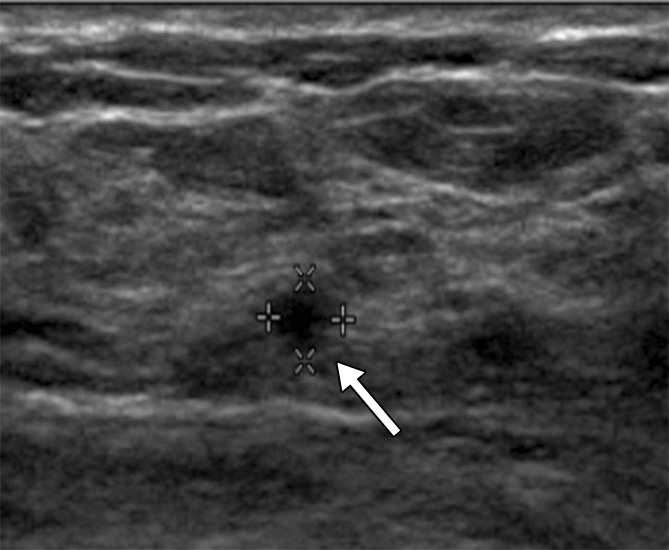
(a) At year 1 screening US, a 4-mm circumscribed oval mass (arrow) was described in a 59-year-old woman and assessed as a BI-RADS category 3 lesion. (b, c) The lesion (arrow) increased in size to (b) 8 mm in year 2 and (c) 12 mm in year 3. It was classified as a BI-RADS category 4a lesion in year 3. This lesion was not detected at mammography until year 3. At pathologic analysis, the lesion was determined to be a 12-mm grade 2 IDC with associated DCIS with a metastatic axillary node (N1).
Figure 3b:
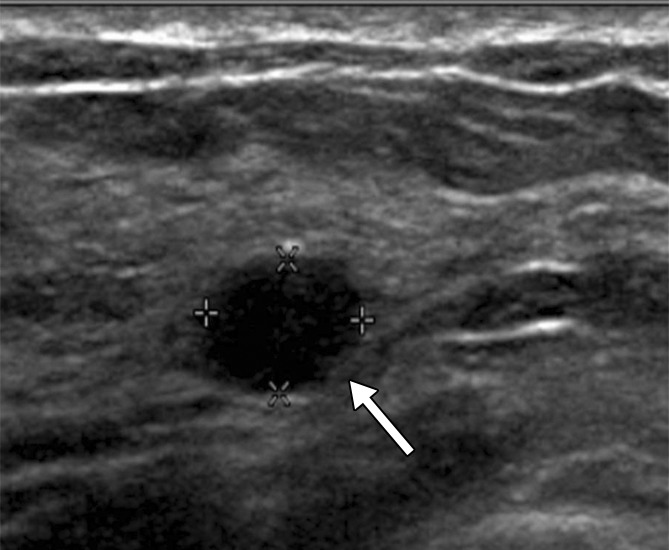
(a) At year 1 screening US, a 4-mm circumscribed oval mass (arrow) was described in a 59-year-old woman and assessed as a BI-RADS category 3 lesion. (b, c) The lesion (arrow) increased in size to (b) 8 mm in year 2 and (c) 12 mm in year 3. It was classified as a BI-RADS category 4a lesion in year 3. This lesion was not detected at mammography until year 3. At pathologic analysis, the lesion was determined to be a 12-mm grade 2 IDC with associated DCIS with a metastatic axillary node (N1).
Figure 3c:
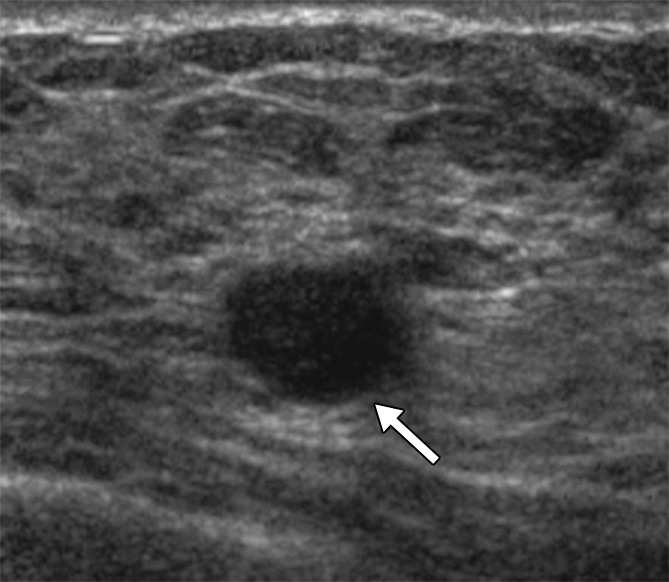
(a) At year 1 screening US, a 4-mm circumscribed oval mass (arrow) was described in a 59-year-old woman and assessed as a BI-RADS category 3 lesion. (b, c) The lesion (arrow) increased in size to (b) 8 mm in year 2 and (c) 12 mm in year 3. It was classified as a BI-RADS category 4a lesion in year 3. This lesion was not detected at mammography until year 3. At pathologic analysis, the lesion was determined to be a 12-mm grade 2 IDC with associated DCIS with a metastatic axillary node (N1).
Figure 4b:
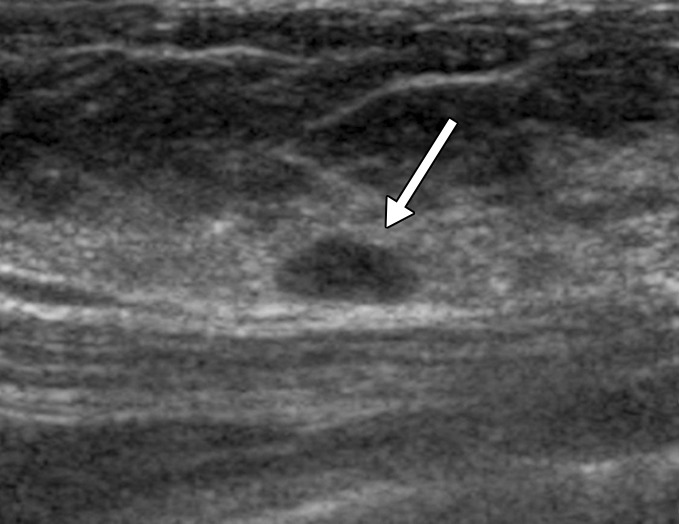
(a, b) US images in a 46-year-old woman with a BI-RADS category 4C lesion in her right breast that was classified as grade 3 invasive ductal cancer at pathologic analysis. At screening US, she also had a 7-mm right breast lesion (arrow in b) and a 7-mm left breast lesion (arrow in a), both of which were classified as BI-RADS category 3; these lesions were not detected at mammography. She elected to undergo bilateral mastectomy (prophylactic on the left side). On the basis of lesion locations, the lesion in the left breast (a) was thought to correlate with DCIS (NX), whereas the lesion in the right breast (b) was thought to correlate with a 10-mm IDC with associated DCIS (N0).
Only one (17%) of six malignant US B3 lesions was identified at mammography when the lesion was initially identified at US. This lesion was considered suspicious at mammography (BI-RADS category 4B), and the mammographic results prompted biopsy (Fig E2 [online]).
Three BI-RADS category 3 lesions that proved to be malignant were detected on the basis of changes seen on follow-up images. One was an invasive lobular carcinoma (N0); one, a low-grade IDC (N0); and one, an intermediate-grade IDC (N1). Of the six cancers identified in BI-RADS category 3 lesions, two (33%) were followed for 1 year, with no short-term 6-month follow-up performed. The single-node positive malignancy in our study had a rate of change that was greater than 20% per 6 months of follow-up (actually >200% in the 1st year); however, this malignancy was not sampled for biopsy until the 2nd year of follow-up. At that point, it had an additional increase of more than 200% in volume due to a lapse in protocol.
Most cancers identified at US were unique from those identified at mammography. Four (67%) of six cancers were not detected at mammography, and one (17%) cancer detected at US in year 1 and sampled for biopsy in year 3 because of change was not detected with mammography until year 3. One (17%) lesion was identified with both mammography and US. Integrated interpretation with mammography had a minimal effect on the recommendation for biopsy in 10 (1.3%) of 745 lesions. One of the five lesions in which the assessment was upgraded to biopsy on the basis of additional imaging performed after integrated interpretation with mammography was a 14-mm US mass that was in fact a 2-mm IDC with associated DS (NX).
Discussion
BI-RADS category 3 lesions are common at screening US; they were seen in nearly 20% of participants in this study after three rounds of screening, and they accounted for approximately 25% of US-detected lesions. Although the occurrence of new US lesions decreased at incidence screening, the proportion of newly detected lesions assessed as BI-RADS category 3 remained stable at approximately 25% for each of the 3 years of screening.
Hooley et al (29) reported BI-RADS category 3 lesions were detected at a rate of 20% (187 of 935 lesions) at initial screening US in women with mammographically dense breasts; this finding was similar to our findings. The percentage of BI-RADS category 3 lesions after work-up of screening mammographic abnormalities has been reported to range from 1.2% to 14.0% (36–40). One reason the detection rate for BI-RADS category 3 lesions is higher on US images than on mammographic images is that lesions ultimately considered to be BI-RADS category 3 lesions after mammographic detection are typically considered BI-RADS category 0 lesions initially. This results in immediate additional evaluation with additional mammographic views, US prior to rendering a BI-RADS category 3 assessment, or both (41,42). For physician-performed US, a BI-RADS category 3 recommendation can be given directly after a screening examination (13). In situations where there is an increased pretest probability, such as an ipsilateral known malignancy, BI-RADS category 3 classification is not appropriate (43). With the advent of whole-breast US images that can be interpreted after the patient has left the facility, follow-up diagnostic US may be required to provide a BI-RADS category 3 assessment. D’ Orsi and Sickles (44) recommended that the separation of screening and diagnostic examinations in auditing outcomes of whole-breast US be maintained.
Fewer than 2% of US BI-RADS category 3 lesions in our study were malignant (0.8%; 95% CI: 0.3%, 1.7%). This is similar to other US studies (7,8) and within the BI-RADS guidance. A similar malignancy rate of less than 2% (range, 0.3%–0.7%) has been validated for particular findings assessed as BI-RADS category 3 lesions at mammography (15–17,45). An important corollary to the low expected rate of malignancy is that the few malignancies found at follow-up should have a prognosis equivalent to that of screen-detected cancers (15). In our study, the average size of the detected invasive cancers among BI-RADS category 3 lesions was 10 mm (range, 2–18 mm), and five of six were invasive. Only one (20%) of five invasive cancers had metastasized to axillary lymph nodes; two were low-grade invasive cancers, two were intermediate-grade invasive cancers, and one was a high-grade invasive cancer. These results are similar to the results for all cancers seen only at screening US in ACRIN 6666, with a median size of 10 mm and one (3.7%) of 27 cancers being node positive (30).
The biopsy rate of US-detected BI-RADS category 3 lesions was 16.6% (124 of 745 lesions), with a positive biopsy rate of 4.0% (five of 124 lesions) in this study. BI-RADS category 3 and BI-RADS category 4a lesions accounted for 454 (71.6%) of the 634 biopsies prompted by US findings while representing 17 (27.9%) of 61 US-only detected cancers in the ACRIN 6666 trial. Across other studies of palpable US lesions classified as BI-RADS category 3, 294 (36%) of 807 were sampled for biopsy (18,43,46–48). In the BE1 study of shear wave elastography (21) 81% of BI-RADS category 3 lesions were biopsied or aspirated in Europe, as were 34% in the United States. In the Hooley et al study (29), biopsies were performed in 63 (6.7%) of 935 lesions; of these, nine (4.8%) were performed in BI-RADS category 3 lesions (n = 187), with no malignancies detected.
The cost of additional studies or biopsy in these probably benign lesions can be substantial (28,49–51). Since BI-RADS category 3 lesions have a low malignancy rate (0.8%; 95% CI: 0.3%, 1.7%) (six of 745 lesions) and since only one (0.1%) lesion had suspicious changes at short-interval follow-up and one (0.1%) lesion had suspicious changes at 1-year follow-up, both of which showed signs of node-negative invasive ductal carcinoma, a recommendation of yearly follow-up for BI-RADS category 3 lesions may be appropriate. Yearly follow-up for US BI-RADS category 3 lesions would substantially decrease the number of follow-up examinations, and thereby improve the cost-benefit ratio of US screening.
Across six prior series (7,52–56), only two (0.23%) of 868 lesions thought to be complicated cysts at US proved to be malignant. A large number of BI-RADS category 3 lesions (n = 183 [24.6%]) in our study were complicated cysts, with only 0.5% being malignant. One N0 lesion was detected because of a suspicious change at 6-month follow-up. These findings suggest complicated cysts with debris can be appropriately followed with routine yearly follow-up, as suggested by Hooley et al (29).
In four prior series, no malignancies were found among 112 lesions described as clustered microcysts (52–54,56). In the ACRIN 6666 study, one of 123 microcysts (0.8%) proved to be a malignancy. This lesion appeared solid with distortion at 2-year screening and may not have been the same lesion (56).
Study limitations included the fact that lesion classification was performed at each site and the fact that BI-RADS recommendations were not always strictly followed. Although the follow-up recommendation for B3 lesions is generally short-term interval follow-up after initial detection and again at 6-month follow-up (if stable), 30% of BI-RADS category 3 lesions at initial detection were given other recommendations. This may be partially explained by the fact that BI-RADS category 3 lesions were prospectively defined; the physician may have upgraded the recommendation based on clinical or patient factors (eg, previous history of breast cancer). Two (33%) of six cancers identified (one at treatment mastectomy for another cancer, counted as a biopsy; one at prophylactic mastectomy) were assumed to correlate with malignancies found at mastectomy based on position. One (17%) of the six cancers was classified as a suspicious change at 1-year follow-up; it changed from clustered microcysts to a solid lesion and may not have been the same lesion. The ACRIN 6666 trial was a well-controlled trial performed by trained physicians in elevated-risk patients, and the results may be different from those seen in usual clinical practice and those obtained with non–physician-performed or automated scanning. The ACRIN 6666 study had a 76% compliance rate for short interval follow-up examinations, which is comparable with that reported by Helvie et al (57) for mammography.
Further prospective validation of yearly follow-up of nonpalpable US BI-RADS category 3 lesions or of the use of elastography to up- or downgrade BI-RADS category 3 and 4a lesions is warranted to assess outcomes (58–61). Ideally, the positive biopsy rate of US-prompted biopsies can be substantially increased while maintaining the cancer detection rate.
In conclusion, with screening breast US becoming more widely used, BI-RADS category 3 masses will account for a substantial number of lesions identified. The present recommendation of short-term interval follow-up leads to additional imaging and biopsies (both physician-requested and patient-requested biopsies), with a low cancer detection rate. Since BI-RADS category 3 lesions have a low malignancy rate (0.8%; 95% CI: 0.3%, 1.7%) (six of 745 lesions) and since only one (0.1%) lesion had suspicious changes at short-interval follow-up and this lesion was node negative, a recommendation of yearly diagnostic follow-up for BI-RADS category 3 lesions detected at screening US may be appropriate.
Advances in Knowledge
■ Breast Imaging Reporting and Data System (BI-RADS) category 3 lesions are common at screening US; they were seen in nearly 20% (n = 519) of 2662 participants across 3 years of screening and accounted for 25% (n = 745) of 2916 US-detected lesions other than simple cysts.
■ The malignancy rate of US BI-RADS category 3 lesions was 0.8% (six of 745 lesions; 95% confidence interval [CI]: 0.3%, 1.7%) in this prospective multicenter trial.
■ Participants with BI-RADS category 3 lesions were more likely to be younger than 50 years (P = .001), of Hispanic or Latino ethnicity (P < .001), and premenopausal (P < .001).
■ Only one (17%) of six US BI-RADS category 3 lesions subsequently diagnosed as malignant was identified at mammography, which was performed 24 months later.
Implications for Patient Care
■ BI-RADS category 3 lesions had a biopsy rate of 16.6%, with 4.0% of such biopsies showing cancer; methods to reduce the number of BI-RADS category 3 lesions sampled for biopsy are needed.
■ The high frequency of BI-RADS category 3 lesions means that after three annual screening rounds, approximately 20% of patients who undergo screening US will be recommended for diagnostic follow-up on the basis of current recommendations.
■ Since BI-RADS category 3 lesions have a low malignancy rate (0.8%; 95% CI: 0.3%, 1.7%) and since only 0.1% of lesions had suspicious changes at short-interval follow-up and another 0.1% showed a suspicious change at 1-year follow-up, both of which were node-negative invasive cancers, a recommendation of yearly follow-up for BI-RADS category 3 lesions may be appropriate.
SUPPLEMENTAL TABLE
SUPPLEMENTAL FIGURES
From the 2011 RSNA Annual Meeting.
Received January 10, 2013; revision requested February 18; revision received April 29; accepted May 15; final version accepted May 29.
Supported by the Avon Foundation.
Funding: This research was supported by the National Institutes of Health (grants CA 80098 and CA 79778).
Disclosures of Conflicts of Interest: R.G.B. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is on the advisory board of Philips Ultrasound and Toshiba Medical Systems; received equipment and research grants from Siemens Ultrasound, Philips Ultrasound, and SuperSonic Imagine; gave lectures for Philips Ultrasound, Siemens Ultrasound, and SuperSonic Imagine; developed a breast university course for Philips Ultrasound; institution received equipment and research grants from Siemens Ultrasound, Philips Ultrasound, and SuperSonic Imagine. Other relationships: none to disclose. Z.Z. No relevant conflicts of interest to disclose. J.B.C. No relevant conflicts of interest to disclose. E.B.M. Financial activities related to the present article: is on the scientific advisory board of Hologic; received a grant from and is on the speaker’s bureau of Siemens Ultrasound; is a consultant for Philips US; is on the scientific advisory board of Toshiba US. Financial activities not related to the present article: is on the clinical advisory committee of Seno; is the medical director of Quantason. Other relationships: none to disclose. W.A.B. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is on the medical advisory board of Philips Healthcare; is a consultant for SuperSonic Imagine and Naviscan; gave lectures for SuperSonic Imagine; prepared educational materials for Gamma Medica; institution received grants from Hologic and GE Medical Systems; institution received equipment support from Gamma Medica and GE Medical Systems. Other relationships: none to disclose.
Abbreviations:
- ACRIN
- American College of Radiology Imaging Network
- BI-RADS
- Breast Imaging Reporting and Data System
- CI
- confidence interval
- DCIS
- ductal carcinoma in situ
- IDC
- invasive ductal carcinoma0
References
- 1.Berg WA, Gilbreath PL. Multicentric and multifocal cancer: whole-breast US in preoperative evaluation. Radiology 2000;214(1):59–66. [DOI] [PubMed] [Google Scholar]
- 2.Moon WK, Noh DY, Im JG. Multifocal, multicentric, and contralateral breast cancers: bilateral whole-breast US in the preoperative evaluation of patients. Radiology 2002;224(2):569–576. [DOI] [PubMed] [Google Scholar]
- 3.Gordon PB, Goldenberg SL. Malignant breast masses detected only by ultrasound: a retrospective review [see comments]. Cancer 1995;76(4):626–630. [DOI] [PubMed] [Google Scholar]
- 4.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008;299(18):2151–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchberger W, Niehoff A, Obrist P, DeKoekkoek-Doll P, Dünser M. Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography. Semin Ultrasound CT MR 2000;21(4):325–336. [DOI] [PubMed] [Google Scholar]
- 6.Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol 2003;181(1):177–182. [DOI] [PubMed] [Google Scholar]
- 7.Kolb TM, Lichy J, Newhouse JH. Occult cancer in women with dense breasts: detection with screening US—diagnostic yield and tumor characteristics. Radiology 1998;207(1):191–199. [DOI] [PubMed] [Google Scholar]
- 8.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 2002;225(1):165–175. [DOI] [PubMed] [Google Scholar]
- 9.Leconte I, Feger C, Galant C, et al. Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. AJR Am J Roentgenol 2003;180(6):1675–1679. [DOI] [PubMed] [Google Scholar]
- 10.Corsetti V, Ferrari A, Ghirardi M, et al. Role of ultrasonography in detecting mammographically occult breast carcinoma in women with dense breasts. Radiol Med (Torino) 2006;111(3):440–448. [DOI] [PubMed] [Google Scholar]
- 11.Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol 2010;20(3):734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendelson EB, Baum JK, Berg WA, et al. Breast Imaging Reporting and Data System, BI-RADS: Ultrasound. Reston, Va: American College of Radiology, 2003. [Google Scholar]
- 13.Mendelson EB, Berg WA, Böhm-Vélez M, et al. Breast Imaging Reporting and Data System, BI-RADS: Ultrasound. 2nd ed. Reston, Va: American College of Radiology; (in press). [Google Scholar]
- 14.Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology 1995;196(1):123–134. [DOI] [PubMed] [Google Scholar]
- 15.Sickles EA. Nonpalpable, circumscribed, noncalcified solid breast masses: likelihood of malignancy based on lesion size and age of patient. Radiology 1994;192(2):439–442. [DOI] [PubMed] [Google Scholar]
- 16.Varas X, Leborgne JH, Leborgne F, Mezzera J, Jaumandreu S, Leborgne F. Revisiting the mammographic follow-up of BI-RADS category 3 lesions. AJR Am J Roentgenol 2002;179(3):691–695. [DOI] [PubMed] [Google Scholar]
- 17.Vizcaíno I, Gadea L, Andreo L, et al. Short-term follow-up results in 795 nonpalpable probably benign lesions detected at screening mammography. Radiology 2001;219(2):475–483. [DOI] [PubMed] [Google Scholar]
- 18.Raza S, Chikarmane SA, Neilsen SS, Zorn LM, Birdwell RL. BI-RADS 3, 4, and 5 lesions: value of US in management—follow-up and outcome. Radiology 2008;248(3):773–781. [DOI] [PubMed] [Google Scholar]
- 19.Graf O, Helbich TH, Hopf G, Graf C, Sickles EA. Probably benign breast masses at US: is follow-up an acceptable alternative to biopsy? Radiology 2007;244(1):87–93. [DOI] [PubMed] [Google Scholar]
- 20.Heinig J, Witteler R, Schmitz R, Kiesel L, Steinhard J. Accuracy of classification of breast ultrasound findings based on criteria used for BI-RADS. Ultrasound Obstet Gynecol 2008;32(4):573–578. [DOI] [PubMed] [Google Scholar]
- 21.Chala L, Endo E, Kim S, et al. Gray-scale sonography of solid breast masses: diagnosis of probably benign masses and reduction of the number of biopsies. J Clin Ultrasound 2007;35(1):9–19. [DOI] [PubMed] [Google Scholar]
- 22.Kim EK, Ko KH, Oh KK, et al. Clinical application of the BI-RADS final assessment to breast sonography in conjunction with mammography. AJR Am J Roentgenol 2008;190(5):1209–1215. [DOI] [PubMed] [Google Scholar]
- 23.Berg WA, Cosgrove DO, Doré CJ, et al. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 2012;262(2):435–449. [DOI] [PubMed] [Google Scholar]
- 24.Costantini M, Belli P, Lombardi R, Franceschini G, Mulè A, Bonomo L. Characterization of solid breast masses: use of the sonographic breast imaging reporting and data system lexicon. J Ultrasound Med 2006;25(5):649–659; quiz 661. [DOI] [PubMed] [Google Scholar]
- 25.Sickles EA. Screening for breast cancer with mammography. Clin Imaging 1991;15(4):253–260. [DOI] [PubMed] [Google Scholar]
- 26.Hall FM. Malignancy in BI-RADS category 3 mammographic lesions. Radiology 2002;225:918–920. [DOI] [PubMed] [Google Scholar]
- 27.Varas X, Leborgne F, Leborgne JH. Nonpalpable, probably benign lesions: role of follow-up mammography. Radiology 1992;184(2):409–414. [DOI] [PubMed] [Google Scholar]
- 28.Lindfors KK, O’Connor J, Acredolo CR, Liston SE. Short-interval follow-up mammography versus immediate core biopsy of benign breast lesions: assessment of patient stress. AJR Am J Roentgenol 1998;171(1):55–58. [DOI] [PubMed] [Google Scholar]
- 29.Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology 2012;265(1):59–69. [DOI] [PubMed] [Google Scholar]
- 30.Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012;307(13):1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American College of Radiology Imaging Network. http://www.acrin.org/TabID/153/Default.aspx. Published November 30, 2007. Accessed December 21, 2012.
- 32.Berg WA, Blume JD, Cormack JB, Mendelson EB. Training the ACRIN 6666 Investigators and effects of feedback on breast ultrasound interpretive performance and agreement in BI-RADS ultrasound feature analysis. AJR Am J Roentgenol 2012;199(1):224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon PB, Gagnon FA, Lanzkowsky L. Solid breast masses diagnosed as fibroadenoma at fine-needle aspiration biopsy: acceptable rates of growth at long-term follow-up. Radiology 2003;229(1):233–238. [DOI] [PubMed] [Google Scholar]
- 34.Berg WA, Sechtin AG, Marques H, Zhang Z. Cystic breast masses and the ACRIN 6666 experience. Radiol Clin North Am 2010;48(5):931–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg WA, Zhang Z, Cormack JB, Mendelson EB. Multiple bilateral circumscribed masses at screening breast US: consider annual follow-up. Radiology doi:10.1148/radiol.13122251. Published online April 24, 2013. Accessed April 24, 2013. [DOI] [PMC free article] [PubMed]
- 36.Yasmeen S, Romano PS, Pettinger M, et al. Frequency and predictive value of a mammographic recommendation for short-interval follow-up. J Natl Cancer Inst 2003;95(6):429–436. [DOI] [PubMed] [Google Scholar]
- 37.Caplan LS, Blackman D, Nadel M, Monticciolo DL. Coding mammograms using the classification “probably benign finding—short interval follow-up suggested”. AJR Am J Roentgenol 1999;172(2):339–342. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology 2006;241(1):55–66. [DOI] [PubMed] [Google Scholar]
- 39.Kerlikowske K, Smith-Bindman R, Abraham LA, et al. Breast cancer yield for screening mammographic examinations with recommendation for short-interval follow-up. Radiology 2005;234(3):684–692. [DOI] [PubMed] [Google Scholar]
- 40.Baum JK, Hanna LG, Acharyya S, et al. Use of BI-RADS 3-probably benign category in the American College of Radiology Imaging Network Digital Mammographic Imaging Screening Trial. Radiology 2011;260(1):61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System, BI-RADS: Mammography. 4th ed. Reston, Va: American College of Radiology, 2003. [Google Scholar]
- 42.Sickles EA, Bassett LW, D’Orsi CJ, et al. BI-RADS Guidance. In: Breast Imaging Reporting and Data System, BI-RADS: Mammography. 5th ed. Reston, Va: American College of Radiology, 2013. [Google Scholar]
- 43.Kim SJ, Ko EY, Shin JH, et al. Application of sonographic BI-RADS to synchronous breast nodules detected in patients with breast cancer. AJR Am J Roentgenol 2008;191(3):653–658. [DOI] [PubMed] [Google Scholar]
- 44.D’Orsi CJ, Sickles EA. To seek perfection or not? that is the question. Radiology 2012;265(1):9–11. [DOI] [PubMed] [Google Scholar]
- 45.Rosen EL, Baker JA, Soo MS. Malignant lesions initially subjected to short-term mammographic follow-up. Radiology 2002;223(1):221–228. [DOI] [PubMed] [Google Scholar]
- 46.Graf O, Helbich TH, Fuchsjaeger MH, et al. Follow-up of palpable circumscribed noncalcified solid breast masses at mammography and US: can biopsy be averted? Radiology 2004;233(3):850–856. [DOI] [PubMed] [Google Scholar]
- 47.Harvey JA, Nicholson BT, Lorusso AP, Cohen MA, Bovbjerg VE. Short-term follow-up of palpable breast lesions with benign imaging features: evaluation of 375 lesions in 320 women. AJR Am J Roentgenol 2009;193(6):1723–1730. [DOI] [PubMed] [Google Scholar]
- 48.Shin JH, Han BK, Ko EY, Choe YH, Nam SJ. Probably benign breast masses diagnosed by sonography: is there a difference in the cancer rate according to palpability? AJR Am J Roentgenol 2009;192(4):W187–W191. [DOI] [PubMed] [Google Scholar]
- 49.Lindfors KK, Rosenquist CJ. Needle core biopsy guided with mammography: a study of cost-effectiveness. Radiology 1994;190(1):217–222. [DOI] [PubMed] [Google Scholar]
- 50.Brenner RJ, Sickles EA. Surveillance mammography and stereotactic core breast biopsy for probably benign lesions: a cost comparison analysis. Acad Radiol 1997;4(6):419–425. [DOI] [PubMed] [Google Scholar]
- 51.Alimoğlu E, Bayraktar SD, Bozkurt S, et al. Follow-up versus tissue diagnosis in BI-RADS category 3 solid breast lesions at US: a cost-consequence analysis. Diagn Interv Radiol 2012;18(1):3–10. [DOI] [PubMed] [Google Scholar]
- 52.Buchberger W, DeKoekkoek-Doll P, Springer P, Obrist P, Dünser M. Incidental findings on sonography of the breast: clinical significance and diagnostic workup. AJR Am J Roentgenol 1999;173(4):921–927. [DOI] [PubMed] [Google Scholar]
- 53.Chang YW, Kwon KH, Goo DE, Choi DL, Lee HK, Yang SB. Sonographic differentiation of benign and malignant cystic lesions of the breast. J Ultrasound Med 2007;26(1):47–53. [DOI] [PubMed] [Google Scholar]
- 54.Daly CP, Bailey JE, Klein KA, Helvie MA. Complicated breast cysts on sonography: is aspiration necessary to exclude malignancy? Acad Radiol 2008;15(5):610–617. [DOI] [PubMed] [Google Scholar]
- 55.Venta LA, Kim JP, Pelloski CE, Morrow M. Management of complex breast cysts. AJR Am J Roentgenol 1999;173(5):1331–1336. [DOI] [PubMed] [Google Scholar]
- 56.Berg WA. Sonographically depicted breast clustered microcysts: is follow-up appropriate? AJR Am J Roentgenol 2005;185(4):952–959. [DOI] [PubMed] [Google Scholar]
- 57.Helvie MA, Pennes DR, Rebner M, Adler DD. Mammographic follow-up of low-suspicion lesions: compliance rate and diagnostic yield. Radiology 1991;178(1):155–158. [DOI] [PubMed] [Google Scholar]
- 58.Barr RG. Sonographic breast elastography: a primer. J Ultrasound Med 2012;31(5):773–783. [DOI] [PubMed] [Google Scholar]
- 59.Barr RG, Lackey AE. The utility of the “bull’s-eye” artifact on breast elasticity imaging in reducing breast lesion biopsy rate. Ultrasound Q 2011;27(3):151–155. [DOI] [PubMed] [Google Scholar]
- 60.Barr RG, Destounis S, Lackey LB, 2nd, Svensson WE, Balleyguier C, Smith C. Evaluation of breast lesions using sonographic elasticity imaging: a multicenter trial. J Ultrasound Med 2012;31(2):281–287. [DOI] [PubMed] [Google Scholar]
- 61.Cho N, Jang M, Lyou CY, Park JS, Choi HY, Moon WK. Distinguishing benign from malignant masses at breast US: combined US elastography and color doppler US—influence on radiologist accuracy. Radiology 2012;262(1):80–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.