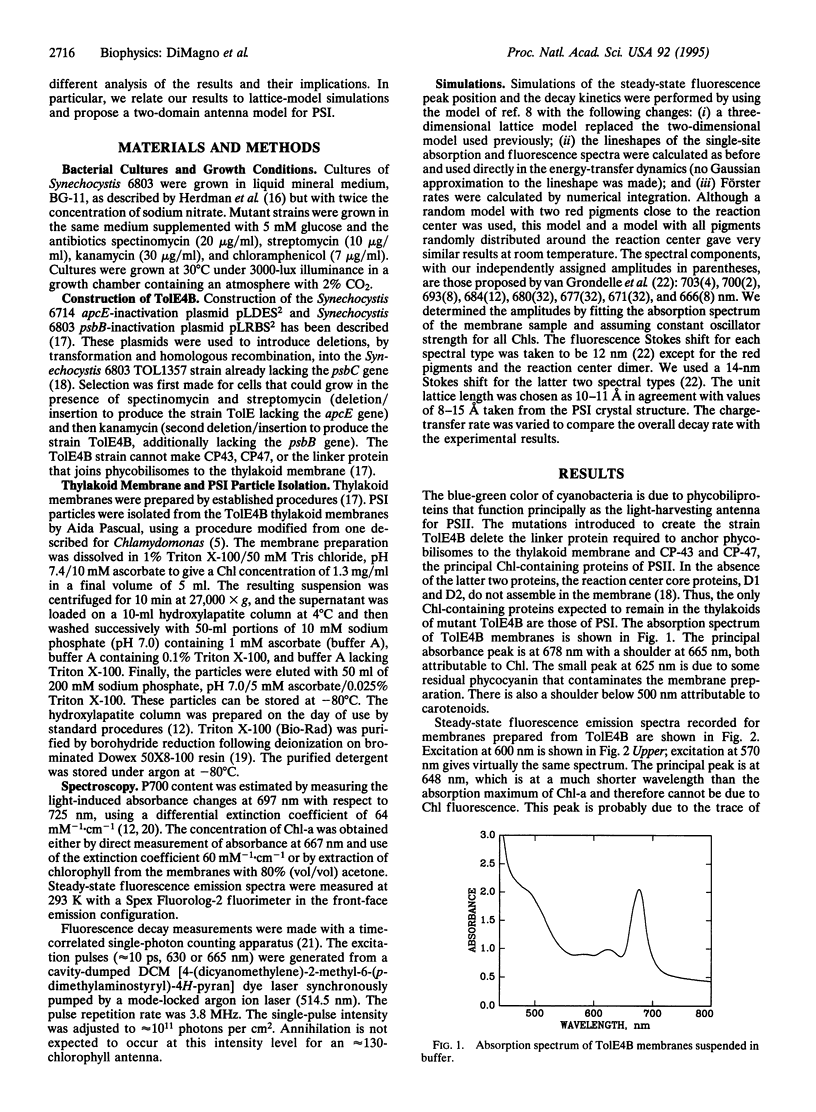
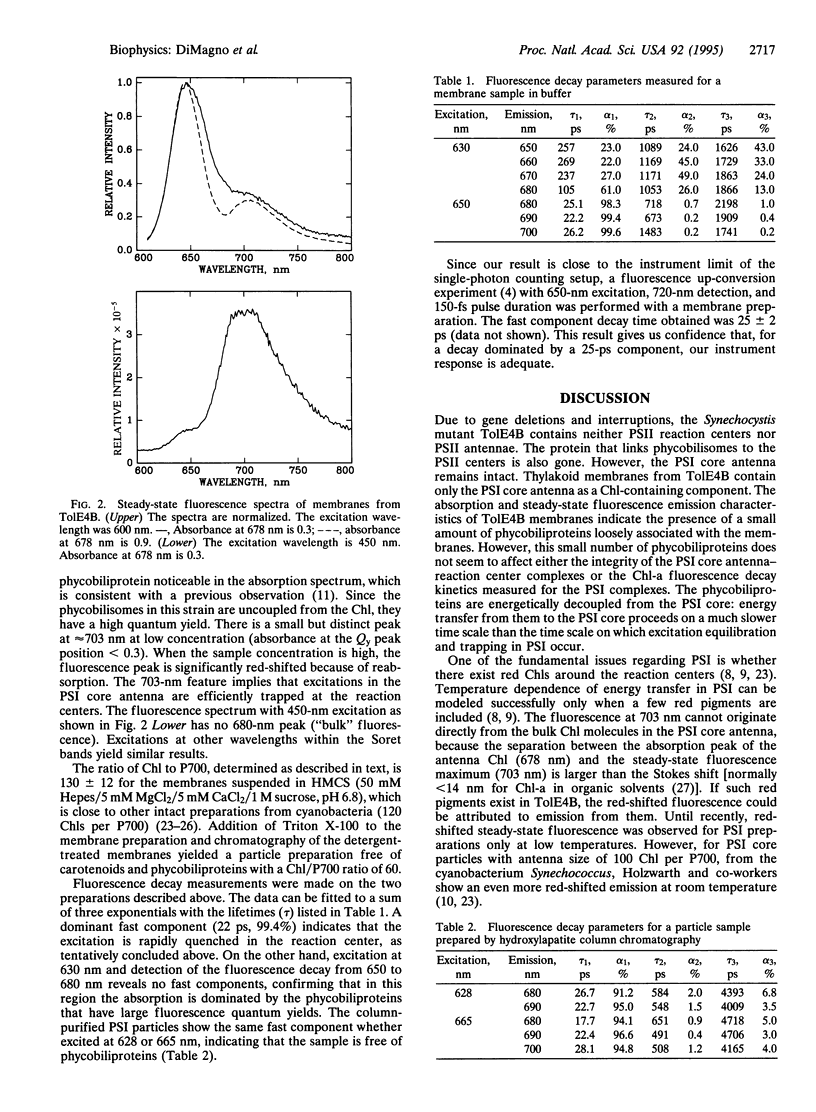
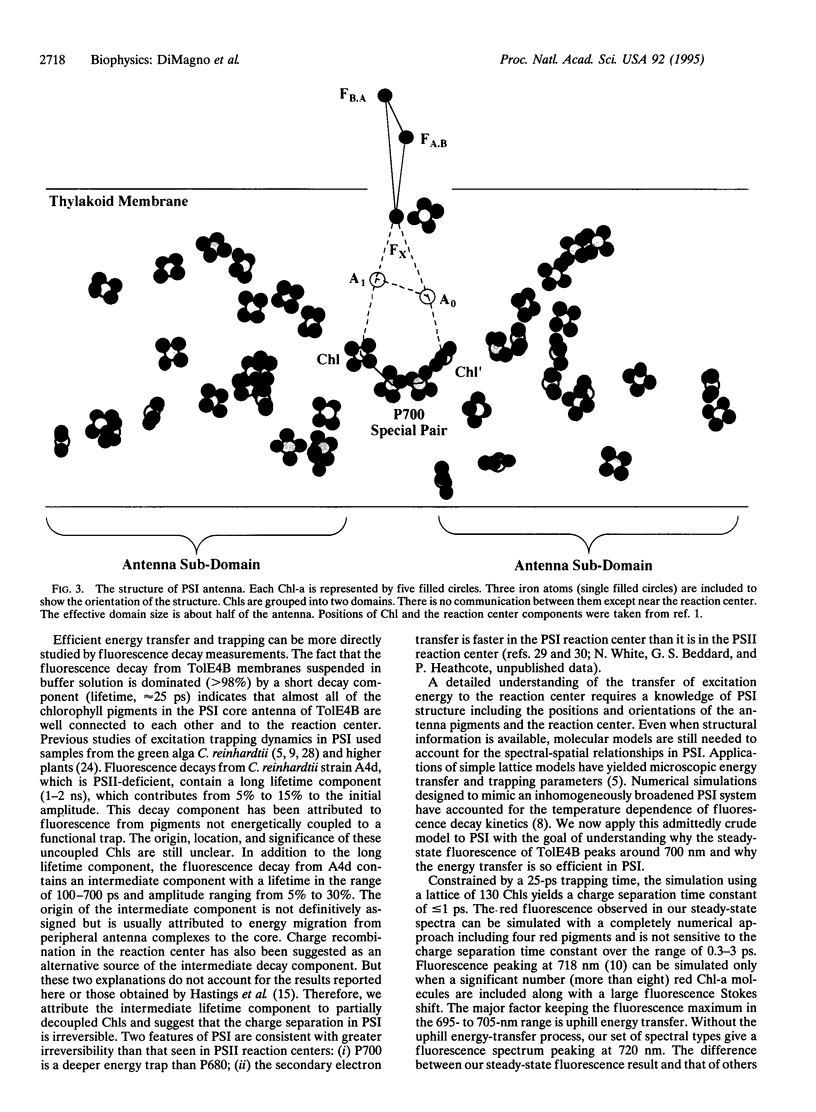
Abstract
A mutant strain of the cyanobacterium Synechocystis 6803, TolE4B, was constructed by genetic deletion of the protein that links phycobilisomes to thylakoid membranes and of the CP43 and CP47 proteins of photosystem II (PSII), leaving the photosystem I (PSI) center as the sole chromophore in the photosynthetic membranes. Both intact membrane and detergent-isolated samples of PSI were characterized by time-resolved and steady-state fluorescence methods. A decay component of approximately 25 ps dominates (99% of the amplitude) the fluorescence of the membrane sample. This result indicates that an intermediate lifetime is not associated with the intact membrane preparation and the charge separation in PSI is irreversible. The decay time of the detergent-isolated sample is similar. The 600-nm excited steady-state fluorescence spectrum displays a red fluorescence peak at approximately 703 nm at room temperature. The 450-nm excited steady-state fluorescence spectrum is dominated by a single peak around 700 nm without 680-nm "bulk" fluorescence. The experimental results were compared with several computer simulations. Assuming an antenna size of 130 chlorophyll molecules, an apparent charge separation time of approximately 1 ps is estimated. Alternatively, the kinetics could be modeled on the basis of a two-domain antenna for PSI, consistent with the available structural data, each containing approximately 65 chlorophyll a molecules. If excitation can migrate freely within each domain and communication between domains occurs only close to the reaction center, a charge separation time of 3-4 ps is obtained instead.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengis C., Nelson N. Purification and properties of the photosystem I reaction center from chloroplasts. J Biol Chem. 1975 Apr 25;250(8):2783–2788. [PubMed] [Google Scholar]
- Golbeck J. H. Structure, function and organization of the Photosystem I reaction center complex. Biochim Biophys Acta. 1987;895(3):167–204. doi: 10.1016/s0304-4173(87)80002-2. [DOI] [PubMed] [Google Scholar]
- Hastings G., Kleinherenbrink F. A., Lin S., Blankenship R. E. Time-resolved fluorescence and absorption spectroscopy of photosystem I. Biochemistry. 1994 Mar 22;33(11):3185–3192. doi: 10.1021/bi00177a007. [DOI] [PubMed] [Google Scholar]
- Hastings G., Kleinherenbrink F. A., Lin S., McHugh T. J., Blankenship R. E. Observation of the reduction and reoxidation of the primary electron acceptor in photosystem I. Biochemistry. 1994 Mar 22;33(11):3193–3200. doi: 10.1021/bi00177a008. [DOI] [PubMed] [Google Scholar]
- Hefti A., Ford R. C., Miller M., Cox R. P., Engel A. Analysis of the structure of photosystem I in cyanobacterial thylakoid membranes. FEBS Lett. 1992 Jan 13;296(1):29–32. doi: 10.1016/0014-5793(92)80396-x. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Jia Y., Jean J. M., Werst M. M., Chan C. K., Fleming G. R. Simulations of the temperature dependence of energy transfer in the PSI core antenna. Biophys J. 1992 Jul;63(1):259–273. doi: 10.1016/S0006-3495(92)81589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. Chlorophyll proteins of photosystem I. Plant Physiol. 1980 May;65(5):814–822. doi: 10.1104/pp.65.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. G., Webb S. P., Alberte R. S., Mets L., Fleming G. R. Antenna structure and excitation dynamics in photosystem I. I. Studies of detergent-isolated photosystem I preparations using time-resolved fluorescence analysis. Biophys J. 1988 May;53(5):733–745. doi: 10.1016/S0006-3495(88)83154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. G., Webb S. P., Mets L., Alberte R. S., Fleming G. R. Antenna size dependence of fluorescence decay in the core antenna of photosystem I: estimates of charge separation and energy transfer rates. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1532–1536. doi: 10.1073/pnas.84.6.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray W. J., Jr, Puvathingal J. M. A simple procedure for removing contaminating aldehydes and peroxides from aqueous solutions of polyethylene glycols and of nonionic detergents that are based on the polyoxyethylene linkage. Anal Biochem. 1985 May 1;146(2):307–312. doi: 10.1016/0003-2697(85)90544-5. [DOI] [PubMed] [Google Scholar]
- Royall D. R., Mahurin R. K., True J. E., Anderson B., Brock I. P., 3rd, Freeburger L., Miller A. Executive impairment among the functionally dependent: comparisons between schizophrenic and elderly subjects. Am J Psychiatry. 1993 Dec;150(12):1813–1819. doi: 10.1176/ajp.150.12.1813. [DOI] [PubMed] [Google Scholar]
- Rögner M., Chisholm D. A., Diner B. A. Site-directed mutagenesis of the psbC gene of photosystem II: isolation and functional characterization of CP43-less photosystem II core complexes. Biochemistry. 1991 Jun 4;30(22):5387–5395. doi: 10.1021/bi00236a009. [DOI] [PubMed] [Google Scholar]
- Shiozawa J. A., Alberte R. S., Thornber J. P. The P700-chlorophyll a-protein. Isolation and some characteristics of the complex in higher plants. Arch Biochem Biophys. 1974 Nov;165(1):388–397. doi: 10.1016/0003-9861(74)90177-5. [DOI] [PubMed] [Google Scholar]
- Werst M., Jia Y., Mets L., Fleming G. R. Energy transfer and trapping in the photosystem I core antenna. A temperature study. Biophys J. 1992 Apr;61(4):868–878. doi: 10.1016/S0006-3495(92)81894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]



