Abstract
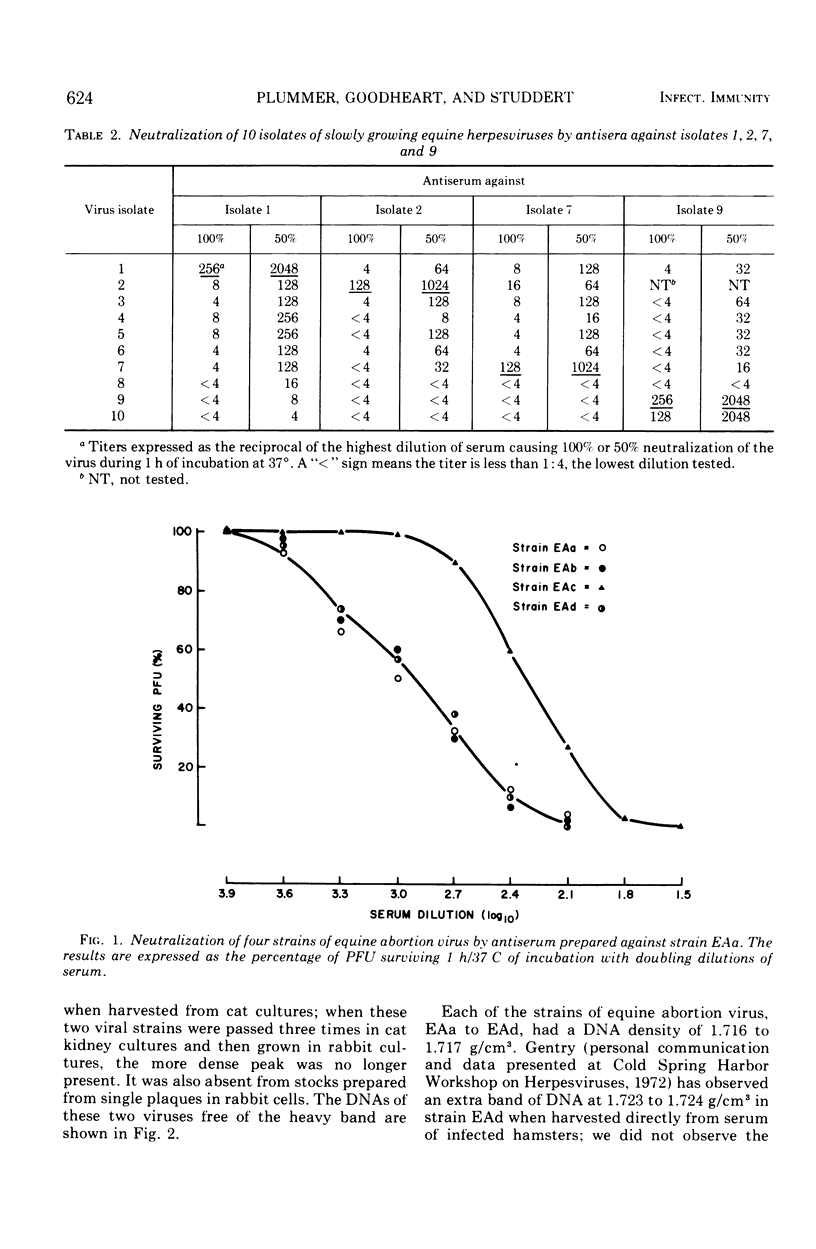
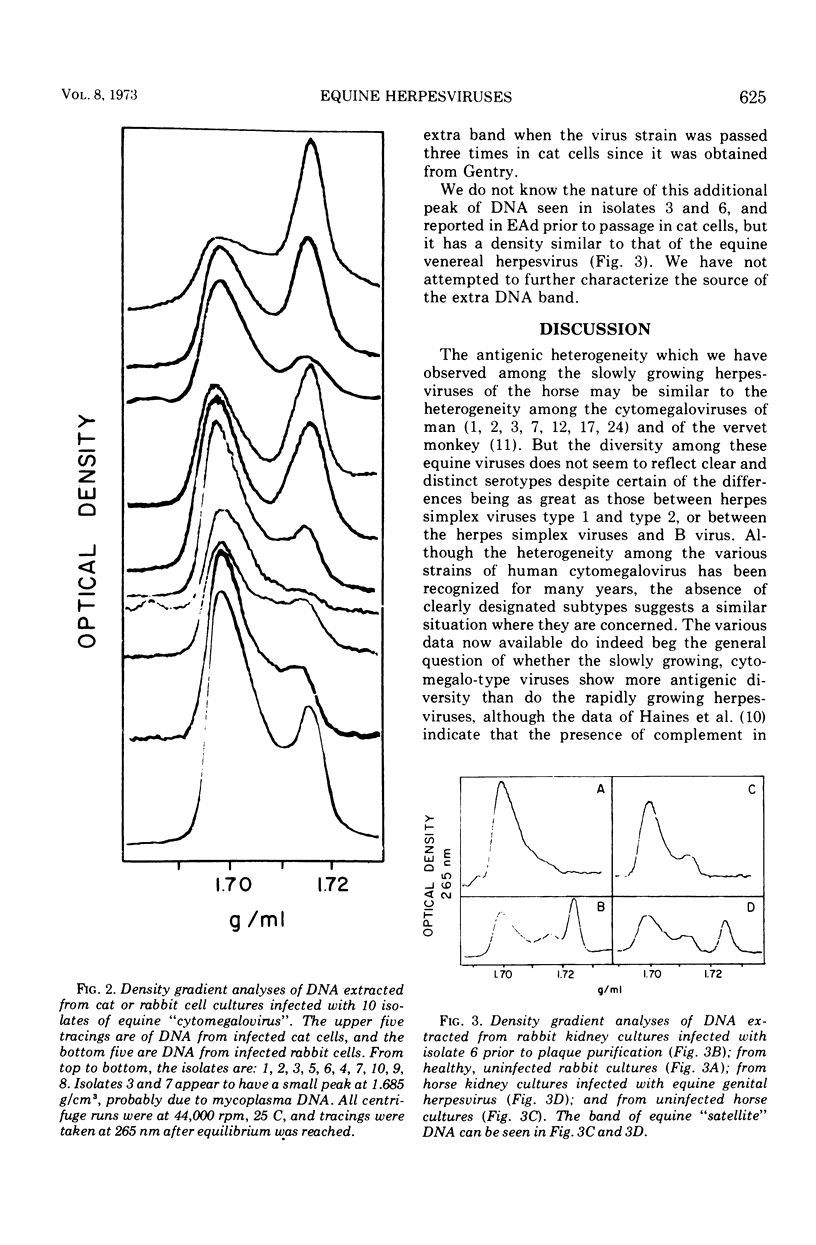
Equine herpesviruses with a deoxyribonucleic acid density of 1.716 to 1.717 g/cm3 were compared with one another by the plaque-reduction test and by the rate of development of cytopathic effect as indicated by plaque size in rabbit kidney cultures. Of the 19 isolates studied, the 9 which had already been tentatively labeled equine abortion viruses were serologically similar to one another; each of them grew more quickly than did any of the other 10 isolates although the mean plaque sizes formed a series of gradations with no clear hiatus which would permit the unequivocal delineation of the abortion viruses from the slowly growing strains. The 10 slowly growing isolates showed antigenic heterogeneity even though complement was present; the neutralizing capacity of an antiserum against the heterologous strains was, in most instances, markedly less than against the homologous strains, the range of the 50% endpoints being much greater than that observed among the equine abortion viruses, or among isolates of herpes simplex type 1. There was no cross neutralization between the equine abortion viruses and any of the 10 slowly growing isolates. An extra band of deoxyribonucleic acid, at 1.723 to 1.725 g/cm3, was present in two of the slowly growing strains when originally grown in rabbit cells, but was no longer present after passage in cat cells. This band occupied the same position as one reported in the hamster-passaged strain of equine abortion virus, and had a density similar to that of the equine genital herpesvirus. Although the taxonomic demarcation of the equine abortion viruses and the slowly growing herpesviruses from one another is still open to question, they can be conveniently labeled equine herpesviruses 1 and 2, respectively; the genital virus would be termed equine herpesvirus 3.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen H. K. Serologic differentiation of human cytomegalovirus strains using rabbit hyperimmune sera. Brief report. Arch Gesamte Virusforsch. 1971;33(1):187–191. doi: 10.1007/BF01254177. [DOI] [PubMed] [Google Scholar]
- Andersen H. K. Studies of human cytomegalovirus strain variations by kinetic neutralization tests. Arch Gesamte Virusforsch. 1972;38(4):297–305. doi: 10.1007/BF01262820. [DOI] [PubMed] [Google Scholar]
- Birnbaum G., Lynch J. I., Margileth A. M., Lonergan W. M., Sever J. L. Cytomegalovirus infections in newborn infants. J Pediatr. 1969 Nov;75(5):789–795. doi: 10.1016/s0022-3476(69)80301-x. [DOI] [PubMed] [Google Scholar]
- DOLL E. R., BRYANS J. T. Incubation periods for abortion in equine viral rhinopneumonitis. J Am Vet Med Assoc. 1962 Aug 1;140:351–354. [PubMed] [Google Scholar]
- DOLL E. R., MCCOLLUM W. H., BRYANS J. W., CROWE E. W. Propagation of equine abortion virus in the embryonated chicken egg. Cornell Vet. 1956 Jan;46(1):97–108. [PubMed] [Google Scholar]
- Dreesman G. R., Benyesh-Melnick M. Spectrum of human cytomegalovirus complement-fixing antigens. J Immunol. 1967 Dec;99(6):1106–1114. [PubMed] [Google Scholar]
- Goodheart C. R., Plummer G., Waner J. L. Density difference of DNA of human herpes simplex viruses, types I and II. Virology. 1968 Jul;35(3):473–475. doi: 10.1016/0042-6822(68)90225-0. [DOI] [PubMed] [Google Scholar]
- HANSHAW J. B., BETTS R. F., SIMON G., BOYNTON R. C. ACQUIRED CYTOMEGALOVIRUS INFECTION: ASSOCIATION WITH HEPATOMEGALY AND ABNORMAL LIVER-FUNCTION TESTS. N Engl J Med. 1965 Mar 25;272:602–609. doi: 10.1056/NEJM196503252721202. [DOI] [PubMed] [Google Scholar]
- Haines H. G., Von Essen R., Benyesh-Melnick M., Melnick J. L. Preparation of specific antisera to cytomegaloviruses in goats. Proc Soc Exp Biol Med. 1971 Dec;138(3):846–849. doi: 10.3181/00379727-138-36003. [DOI] [PubMed] [Google Scholar]
- Hampar B., Martos L. M., Ablashi D. V., Siguënza R. F., Wells G. A. Differentiation of cross-reacting simian cytomegalovirus strains by late 19S rabbit neutralizing antibodies. J Immunol. 1969 Nov;103(5):1155–1156. [PubMed] [Google Scholar]
- Hsiung G. D., Fischman H. R., Fong C. K., Green R. H. Characterization of a cytomegalo-like virus isolated from spontaneously degenerated equine kidney cell culture. Proc Soc Exp Biol Med. 1969 Jan;130(1):80–84. doi: 10.3181/00379727-130-33492. [DOI] [PubMed] [Google Scholar]
- Karpas A. Characterization of a new herpes-like virus isolated from foal kidney. Ann Inst Pasteur (Paris) 1966 May;110(5):688–696. [PubMed] [Google Scholar]
- Ludwig H., Biswal N., Bryans J. T., McCombs R. M. Some properties of the DNA from a new equine herpesvirus. Virology. 1971 Aug;45(2):534–537. doi: 10.1016/0042-6822(71)90357-6. [DOI] [PubMed] [Google Scholar]
- MEDEARIS D. N., Jr OBSERVATIONS CONCERNING HUMAN CYTOMEGALOVIRUS INFECTION AND DISEASE. Bull Johns Hopkins Hosp. 1964 Mar;114:181–211. [PubMed] [Google Scholar]
- PLUMMER G., WATERSON A. P. Equine herpes viruses. Virology. 1963 Mar;19:412–416. doi: 10.1016/0042-6822(63)90083-7. [DOI] [PubMed] [Google Scholar]
- Pascoe R. R., Spradbrow P. B., Bagust T. J. An equine genital infection resembling coital exanthema associated with a virus. Aust Vet J. 1969 Apr;45(4):166–170. doi: 10.1111/j.1751-0813.1969.tb01922.x. [DOI] [PubMed] [Google Scholar]
- Plummer G., Bowling C. P., Goodheart C. R. Comparison of four horse herpesviruses. J Virol. 1969 Nov;4(5):738–741. doi: 10.1128/jvi.4.5.738-741.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL W. C., CRAWFORD L. V. PROPERTIES OF THE NUCLEIC ACIDS FROM SOME HERPES GROUP VIRUSES. Virology. 1964 Feb;22:288–292. doi: 10.1016/0042-6822(64)90017-0. [DOI] [PubMed] [Google Scholar]
- Studdert M. J., Turner A. J., Peterson J. E. Equine herpesviruses. I. Isolation and characterisation of equine rhinopneumonitis virus and other equine herpesviruses from horses. Aust Vet J. 1970 Mar;46(3):83–89. doi: 10.1111/j.1751-0813.1970.tb15927.x. [DOI] [PubMed] [Google Scholar]
- Turner A. J., Studdert M. J., Peterson J. E. Equine herpes viruses. 2. Persistence of equine herpesviruses in experimentally infected horses and the experimental induction of abortion. Aust Vet J. 1970 Mar;46(3):90–98. doi: 10.1111/j.1751-0813.1970.tb15928.x. [DOI] [PubMed] [Google Scholar]
- WELLER T. H., HANSHAW J. B., SCOTT D. E. Serologic differentiation of viruses responsible for cytomegalic inclusion disease. Virology. 1960 Sep;12:130–132. doi: 10.1016/0042-6822(60)90156-2. [DOI] [PubMed] [Google Scholar]


