Abstract
Background
Enhanced arginine vasopressin (AVP) levels are associated with increased mortality during end-stage human heart failure (HF), and cardiac AVP type 1A receptor (V1AR) expression becomes increased. Additionally, mice with cardiac-restricted V1AR overexpression develop cardiomyopathy and decreased β-adrenergic receptor (βAR) responsiveness. This led us to hypothesize that V1AR signaling regulated βAR responsiveness and in doing so contributes to HF development.
Methods and Results
Transaortic constriction resulted in decreased cardiac function and βAR density and increased cardiac V1AR expression, effects reversed by a V1AR-selective antagonist. Molecularly, V1AR stimulation led to decreased βAR ligand affinity, as well as βAR-induced Ca2+ mobilization and cAMP generation in isolated adult cardiomyocytes, effects recapitulated via ex vivo Langendorff analysis. V1AR-mediated regulation of βAR responsiveness was demonstrated to occur in a previously unrecognized Gq protein-independent/GRK-dependent manner.
Conclusions
This newly discovered relationship between cardiac V1AR and βAR may be informative for the treatment of patients with acute decompensated HF and elevated AVP.
Keywords: vasopressin type 1A receptor, β-adrenergic receptor, myocardium, contractility, cardiomyopathy
Introduction
The neurohormone arginine vasopressin (AVP) is elevated in patients with heart failure (HF) and there is a direct relationship between plasma levels of AVP and disease severity and mortality1-5. AVP is released from the hypothalamus in response to changes in arterial pressure and plasma osmolality. Subsequently, AVP acts at three related but distinct G protein-coupled receptors (GPCR): V1AR (heart, vascular smooth muscle, myometrium, central nervous system and liver), V1BR (anterior pituitary) and V2R (vascular endothelial cells and renal tubule collecting ducts). AVP acts at V1ARs to induce peripheral vasoconstriction and cardiac hypertrophy via Gq protein-mediated signaling. Activation of the V2R leads to release of von Willebrand factor from vascular endothelial cells and insertion of aquaporin 2 into collecting duct cells, thereby resulting in increased transepithelial water permeability, water retention and urine concentration via Gs protein-mediated signaling6. Therefore, either inappropriate or persistent AVP release causes profound hyponatremia, itself a risk factor for increased death and hospitalization in HF patients 7. Because of the adverse clinical consequences associated with hyponatremia, basic science investigations and drug development have focused on V2R antagonists, which have been approved for the treatment of the hyponatremia associated with HF.
The physiologic effects of AVP on the heart have been far less clear than its effects on the kidney owing in part to the fact that AVP mediates peripheral vasoconstrictor through activation of the V1AR8-10. To better understand the role of V1AR in the heart independent of its effects on the vasculature, we previously created transgenic mice with inducible and cardiac-restricted overexpression of V1AR (V1AR-TG). These mice developed LV hypertrophy, dilatation, diminished contractile performance and reprogramming of the HF gene profile in a Gq protein-dependent manner11, findings consistent with effects observed with other Gq protein-coupled receptors12. However, these results did not clarify the potential acute effects of V1AR activation in the heart because the HF phenotype was only observed in the V1AR-TG mice 20 weeks following transgene activation.
Assessing the acute effects of V1AR signaling on the heart and identifying its cognate signaling pathways is relevant because of the recent observations that V1AR density is significantly increased in hearts from patients with end-stage HF13 and we showed in our V1AR-TG model that enhanced cardiac V1AR expression diminished the hemodynamic response to βAR stimulation in vivo11. Thus, if V1AR signaling acts to impair βAR activation, even modest increases in AVP might have substantial effects on myocardial performance. Here, using cultured adult myocytes, genetically engineered non-myoctes, ex vivo Langendorff-perfused hearts and adult mice with HF secondary to trans-aortic constriction (TAC), we report that AVP acutely inhibits βAR-mediated cardiac contractility via a novel G-protein receptor kinase (GRK)-dependent and Gq protein-independent mechanism. These results may explain the increased mortality observed in patients with acute heart failure and elevated AVP levels and provide support for the potential use of a V1AR antagonist in the treatment of these patients.
Methods
Materials
Angiotensin II (A9525), arginine vasopressin (V9879), CGP 20712A (C231), dimethyl sulfoxide (D4540), forskolin (F6886), ICI 119,551 (I127), 3-isobutyl-1-methylxanthine (I5879), isoproterenol (I6504), rolipram (R6520) and SR 49059 (S5701) were purchased from Sigma-Aldrich (St. Louis, MO). Xtremegene 9 DNA transfection reagent was purchased from Roche Applied Science (Indianapolis, IN). UBO-QIC was purchased from Prof. Evi Kostenis, University of Bonn, Germany.
Animal Protocols
All experiments were performed following the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Temple University IACUC (ACUP#4031). GqI-TG mice have cardiac-restricted over-expression of a peptide derived from a carboxyl-terminal peptide of the α-subunit of Gq protein14 and V1AR-TG mice have controlled and cardiac-restricted over-expression of the V1AR11. Wild-type (WT, 75%C57Bl6/J/25%FVB) littermate controls were used where appropriate.
Transverse Aortic Constriction (TAC)
Pressure overload was produced by transverse aortic constriction (TAC) as previously described15. After anesthesia with isoflurane (2.5%), the chest was opened and an aortic band was created in 8 wk old wild-type mice by placing a ligature (7-0 nylon suture) securely between the origin of the right innominate and left common carotid arteries with a 27-gauge needle as a guide. The sham procedure was identical except that the aorta was not ligated. Post-procedure the chest was closed and the animal was allowed to recover after anesthesia. Doppler velocity was measured in the right (RCA) and the left (LCA) carotid arteries and RCA/LCA velocity ratio was calculated to ensure that TAC produced equal aortic pressure gradient in all experimental groups.
Echocardiography
Left ventricular (LV) function was evaluated in mice before TAC and at weekly intervals after TAC using a VisualSonic Vevo 770 imaging system and a 707 scan head (Miami, FL) as previously described16. After mice were lightly sedated with isoflurane (2.0%), a parasternal short-axis view was obtained for LV M-mode imaging at the papillary muscle level. Three independent M-mode images were used for measurements of LV end-diastolic internal diameter (LVEDD) and LV end-systolic internal diameter (LVESD) in two consecutive beats according to the American Society of Echocardiography leading edge method. Fractional shortening (FS) was calculated as FS% = [(LVEDD − LVESD)/LVEDD]×100. Anterior (AWT) and Posterior Wall thickness (PWT) were also measured.
Subcutaneous implantation of ALZET minipumps
1 week after sham or TAC surgery, the mice were anesthetized with isoflurane (1.5-2.5%) and underwent subcutaneous implantation with minipumps (ALZET, Cupertino CA, model #2006) containing SR49059 (1 mg/kg/day) or vehicle control (0.1% DMSO in sterile saline) and were subsequently monitored for 6 weeks.
Cardiac membrane preparation and radioligand binding for V1AR and βAR
Membrane preparation and radioligand binding assays were performed as described previously13. Cleaned and minced myocardium was homogenized in ice-cold buffer (mmol/L: Tris-HCl 10, pH 7.4, EDTA 10) with a Polytron homogenizer (Brinkmann Instruments), filtered through three layers of cheesecloth and centrifuged at 1000 × g for 10 minutes at 4°C. The supernatant was then filtered through two layers of cheesecloth and centrifuged at 45,000 × g for 30 minutes at 4°C to yield membranes. The plasma membrane pellet was adjusted to a final concentration of 1 mg/mL protein with binding buffer (mmol/L: Tris 50, EDTA 1, pH 7.4). Protein concentration was determined by the Lowry method.
Membrane preparations (25ug protein for βAR-binding or 40 μg protein for V1AR-binding) were incubated with 125I-cyanopindolol (Cyp, PerkinElmer, Waltham, MA; 4 to 300 pmol/L) or 125I-p-AVP (PerkinElmer, Waltham, MA; 0.67 to 150 pmol/L) in incubation buffer (mmol/L: Tris 50, EDTA 5, 0.1% BSA). Incubations were performed either alone or with propranolol (βAR non-specific antagonist, 10μmol/L) or SR49059 (V1AR-selective antagonist, 5 μmol/L), to determine nonspecific binding for subtraction from total binding to calculate specific binding. The incubation was carried out at room temperature (25°C) for 2 hours in a total volume of 250μL (βAR) or 100 μL (V1AR) in which steady state kinetics were achieved in specific binding. The reaction was terminated by the addition of 4°C incubation buffer and vacuum filtration through glass fiber filters (Whatman GF/C, Brandel, Inc). Each filter was washed three times with 7 ml of ice-cold 10 mmol/L Tris-HCl plus 0.1% BSA. Radioactivity of the wet filters was determined in a Gamma counter. All assays were performed in duplicate. Receptor density was normalized to milligrams of membrane protein. Kd and the maximal number of binding sites (Bmax) were determined by Scatchard analysis of saturation binding isotherms with Prism 5.0.
Isolation of adult murine cardiac myocytes and intracellular Ca2+ transient measurements
Cardiac myocytes were isolated from the septum and LV free wall of 8-12 week old male WT mice as previously described17 and intracellular Ca2+ transient measurements were performed as previously described18. Briefly, mice were heparinized (1,500 U/kg ip) and anesthetized (pentobarbital sodium, 50 mg/kg ip). Excised hearts were mounted on a steel cannula and retrograde perfused (100 cmH2O, 37°C) with Ca2+-free bicarbonate buffer followed by enzymatic digestion (collagenases B and D, protease XIV). Isolated myocytes were plated on laminin-coated glass coverslips, and the Ca2+ concentration of the buffer was incrementally increased (0.05, 0.125, 0.25, 0.5 mmol/L) with 10 min of exposure at each concentration. The final Ca2+ buffer was then aspirated and replaced with MEM (Sigma-Aldrich) containing 1.2 mmol/L Ca2+, 2.5% FBS, and 1% penicillin/streptomycin. The myocytes were exposed to 0.67 μmol/L Fura 2-AM for 15 min at 37°C, then field-stimulated to evoke intracellular Ca2+ transients (1 Hz, 37°C) in medium 199 containing 1.8 mmol/L extracellular Ca2+.
Adult feline left ventricular myocyte (AFVM) isolation and infection
Adult feline left ventricular myocytes were isolated as previously described19. Felines were anesthetized with sodium pentobarbital and hearts were rapidly excised, cannulated, and mounted on a constant flow Langendorff apparatus. Hearts were rinsed with a physiological Krebs-Henseleit buffer (KHB) and then retrograde perfused with collagenase containing KHB. When the tissue softened, the left ventricle was isolated and gently minced, filtered, and equilibrated in KHB with 0.2 mmol/L CaCl2, and 1% bovine serum albumin (BSA) at room temperature. Isolated myocytes were washed with serum-free culture medium (Medium 199, Sigma) supplemented with penicillin-streptomycin-glutamine (Gibco) and seeded on 10mm glass coverslip-containing 35mm culture dishes (MatTek Corporation, MA) coated with laminin (BD Bioscience). 5×104 cells/10mm coverslip were infected with adenovirus containing the cAMP FRET reporter ICUE320 (Ad-ICUE3) at a multiplicity of infection (MOI) of 40 for 36 hr.
Site-directed mutagenesis of human V1AR to attain GRK phosphorylation-deficient V1AR (GRK−V1AR)
The nucleotide sequences encoding the 17 carboxy-terminal serine (S352, S362, S380, S382, S389, S393, S393, S404, S407, S408, S410, S417) and threonine (T378, T386, T395, T398, T418) residues in HA-tagged human V1AR underwent mutagenesis to alanine-encoding sequences using Stratagene Quikchange II XL Site-Directed Mutagenesis Kit as per kit instructions (primers listed in Supplemental Table 1).
Cell culture and transfection
HEK 293 cells stably expressing FLAG-tagged β1AR21, HA-tagged angiotensin II type 1A receptor (AT1R)22 or human U2S osteosarcoma cells were grown in 10% FBS and 1% PSF-containing MEM. 5×104 cells/coverslip were seeded on 10mm glass coverslip-containing 35mm dishes as described above and transfected for 24 hr with 1μg WTV1AR (for β1AR cells or U2S cells), GRK−V1AR (for β1AR cells) or WT-β1AR (for AT1R cells) with 1 μg of either ICUE3 or the diacylglycerol FRET reporter (DAGR) using a 3:1 ratio of X-tremeGENE 9 to DNA.
Fluorescent resonance energy transfer (FRET) measurements
AFVM or HEK 293 cells expressing FRET reporters were rinsed and media was replaced with imaging buffer (in mmol/L: NaCl 125, KCl 5, MgCL2 1.5, CaCl2 1.5, glucose 10, HEPES [pH 7.4] 10 and BSA at 0.2%) prior to imaging using a Leica DMI4000B inverted microscope with a Leica DFC365 FX 1.4-megapixel monochrome digital camera with CFP excitation and CFP and YFP emissions measured every 2 sec. Cells were pretreated for 5 min with buffer or antagonists. After 30 sec of baseline reads the cells were stimulated with buffer, AVP or Ang II, followed by ISO at 90 sec. Single cell measurements at 20X magnification were used to assess changes in FRET and each treatment condition was performed in a minimum of 3 independent cell preparations. Quantification of the changes in FRET ratio were calculated as change in CFP emission/YFP emission (ICUE3) or YFP emission/CFP emission (DAGR) over time, normalized to baseline.
Langendorff perfusion system
The isolated heart perfusion technique was described previously23. Briefly, hearts were perfused under constant pressure (80 mmHg) with a solution containing (in mmol/L): NaCl (13.8), NaHCO3 (22), KCl (4.7), KH2PO4 (1.2), MgSO4 (1.1), glucose (1.1), CaCl2 (2), Na pyruvate (2). A balloon was placed in the LV, connected to a Millar pressure transducer and an ADInstruments Physiograph (Colorado Springs, CO) and filled with H2O to set the LV end-diastolic pressure (LVEDP) at 10 mmHg. Hearts were maintained at a temperature of 37 °C and were paced at a rate of 480 beats per min. After a 15 min stabilization period, pharmacologic agents were added to the perfusion media at 5 min intervals. LV pressure (LVP), LVEDP, and the maximum rate of positive and negative change in LV pressure (±LV dP/dt) were recorded. LVDP was calculated by subtracting the LVEDP from the LV systolic pressure. Data were analyzed with LabChart Pro-6.0 (ADInstruments).
Statistical Analysis
Data is summarized as mean±SEM for continuous variables. All experiments in which the same subject (mouse or heart) was tested over time or using different drug concentrations were analyzed using two-way repeated measures ANOVA with Bonferroni multiple comparisons adjustments. The number of comparisons with Bonferroni post-test adjustments is indicated in figure legends along with p-values whenever applicable. Pair-wise two-group comparisons with non-repeated measures data were analyzed using non-parametric Wilcoxon Rank-Sum tests and exact test p-values were reported when the combined sample size was less than 58 as well as whenever feasible for larger sample sizes. Two-sample t-tests or oneway ANOVA with Bonferroni post-test adjustments were also performed as sensitivity analyses, but the results were not reported herein. A p-value of p<0.05 was considered statistically significant. Prism 5.0 (Graph Pad Software Inc) or SAS version 9.3 (SAS Institute, Inc., Cary, NC) were used for statistical analyses.
Results
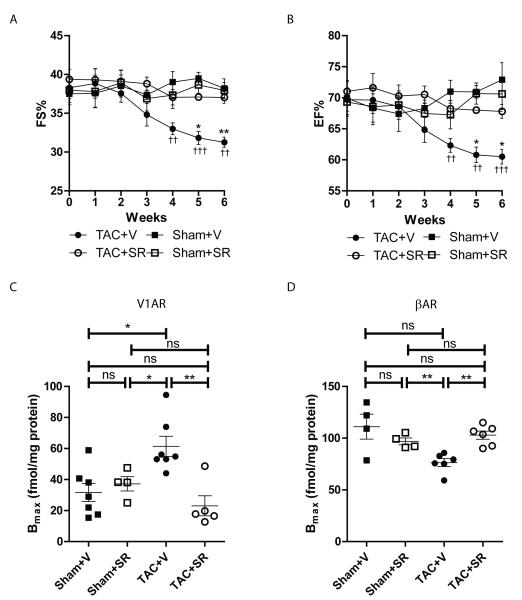
The V1AR-selective antagonist SR 49059 preserves cardiac function and restores V1AR and βAR expression levels during the development of pressure overload-induced hypertrophy in vivo
We previously reported that V1AR expression increases in end-stage human heart failure13 and that mice with cardiac-restricted overexpression of V1AR (V1AR-TG) undergo progressive development of cardiomyopathy and decreased βAR responsiveness with age11. To determine if changes in endogenous V1AR expression are associated with the development of cardiomyopathy and βAR dysfunction, WT mice underwent TAC in conjunction with osmotic minipump-mediated delivery of the V1AR-selective antagonist SR 49059. As expected, TAC decreased cardiac function and increased cardiac hypertrophy, however co-administration of SR 49059 preserved fractional shortening (Fig. 1A) and ejection fraction (Fig. 1B) without impacting hypertrophy (Supplemental Figs. 1A, 1B). Cardiac V1AR and βAR expression were also assessed in each of the cohorts via radioligand binding. Interestingly, TAC induced a significant 2-fold increase in V1AR expression, similar to that observed in human hearts13, which was inhibited by SR 49059 (Fig. 1C). βAR downregulation is associated with the development and progression of heart failure24, and in response to 7 weeks of TAC in this study was reduced by ~30% (Fig. 1D). However, concomitant treatment of TAC mice with SR 49059 completely restored cardiac βAR expression to normal levels. These results confirm the involvement of endogenous V1AR signaling in the progression of heart failure and indicates that a loss of βAR density in the presence of chronically enhanced V1AR expression may account for the diminished βAR responsiveness we previously observed in V1AR-TG mice11.
Figure 1.
V1AR antagonist SR 49059 protects against TAC-induced cardiac dysfunction. In response to TAC, fractional shortening (A) and ejection fraction (B) were significantly decreased in vehicle-treated mice (TAC+V) compared to vehicle-treated sham surgery mice (Sham+V), effects prevented with SR 49059 (1mg/kg/day, starting 1 week post-TAC). ††p<0.01, †††p<0.001 versus Sham+V, *p<0.05, **p<0.01 versus TAC+SR at corresponding weeks, twoway repeated measures ANOVA with Bonferroni multiple comparisons test (6 comparisons total). N = 9 (Sham+V), 8 (Sham+SR), 13 (TAC+V), 14 (TAC+SR). As shown by radioligand binding analysis, TAC increases V1AR membrane density (C, N = 7 (Sham+V), 4 (Sham+SR), 7 (TAC+V), 5 (TAC+SR)) and decreases βAR membrane density (D, N = 4 (Sham+V), 4 (Sham+SR), 6 (TAC+V), 6 (TAC+SR)), effects prevented by treatment with SR 49059. *p<0.05, **p<0.01, ns = not significant, Exact Wilcoxan Rank-Sum Test.
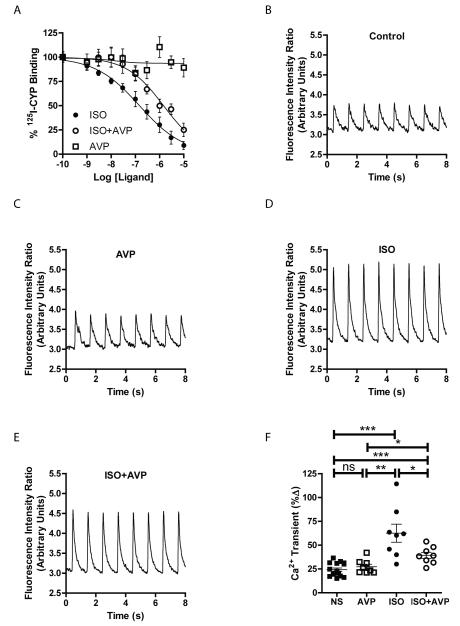
AVP negatively regulates endogenous cardiac β1AR activation
To begin to understand a possible influence of cardiac-expressed V1AR on βAR signaling more acutely, we began to study the molecular relationship between these receptors. Initially, we performed 125I-CYP competition binding analysis with increasing concentrations of isoproterenol (ISO)±AVP in adult mouse heart membrane preparations. Although AVP alone did not alter 125I-CYP binding to βAR, AVP induced a significant rightward shift in the ability of ISO to displace 125I-CYP from βAR (IC50 of ISO from 0.14μM to 1.4μM, p<0.0001 [2-tailed t-test], Fig. 2A), indicative of a loss in affinity of βAR for ISO that could be associated with diminished receptor coupling to downstream G protein-dependent signaling25, 26. Since Gs protein-dependent Ca2+ mobilization is enhanced downstream of βAR in cardiomyocytes, we measured field stimulation-induced Ca2+ transient responses in Fura2-loaded adult mouse left ventricular myocytes in the presence or absence of AVP and ISO (Fig. 2B-E). Although AVP did not evoke a change in Ca2+ mobilization alone even at μmolar concentrations (Supplemental Fig. 1C), ISO-induced Ca2+ transients were significantly reduced in the presence of AVP (Fig. 2F).
Figure 2.
AVP negatively impacts mouse cardiac βAR ligand binding and Ca2+ transients in adult mouse cardiomyocytes. A) Competition radioligand binding on adult mouse left ventricular membranes shows that AVP (0.5 μM) decreases the affinity of isoproterenol (ISO) for βAR (red) compared to ISO alone (blue), while increasing concentrations of AVP alone (green) do not alter βAR ligand binding. N = 4 per ligand concentration. Representative tracings from field stimulation-induced Ca2+ transients in Fura2-loaded adult mouse left ventricular myocytes in response to non-stimulated control (B), AVP (10 nM, C), ISO (50 nM, D) or AVP+ISO (E). F) Summary of the changes in Ca2+ transients indicates a significant decrease in ISO-mediated transients in the presence of AVP. *p<0.05, **p<0.01, ***p<0.001, ns = not significant, Exact Wilcoxan Rank-Sum Test. N = 16 (NS), 8 (AVP, ISO, AVP+ISO).
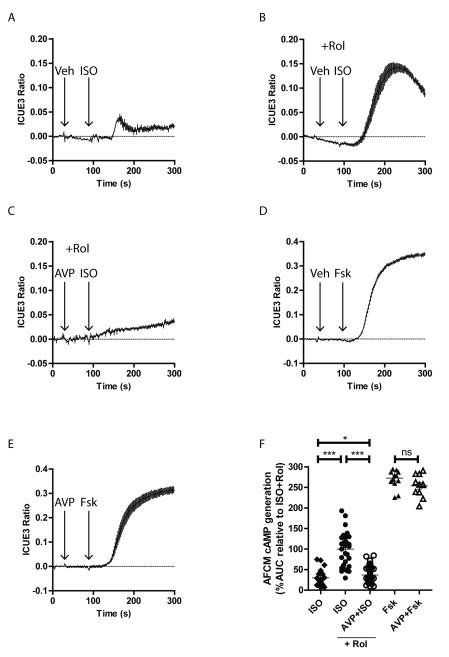
βAR-induced Ca2+ transients occur in response to Gs protein-dependent generation of cAMP, thus we tested whether AVP stimulation impacts ISO-mediated cAMP production in adult feline left ventricular myocytes (AFVM) infected with adenovirus encoding the fluorescent cAMP biosensor ICUE320. Stimulation of AFVM with ISO produced only a small increase in cAMP production (Fig. 3A). Since βAR-dependent cAMP signaling is tightly controlled by phosphodiesterase 4 (PDE4) variants27, we pretreated AFVM with the PDE4-selective antagonist rolipram which greatly enhanced the ISO-induced cAMP generation (Fig. 3B). Treatment of AFVM with AVP one minute prior to ISO addition blocked the cAMP response regardless of PDE4 inhibition (Fig. 3C), suggesting that V1AR stimulation impacts βAR signaling at least at the level of cAMP generation and not cAMP degradation. To test whether cAMP generation is altered by AVP, AFVM were stimulated with the adenylyl cyclase activator forskolin±AVP (Figs. 3D, 3E). AVP did not alter cAMP production in response to direct activation of adenylyl cyclase (Fig. 3F), suggesting that V1AR stimulation impacts βAR-mediated cAMP generation at the receptor-G protein level.
Figure 3.
AVP reduces ISO-mediated cAMP generation in adult feline ventricular myocytes. cAMP generation responses were monitored in AFVM infected with the fluorescent biosensor ICUE3 in response to vehicle (Veh)+ISO (100 nM, A), rolipram (Rol, 1 μM)+Veh+ISO (B), Rol+AVP (1 μM)+ISO (C), Veh+forskolin (Fsk 1 μM, D) or AVP+Fsk (E). F) Summarized area under the curve (AUC) data relative to the Rol+Veh+ISO condition show that AVP pretreatment significantly reduces ISO-mediated cAMP generation, while not affecting Fsk-mediated cAMP generation. *p<0.05, ***p<0.001, ns = not significant, Exact Wilcoxan Rank-Sum Test. N = 20 (ISO), 31 (Rol+ISO), 58 (Rol+AVP+ISO), 13 (Fsk), 11 (AVP+Fsk).
β1AR and β2AR are the predominant βAR isoforms present in normal adult cardiomyocytes that enhance cAMP generation, though β1AR was predominantly responsible for cAMP generation in AFVM (Supplemental Figs. 2A, 2B). To determine if AVP stimulation can also modulate β2AR responsiveness, we tested the cAMP generation response to ISO stimulation in the presence or absence of AVP in human U2S osteosarcoma cells, which express βAR at similar levels as myocardium21 and predominately signal via β2AR (Supplemental Figs. 2C, 2D). U2S cells were transfected with V1AR and either ICUE3 or the fluorescent diacylglycerol (DAG) biosensor DAGR28. Despite the ability of AVP to evoke V1AR-dependent signaling in these cells (Supplemental Fig. 2E), AVP was unable to alter ISO-mediated cAMP generation (Supplemental Figs. 2F, 2G), suggesting that V1AR-dependent effects on cardiac βAR signaling are β1AR-selective.
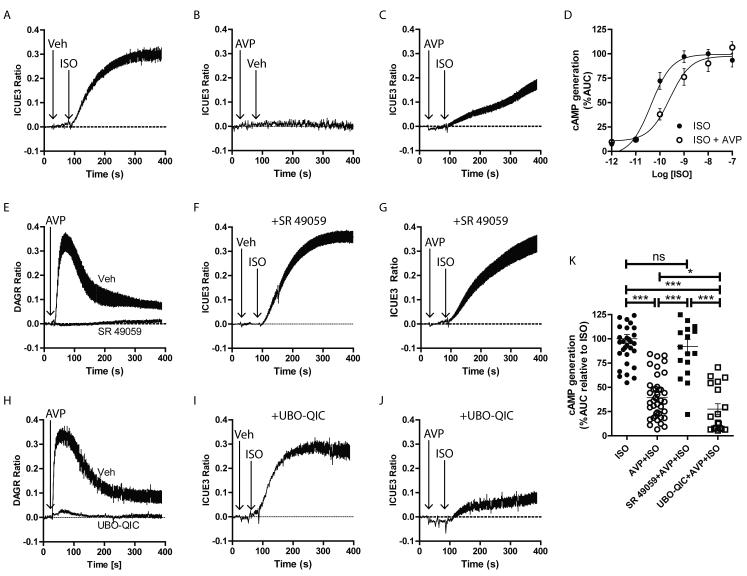
V1AR-mediated regulation of β1AR signaling is Gq protein-independent
To assess the mechanism by which V1AR stimulation decreases β1AR signaling, we used HEK 293 cells stably expressing β1AR21 and transiently expressing V1AR and either ICUE3 or DAGR. In these cells, AVP induced a concentration-dependent increase in DAG production (Supplemental Figs. 3A, 3B) with a maximal effect produced by 1μM, used subsequently to test the effects of V1AR stimulation on β1AR responsiveness. ISO stimulation rapidly enhanced cAMP production (Fig. 4A) while AVP alone had no impact (Fig. 4B). As observed in AFVM, AVP pretreatment greatly reduced the ISO-mediated cAMP production (Fig. 4C), and similar to the binding data above, AVP induced a competitive rightward shift in ISO-mediated cAMP generation (Fig. 4D), altering the EC50 of ISO-dependent cAMP generation from 43pM to 256pM. While SR 49059 pretreatment did not prevent ISO-mediated cAMP production in the absence of AVP (Fig. 4F), it completely blocked AVP-dependent DAG production (Fig. 4E) and, importantly, restored ISO-dependent cAMP production in the presence of AVP (Fig. 4G), confirming AVP-mediated regulation of β1AR occurs via V1AR.
Figure 4.
AVP effects on βAR-mediated cAMP generation are Gq protein-independent. cAMP and DAG generation responses were monitored in HEK 293 cells stably expressing β1AR and transiently transfected with V1AR and either ICUE3 or DAGR. ISO (100 pM, A), but not AVP (1 μM, B), increases cAMP generation. AVP pretreatment reduces ISO-mediated cAMP production (C) in a competitive manner (D). E) AVP (1 μM) induces a rapid DAG generation response that is blocked by SR 49059 (10 μM). Pretreatment of the cells with SR 49059 does not block ISO-induced cAMP generation (F) but does prevent the AVP-dependent reduction in the ISO response (G). H) The rapid DAG response to AVP stimulation is blocked by UBO-QIC (10 nM). Pretreatment of the cells with UBO-QIC neither blocks ISO-induced cAMP generation (I) nor the AVP-dependent reduction in the ISO response (J). K) Summarized area under the curve (AUC) results relative to ISO treatment alone, *p<0.05, ***p<0.001, ns = not significant, Exact Wilcoxan Rank-Sum Test. N = 34 (ISO), 40 (AVP+ISO), 18 (SR+AVP+ISO), 18 (QIC+AVP+ISO).
To test whether the acute effect of AVP on β1AR signaling is a common response to stimulation of Gq protein-coupled receptors (GqPCRs), we assessed the ability of the angiotensin II type 1A receptor (AT1R), known to chronically modulate βAR signaling29, to alter β1AR-mediated cAMP generation in HEK 293 cells overexpressing both AT1R and β1AR. Similar to AVP, angiotensin II (Ang II) induced a concentration-dependent increase in DAG production that achieved maximal effect by 1μM (Supplemental Figs. 3C, 3D). However, pretreatment of the cells with Ang II was unable to modulate β1AR-mediated cAMP production (Supplemental Figs. 3E, 3F), suggesting that the acute effect of V1AR signaling on β1AR responsiveness is selective with regard to other GqPCRs. Since chronic Gq protein-dependent signaling has been shown to mediate βAR desensitization29-31, we used the small molecule inhibitor of Gq protein UBOQIC32 to assess the impact of acute Gq protein-dependent V1AR signaling on βAR responsiveness. As expected, UBO-QIC blocked AVP-Gq protein-dependent DAG production (Fig. 4H) with no impact on ISO-mediated cAMP production alone (Fig. 4I). Interestingly, UBO-QIC pretreatment was unable to restore ISO-mediated cAMP responsiveness in the presence of AVP (Fig. 4J). Thus, although AVP signaling through V1AR negatively regulates β1AR-dependent cAMP formation, it does so in a Gq protein-independent manner (Fig. 4K).
V1AR-mediated regulation of β1AR signaling is GRK-dependent
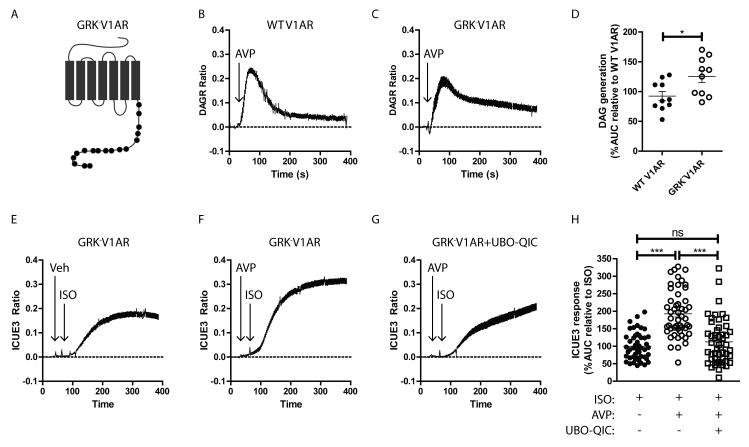
We next tested whether Gq protein-independent V1AR signaling through GRKs reduces β1AR responsiveness. Multiple GRK isoforms have been shown to play distinct roles in mediating receptor signaling responses in different tissues33, but generalized ablation of GRKs would impact βAR signaling regardless of the role of V1AR in regulating βAR desensitization. Thus, to specifically explore the impact of GRK-dependent V1AR signaling, we developed a V1AR construct lacking all possible C-terminal GRK phosphorylation sites (GRK−V1AR, Fig. 5A, Supplemental Table 1). Transfection of HEK 293 cells stably expressing β1AR with either wild-type (WT)-V1AR or GRK−V1AR resulted in similar levels of membrane expression and AVP affinity (Supplemental Figs. 4A, 4B). Stimulation of WT-V1AR (Fig. 5B) and GRK−V1AR (Fig. 5C) with AVP each induced rapid DAG formation, with GRK−V1AR producing a small but significantly enhanced accumulation of DAG over WT-V1AR (Fig. 5D), consistent with prolonged Gq protein-dependent activity in the absence of GRK phosphorylation. Interestingly, in contrast to WT-V1AR, which reduced ISO-mediated cAMP generation (Fig. 4C), AVP stimulation of GRK−V1AR significantly enhanced ISO-mediated cAMP formation, an effect that was blocked by Gq protein inhibition with UBO-QIC (Figs. 5E-H), suggesting that in the absence of GRK-dependent regulation of V1AR signaling, Gq protein-dependent V1AR signaling promotes β1AR sensitivity to adrenergic stimulation. These results also suggest that when both Gq protein- and GRK-dependent V1AR signaling mechanisms are present, the GRK-dependent branch is dominant with regard to the regulation of β1AR responsiveness.
Figure 5.
GRK phosphorylation-deficient V1AR retains Gq protein-coupling and augments βAR responsiveness. A) Schematic of the GRK-V1AR, where black circles indicate serine/threonine sites mutated to alanine, corresponding to those in Supplemental Table 1. HEK 293 cells stably expressing β1AR were transiently transfected with DAGR or ICUE3 with either WT V1AR or GRK-V1AR. Both WT V1AR (B) and GRK-V1AR (C) induce DAG formation responses to AVP (1 μM). D) DAG formation area under the curve (AUC) responses relative to WT V1AR indicates stimulation of GRK-V1AR leads to more DAG accumulation. *p<0.05, Exact Wilcoxan Rank-Sum Test. N = 10 each. E-G) Stimulation of GRK-V1AR with AVP (1 μM) leads to enhanced ISO-mediated cAMP formation, an effect blocked by the Gq protein inhibitor UBO-QIC. H) Summarized area under the curve (AUC) results relative to ISO treatment of GRK-V1AR, ***p<0.001, Exact Wilcoxan Rank-Sum Test. N = 48 (ISO), 50 (AVP+ISO), 50 (UBO-QIC+AVP+ISO).
Endogenous and overexpressed V1AR decrease βAR-dependent ex vivo cardiac contractility
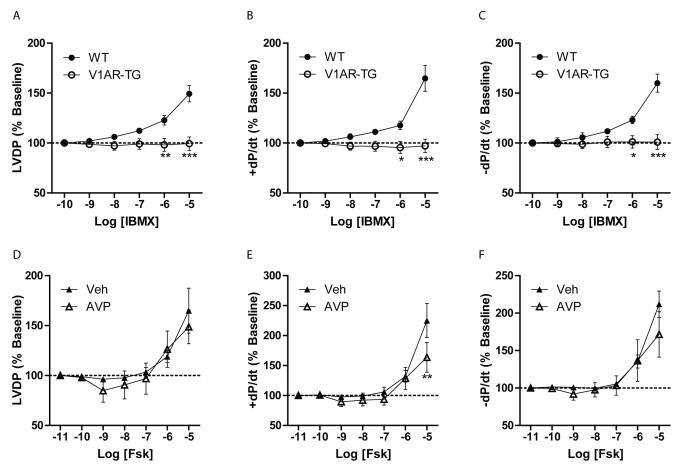
Since ISO-induced Ca2+ transients were reduced in the presence of AVP, and we previously reported diminished ISO-dependent cardiac contractility in hearts with V1AR overexpression11, we next assessed the impact of V1AR signaling on βAR function in the whole heart. Ex vivo Langendorff analysis was performed using WT versus V1AR-TG mouse hearts (which contain ~5-fold higher V1AR expression but a similar AVP affinity, Supplemental Figs. 4C, 4D). Contractility, including LVDP, +dP/dt and -dP/dt, was compared between WT and V1AR-TG hearts in response to increasing concentration of the non-selective PDE inhibitor IBMX. While IBMX infusion at higher concentrations increased contractility in WT hearts, V1AR-TG hearts were resistant to IBMX even at the highest concentrations tested (Figs. 6A-C), suggesting that basal cAMP generation in the heart is suppressed by V1AR overexpression. To determine if adenylyl cyclase activity is negatively regulated by V1AR signaling, WT hearts underwent infusion with forskolin±AVP (1nM) pretreatment. In accordance with the FRET experiments, pretreatment of WT hearts with AVP had little to no significant impact on forskolin-mediated effects on contractility (Figs. 6D-F), confirming that in the whole heart, V1AR-mediated effects on contractile signaling occur proximal to adenylyl cyclase activation.
Figure 6.
V1AR overexpression blocks basal cardiac contractility ex vivo, but V1AR activation does not impact adenylyl cyclase activity. Cardiac contractile parameters were measured in ex vivo Langendorff preparations from wild-type (WT) mice or mice with cardiac-restricted V1AR expression (V1AR-TG). Infusion of increasing concentrations of the non-selective phosphodiesterase inhibitor IBMX led to an increase in contractility in WT hearts as measured by LVDP (A), +dP/dt (B) and -dP/dt (C), expressed as % of baseline, effects that were absent in V1AR-TG hearts. *P<0.05, **P<0.01, ***p<0.001 versus WT hearts at corresponding concentration, two-way repeated measures ANOVA with Bonferroni post-test. N = 5 hearts each. WT hearts received increasing concentrations of the adenylyl cyclase activator forskolin (Fsk), which enhanced LVDP (D), +dP/dt (E) and −dP/dt (F) in either the presence or absence of AVP (1 nM) pretreatment. **P<0.01 versus Veh-treated hearts at corresponding concentration, twoway ANOVA with Bonferroni multiple comparisons test. N = 5 hearts each.
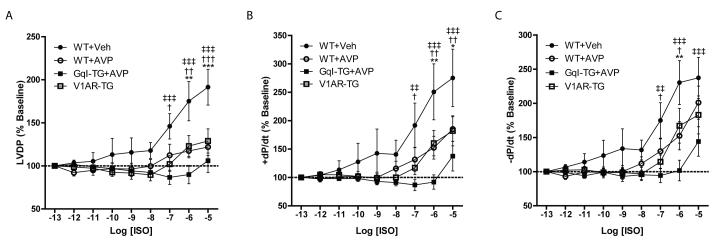
To assess βAR responsiveness directly, hearts were perfused with increasing concentrations of ISO alone (WT and V1AR-TG) or following AVP pretreatment (WT+AVP). In comparison to the WT hearts alone, both V1AR-TG hearts and WT+AVP hearts produced significantly diminished responses to ISO perfusion, including LVDP, +dP/dt and -dP/dt (Figs. 7A-C), suggesting that cardiac overexpression of V1AR mimics the effects of exogenous AVP on βAR responsiveness. Decreased ISO responsiveness in the presence of AVP cannot be explained by non-cardiac effects since AVP produced only a small concentration-dependent decrease in cardiac hemodynamics (~20% from baseline, Supplemental Figs. 4E-G), whereas AVP diminished ISO-mediated contractility by ~70% at maximal [ISO]. The modest diminution in ex vivo cardiac contractility in the presence of AVP has been previously reported to be secondary to reduced coronary blood flow34. Additionally, we tested the effect of AVP on ISO responsiveness in isolated hearts of mice overexpressing the Gq protein inhibitory peptide GqI14. Similar to the FRET data above, Gq protein inhibition did not prevent AVP from dampening ISO-mediated contractility in the isolated hearts (Figs. 7A-C). In fact, GqI overexpression resulted in an even more statistically significant AVP-dependent ablation of ISO-mediated contractility. Altogether our data highlights a previously unknown role for cardiac V1AR-mediated desensitization of βAR signaling via a novel Gq protein-independent, GRK-dependent mechanism.
Figure 7.
Either V1AR overexpression or stimulation can block ISO-mediated cardiac contractility ex vivo, even in the presence of genetic Gq protein inhibition. Cardiac contractile parameters were measured in ex vivo Langendorff preparations from WT or V1AR-TG mice. Infusion of increasing concentrations of ISO led to an increase in contractility in WT hearts as measured by LVDP (A), +dP/dt (B) and -dP/dt (C), expressed as % of baseline. These effects were blocked in V1AR-TG hearts, in WT hearts pretreated with AVP (1 nM) and in GqI-expressing hearts pretreated with AVP. *p<0.05, **P<0.01, ***p<0.001 versus WT+AVP, †p<0.05, ††p<0.01, †††p<0.001 versus V1AR-TG, and ‡‡p<0.01, ‡‡‡p<0.001 versus GqITG+AVP at corresponding concentration, two-way repeated measures ANOVA with Bonferonni multiple comparisons test (6 comparisons total). N = 5 (WT+Veh), 5 (WT+AVP), 8 (V1AR-TG), 7 (GqI-TG+AVP) hearts each.
Discussion
Neurohormone-binding GPCRs, their cognate G proteins and downstream signaling effectors, including GRKs, play a critical role in the development of the complex phenotype of the failing heart: cardiac hypertrophy, dilatation and apoptosis. Similarly, activation of V1AR also contributes to the development of HF as both constitutive and controlled cardiac-restricted over-expression of the V1AR in mice results in the development of cardiac hypertrophy, dilatation and diminished performance through activation of Gq protein-dependent signaling11. In this study we demonstrate for the first time that AVP is a potent inhibitor of βAR signaling in cultured adult cardiomyocytes and in a murine ex vivo Langendorff preparation through a GRK-dependent but Gq protein-independent manner. Interestingly, the impact of V1AR stimulation on βAR responsiveness appears to be selective not only with regard to GqPCRs, since AT1R did not reduce ISO-mediated signaling, but also βAR subtype, as only β1AR- but not β2AR-dependent signaling was negatively regulated by AVP treatment. Furthermore, we show that mice with HF secondary to TAC recapitulate the V1AR molecular phenotype found in humans with HF: a two-fold increase in myocardial V1AR density and decreased βAR expression. Interestingly, while the hypertrophic response to TAC was unchanged in the presence of the V1AR antagonist SR 49059, likely due to enhanced activity of other neurohormone pathways, SR 49059 normalized expression levels of both V1AR and βAR and significantly improved the ejection fraction in TAC mice compared with vehicle controls. These findings provide important information regarding the impact of AVP during the development of heart failure and provide new rationale for treatment strategies aimed at supporting βAR responsiveness.
An increase in the expression of cardiac Gq protein has been shown to inhibit βAR signaling30, thus our finding that activation of the V1AR inhibits cardiac β1AR-mediated cAMP signaling through a GRK-dependent but Gq protein-independent pathway was unexpected and contrasts with studies of other cardiac GPCRs. For instance, although both the angiotensin II type 1A receptor (AT1R) and V1AR have been shown to enhance V2R-Gs protein-mediated cAMP formation in Cho cells, this effect was Gq protein-dependent35, 36. Similarly, β2AR-mediated cAMP formation was increased by stimulation of several Gq protein-coupled receptors in cardiac fibroblasts37, effects that have been attributed to PKC-dependent effects such as enhanced Gs protein-adenylyl cyclase coupling. Although desensitization of one GPCR upon activation of another distinct GPCR has been shown, for instance opioid receptor-like 1 (ORL1)-mediated desensitization of the μ opioid receptor and M3 muscarinic receptor-mediated desensitization of β2AR, these effects have also been shown to be PKC-dependent, even when involving GRK signaling38, 39. Similarly, while mechanical stretch activates the AT1R with subsequent up-regulation of GRK2 and reduction in βAR signaling in neonatal rat ventricular myocytes, inhibition of either AT1R-Gq protein coupling or PKC-dependent phosphorylation of GRK2 restored normal βAR signaling40.
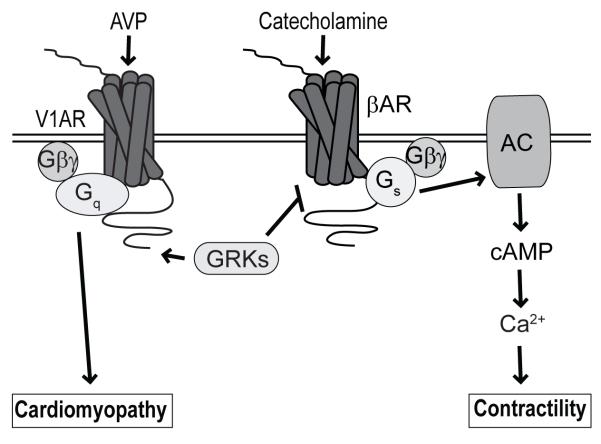
Here, GRK-mediated regulation of β1AR signaling occurred even in the absence of Gq protein activity and we only observed a V1AR-mediated increase in β1AR signaling when all possible C-terminal GRK phosphorylation sites were mutated to alanine. These results suggest that GRK-dependent V1AR signaling predominately reduces β1AR activity and that β1AR-enhancing Gq protein-dependent V1AR effects are either not normally present or unable to significantly impact β1AR activity in the presence of intact V1AR coupling to GRK. Additionally, GqI peptide-mediated inhibition of Gq protein activity did not impact the ability of AVP to inhibit βAR responsiveness in isolated perfused hearts, but led to a more significant reduction in βAR-induced cardiac contractility than in WT hearts with intact Gq protein-dependent signaling. These acute results were disparate with our earlier finding that GqI peptide overexpression blocked the development of chronic heart failure 20 weeks after cardiac-restricted and controlled over-expression of the V1AR11. Thus, the acute effects of AVP-V1AR signaling on βAR-mediated cardiac contractility occur via GRK-dependent mechanisms, while the chronic effects of AVP-V1AR signaling on cardiac morphology occur via Gq protein-dependent signaling. Although the GRK-V1AR construct reveals a required role for GRK-dependent regulation of βAR activity after acute V1AR stimulation, the precise mechanism of this effect remains to be defined. Since GRKs are primarily known for their role in phosphorylation-mediated GPCR desensitization, a likely molecular explanation exists in which V1AR stimulation results in enhanced association of active GRK at the plasma membrane, enabling more rapid GRK-mediated phosphorylation of βAR upon subsequent catecholamine stimulation, thereby enhancing the kinetics of βAR-Gs protein uncoupling and desensitization (Fig. 8). βAR-Gs protein uncoupling would account for the rightward shift in βAR ligand affinity25, 26 as well as the diminished cAMP generation, Ca2+ mobilization and contractile responses to ISO stimulation observed in this study. The fact that this effect on βAR responsiveness occurs selectively in response to stimulation of V1AR, and not AT1R, suggests that beyond the requirement for GRK activity, close proximity of V1AR and β1AR, possibly via co-localization or heterodimerization, may be an essential component of this desensitizing mechanism. However, follow-up studies will be required to address these possible scenarios.
Figure 8.
Gq protein- versus GRK-dependent cardiac V1AR signaling. Increased levels of AVP lead to enhanced stimulation of Gq protein-dependent V1AR signaling to promote cardiomyopathy, whereas GRK-dependent V1AR signaling reduces βAR-mediated signaling and downstream contractility. AC, adenylyl cyclase.
That GRK-dependent V1AR signaling can mediate acute effects of AVP in cardiomyocytes is consistent with our earlier report demonstrating that V1AR stimulation results in rapid and sustained activation of both Gq protein/PKC-dependent and GRK2/βarr1-dependent ERK1/2 signaling in cardiac-like H9c2 cells41. However, while V1AR/GRK2/βarr1-dependent signaling also participated in cellular protection against hypoxia/re-oxygenation, our current results suggest that GRK-dependent V1AR signaling may ultimately be maladaptive in vivo in part via its rapid inhibition of βAR responsiveness. GRKs, in particular GRK2 and GRK5, have been demonstrated to regulate a number of maladaptive effects during the development of HF, including βAR desensitization, and show promise as therapeutic targets for the treatment of HF, alone or in conjunction with conventional β-blockade42. While we have demonstrated GRK-dependent V1AR signaling impacts βAR responsiveness and cardiac contractility during HF, the potential roles for specific GRK isoforms in these processes remain unknown. GRKs are ubiquitous, interact with multiple GPCRs and their action at particular GPCRs is ligand and substrate-specific43, 44. Therefore, using more fine-tuned molecular and genetic approaches, it will be desirable to discriminate the impact of specific cardiac GRK isoforms in the regulation of V1AR-mediated effects on βAR responsiveness versus potential pro-survival signaling during the development of HF.
Both basic science investigation and drug development has primarily focused on the renal V2R and not on the cardiac V1AR. However, the clinical effects of V2R antagonists in patients with HF have been disappointing3, 45, 46. The selective V2R antagonist tolvaptan raised serum Na+ levels in patients with HF but failed to affect the dual primary endpoints of all-cause mortality or cardiovascular deaths or HF hospitalizations in a well-powered trial45. The highly-selective V2R antagonist lixivaptan was associated with an increase in deaths within the first 10 days of administration of drug when administered to patients with an acute exacerbation of HF, nearly all of the deaths being secondary to worsening HF47. Since both tolvaptan and lixivaptan increase plasma AVP levels, it is possible that the adverse results with lixivaptan might be explained by AVP-induced inhibition of βAR signaling in a group of patients with elevated levels of AVP (hyponatremia) and limited inotropic reserve. Others have demonstrated in a mouse model of ischemia-reperfusion injury that AVP infusion significantly increased myocardial dysfunction and mortality in comparison with both saline and dobutamine48. These results are consistent with our observation that cardiac contractility is diminished in hearts of animals with HF and elevated plasma AVP. By contrast, AVP has been shown to improve survival in swine after experimental cardiac arrest, presumably due to enhanced myocardial blood flow secondary to enhanced peripheral vasoconstriction49-53. Unfortunately, AVP has not consistently proven beneficial in humans in large clinical trials of patients who have sustained cardiogenic shock54-57. Thus, further exploration of the role of AVP in distinct forms of cardiovascular stress is warranted.
In summary, we present data that shows for the first time that AVP stimulation: 1) acutely inhibits βAR-dependent cAMP generation and Ca2+ mobilization in adult cardiomyocytes and ex vivo cardiac contractility, 2) activates a previously unknown GRK-dependent V1AR signaling mechanism to diminish βAR responsiveness, and 3) chronically impacts in vivo contractile function and βAR expression in mice during TAC-induced hypertrophy. These results have led us to hypothesize that V1AR antagonists might be useful in treatment of patients with acute HF and high circulating levels of AVP.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by NIH grants HL105414 (to D.G.T.), HL091799 (to W.J.K, A.M.F and S.R.H.), HL074854 (to J.Y.C.), HL033921 and HL091804 (to S.R.H), a PA Health Research Formula Fund (SAP#4100060220 to A.M.F.), and AHA postdoctoral (to L.A.G.) and predoctoral (to C.A.M.) fellowships.
Footnotes
Conflict of Interest Disclosures: DGT, WJK, SHR and AMF have equity in Renovacor, Inc., which has neither funded this study nor has a relevant product related to this study.
References
- 1.Bell NH, Schedl HP, Bartter FC. An Explanation for Abnormal Water Retention and Hypoosmolality in Congestive Heart Failure. Am J Med. 1964;36:351–60. doi: 10.1016/0002-9343(64)90161-5. [DOI] [PubMed] [Google Scholar]
- 2.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin-Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD) Circulation. 1990;82:1724–9. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Pang PS, Ambrosy AP, Lan G, Schmidt P, Filippatos G, Konstam M, Swedberg K, Cook T, Traver B, Maggioni A, Burnett J, Grinfeld L, Udelson J, Zannad F. A comprehensive, longitudinal description of the in-hospital and post-discharge clinical, laboratory, and neurohormonal course of patients with heart failure who die or are re-hospitalized within 90 days: analysis from the EVEREST trial. Heart Fail Rev. 2012;17:485–509. doi: 10.1007/s10741-011-9280-0. [DOI] [PubMed] [Google Scholar]
- 4.Szatalowicz VL, Arnold PE, Chaimovitz C, Bichet D, Berl T, Schrier RW. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med. 1981;305:263–6. doi: 10.1056/NEJM198107303050506. [DOI] [PubMed] [Google Scholar]
- 5.Voors AA, von Haehling S, Anker SD, Hillege HL, Struck J, Hartmann O, Bergmann A, Squire I, van Veldhuisen DJ, Dickstein K, Investigators O. C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL study. Eur Heart J. 2009;30:1187–94. doi: 10.1093/eurheartj/ehp098. [DOI] [PubMed] [Google Scholar]
- 6.Knepper MA. Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol. 1997;272:F3–12. doi: 10.1152/ajprenal.1997.272.1.F3. [DOI] [PubMed] [Google Scholar]
- 7.Klein L, O’Connor CM, Leimberger JD, Gattis-Stough W, Pina IL, Felker GM, Adams KF, Jr., Califf RM, Gheorghiade M, Investigators O-C. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation. 2005;111:2454–60. doi: 10.1161/01.CIR.0000165065.82609.3D. [DOI] [PubMed] [Google Scholar]
- 8.Goldsmith SR. Vasopressin as vasopressor. Am J Med. 1987;82:1213–9. doi: 10.1016/0002-9343(87)90228-2. [DOI] [PubMed] [Google Scholar]
- 9.Naitoh M, Suzuki H, Murakami M, Matsumoto A, Arakawa K, Ichihara A, Nakamoto H, Oka K, Yamamura Y, Saruta T. Effects of oral AVP receptor antagonists OPC-21268 and OPC-31260 on congestive heart failure in conscious dogs. Am J Physiol. 1994;267:H2245–54. doi: 10.1152/ajpheart.1994.267.6.H2245. [DOI] [PubMed] [Google Scholar]
- 10.Yatsu T, Kusayama T, Tomura Y, Arai Y, Aoki M, Tahara A, Wada K, Tsukada J. Effect of conivaptan, a combined vasopressin V(1a) and V(2) receptor antagonist, on vasopressin-induced cardiac and haemodynamic changes in anaesthetised dogs. Pharmacol Res. 2002;46:375–81. doi: 10.1016/s1043661802002062. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Chan TO, Myers V, Chowdhury I, Zhang XQ, Song J, Zhang J, Andrel J, Funakoshi H, Robbins J, Koch WJ, Hyslop T, Cheung JY, Feldman AM. Controlled and cardiac-restricted overexpression of the arginine vasopressin V1A receptor causes reversible left ventricular dysfunction through Galphaq-mediated cell signaling. Circulation. 2011;124:572–81. doi: 10.1161/CIRCULATIONAHA.111.021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilley DG. G protein-dependent and G protein-independent signaling pathways and their impact on cardiac function. Circ Res. 2011;109:217–30. doi: 10.1161/CIRCRESAHA.110.231225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu W, Tilley DG, Myers VD, Tsai EJ, Feldman AM. Increased Vasopressin 1A Receptor Expression in Failing Human Hearts. J Am Coll Cardiol. 2014;63:375–6. doi: 10.1016/j.jacc.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–7. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 15.Funakoshi H, Chan TO, Good JC, Libonati JR, Piuhola J, Chen X, MacDonnell SM, Lee LL, Herrmann DE, Zhang J, Martini J, Palmer TM, Sanbe A, Robbins J, Houser SR, Koch WJ, Feldman AM. Regulated overexpression of the A1-adenosine receptor in mice results in adverse but reversible changes in cardiac morphology and function. Circulation. 2006;114:2240–50. doi: 10.1161/CIRCULATIONAHA.106.620211. [DOI] [PubMed] [Google Scholar]
- 16.Hamad EA, Zhu W, Chan TO, Myers V, Gao E, Li X, Zhang J, Song J, Zhang XQ, Cheung JY, Koch W, Feldman AM. Cardioprotection of controlled and cardiac-specific over-expression of A(2A)-adenosine receptor in the pressure overload. PLoS One. 2012;7:e39919. doi: 10.1371/journal.pone.0039919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Gao E, Rabinowitz J, Song J, Zhang XQ, Koch WJ, Tucker AL, Chan TO, Feldman AM, Cheung JY. Regulation of in vivo cardiac contractility by phospholemman: role of Na+/Ca2+ exchange. Am J Physiol Heart Circ Physiol. 2011;300:H859–68. doi: 10.1152/ajpheart.00894.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Most P, Seifert H, Gao E, Funakoshi H, Volkers M, Heierhorst J, Remppis A, Pleger ST, DeGeorge BR, Jr., Eckhart AD, Feldman AM, Koch WJ. Cardiac S100A1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation. 2006;114:1258–68. doi: 10.1161/CIRCULATIONAHA.106.622415. [DOI] [PubMed] [Google Scholar]
- 19.Silver LH, Hemwall EL, Marino TA, Houser SR. Isolation and morphology of calcium-tolerant feline ventricular myocytes. Am J Physiol. 1983;245:H891–6. doi: 10.1152/ajpheart.1983.245.5.H891. [DOI] [PubMed] [Google Scholar]
- 20.Allen MD, DiPilato LM, Rahdar M, Ren YR, Chong C, Liu JO, Zhang J. Reading dynamic kinase activity in living cells for high-throughput screening. ACS Chem Biol. 2006;1:371–6. doi: 10.1021/cb600202f. [DOI] [PubMed] [Google Scholar]
- 21.Tilley DG, Kim IM, Patel PA, Violin JD, Rockman HA. beta-Arrestin mediates beta1-adrenergic receptor-epidermal growth factor receptor interaction and downstream signaling. J Biol Chem. 2009;284:20375–86. doi: 10.1074/jbc.M109.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tilley DG, Nguyen AD, Rockman HA. Troglitazone stimulates beta-arrestin-dependent cardiomyocyte contractility via the angiotensin II type 1A receptor. Biochem Biophys Res Commun. 2010;396:921–6. doi: 10.1016/j.bbrc.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonnell SM, Garcia-Rivas G, Scherman JA, Kubo H, Chen X, Valdivia H, Houser SR. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circ Res. 2008;102:e65–72. doi: 10.1161/CIRCRESAHA.108.174722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bristow MR. Treatment of chronic heart failure with beta-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ Res. 2011;109:1176–94. doi: 10.1161/CIRCRESAHA.111.245092. [DOI] [PubMed] [Google Scholar]
- 25.Pontier SM, Percherancier Y, Galandrin S, Breit A, Gales C, Bouvier M. Cholesterol-dependent separation of the beta2-adrenergic receptor from its partners determines signaling efficacy: insight into nanoscale organization of signal transduction. J Biol Chem. 2008;283:24659–72. doi: 10.1074/jbc.M800778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980;255:7108–17. [PubMed] [Google Scholar]
- 27.Fu Q, Chen X, Xiang YK. Compartmentalization of beta-adrenergic signals in cardiomyocytes. Trends Cardiovasc Med. 2013;23:250–6. doi: 10.1016/j.tcm.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Violin JD, Dewire SM, Barnes WG, Lefkowitz RJ. G protein-coupled receptor kinase and beta-arrestin-mediated desensitization of the angiotensin II type 1A receptor elucidated by diacylglycerol dynamics. J Biol Chem. 2006;281:36411–9. doi: 10.1074/jbc.M607956200. [DOI] [PubMed] [Google Scholar]
- 29.Harris DM, Chen X, Pesant S, Cohn HI, MacDonnell SM, Boucher M, Vinge LE, Raake P, Moraca SR, Li D, Most P, Houser SR, Koch WJ, Eckhart AD. Inhibition of angiotensin II Gq signaling augments beta-adrenergic receptor mediated effects in a renal artery stenosis model of high blood pressure. J Mol Cell Cardiol. 2009;46:100–7. doi: 10.1016/j.yjmcc.2008.09.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorn GW, 2nd, Tepe NM, Wu G, Yatani A, Liggett SB. Mechanisms of impaired beta-adrenergic receptor signaling in G(alphaq)-mediated cardiac hypertrophy and ventricular dysfunction. Mol Pharmacol. 2000;57:278–87. [PubMed] [Google Scholar]
- 31.Jenie RI, Nishimura M, Fujino M, Nakaya M, Mizuno N, Tago K, Kurose H, Itoh H. Increased ubiquitination and the crosstalk of G protein signaling in cardiac myocytes: involvement of Ric-8B in Gs suppression by Gq signal. Genes Cells. 2013;18:1095–106. doi: 10.1111/gtc.12099. [DOI] [PubMed] [Google Scholar]
- 32.Jacobsen SE, Norskov-Lauritsen L, Thomsen AR, Smajilovic S, Wellendorph P, Larsson NH, Lehmann A, Bhatia VK, Brauner-Osborne H. Delineation of the GPRC6A receptor signaling pathways using a mammalian cell line stably expressing the receptor. J Pharmacol Exp Ther. 2013;347:298–309. doi: 10.1124/jpet.113.206276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamal FA, Travers JG, Blaxall BC. G protein-coupled receptor kinases in cardiovascular disease: why “where” matters. Trends Cardiovasc Med. 2012;22:213–9. doi: 10.1016/j.tcm.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Ryckwaert F, Virsolvy A, Fort A, Murat B, Richard S, Guillon G, Colson PH. Terlipressin, a provasopressin drug exhibits direct vasoconstrictor properties: consequences on heart perfusion and performance. Crit Care Med. 2009;37:876–81. doi: 10.1097/CCM.0b013e31819b8199. [DOI] [PubMed] [Google Scholar]
- 35.Klingler C, Ancellin N, Barrault MB, Morel A, Buhler JM, Elalouf JM, Clauser E, Lugnier C, Corman B. Angiotensin II potentiates vasopressin-dependent cAMP accumulation in CHO transfected cells. Mechanisms of cross-talk between AT1A and V2 receptors. Cell Signal. 1998;10:65–74. doi: 10.1016/s0898-6568(97)00077-6. [DOI] [PubMed] [Google Scholar]
- 36.Klingler C, Ancellin N, Barrault MB, Morel A, Corman B. Potentiation of receptor-mediated cAMP production: role in the cross-talk between vasopressin V1a and V2 receptor transduction pathways. Biochem J. 1998;330(Pt 2):1023–8. doi: 10.1042/bj3301023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meszaros JG, Gonzalez AM, Endo-Mochizuki Y, Villegas S, Villarreal F, Brunton LL. Identification of G protein-coupled signaling pathways in cardiac fibroblasts: cross talk between G(q) and G(s) Am J Physiol Cell Physiol. 2000;278:C154–62. doi: 10.1152/ajpcell.2000.278.1.C154. [DOI] [PubMed] [Google Scholar]
- 38.Budd DC, Challiss RA, Young KW, Tobin AB. Cross talk between m3-muscarinic and beta(2)-adrenergic receptors at the level of receptor phosphorylation and desensitization. Mol Pharmacol. 1999;56:813–23. [PubMed] [Google Scholar]
- 39.Mandyam CD, Thakker DR, Christensen JL, Standifer KM. Orphanin FQ/nociceptin-mediated desensitization of opioid receptor-like 1 receptor and mu opioid receptors involves protein kinase C: a molecular mechanism for heterologous cross-talk. J Pharmacol Exp Ther. 2002;302:502–9. doi: 10.1124/jpet.102.033159. [DOI] [PubMed] [Google Scholar]
- 40.Malhotra R, D’Souza KM, Staron ML, Birukov KG, Bodi I, Akhter SA. G alpha(q)-mediated activation of GRK2 by mechanical stretch in cardiac myocytes: the role of protein kinase C. J Biol Chem. 2010;285:13748–60. doi: 10.1074/jbc.M110.109272. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Zhu W, Tilley DG, Myers VD, Coleman RC, Feldman AM. Arginine vasopressin enhances cell survival via a G protein-coupled receptor kinase 2/beta-arrestin1/extracellular-regulated kinase 1/2-dependent pathway in H9c2 cells. Mol Pharmacol. 2013;84:227–35. doi: 10.1124/mol.113.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang ZM, Gold JI, Koch WJ. G protein-coupled receptor kinases in normal and failing myocardium. Front Biosci. 2011;16:3047–60. doi: 10.2741/3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinge LE, Andressen KW, Attramadal T, Andersen GO, Ahmed MS, Peppel K, Koch WJ, Freedman NJ, Levy FO, Skomedal T, Osnes JB, Attramadal H. Substrate specificities of g protein-coupled receptor kinase-2 and -3 at cardiac myocyte receptors provide basis for distinct roles in regulation of myocardial function. Mol Pharmacol. 2007;72:582–91. doi: 10.1124/mol.107.035766. [DOI] [PubMed] [Google Scholar]
- 44.Heitzler D, Durand G, Gallay N, Rizk A, Ahn S, Kim J, Violin JD, Dupuy L, Gauthier C, Piketty V, Crepieux P, Poupon A, Clement F, Fages F, Lefkowitz RJ, Reiter E. Competing G protein-coupled receptor kinases balance G protein and beta-arrestin signaling. Mol Syst Biol. 2012;8:590. doi: 10.1038/msb.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konstam MA, Gheorghiade M, Burnett JC, Jr., Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan I. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–31. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 46.Lanfear DE, Sabbah HN, Goldsmith SR, Greene SJ, Ambrosy AP, Fought AJ, Kwasny MJ, Swedberg K, Yancy CW, Konstam MA, Maggioni AP, Zannad F, Gheorghiade M, Investigators Et. Association of arginine vasopressin levels with outcomes and the effect of V2 blockade in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail. 2013;6:47–52. doi: 10.1161/CIRCHEARTFAILURE.112.970012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldman AM, Hamad E, Tsai EJ, Zhu W, Tilley DG, Alvarez R, Cheung JY. Vasopressin antagonists for patients with acute heart failure: interpreting new clinical and translational data. Clin Pharmacol Ther. 2014;95:373–5. doi: 10.1038/clpt.2013.240. [DOI] [PubMed] [Google Scholar]
- 48.Indrambarya T, Boyd JH, Wang Y, McConechy M, Walley KR. Low-dose vasopressin infusion results in increased mortality and cardiac dysfunction following ischemia-reperfusion injury in mice. Crit Care. 2009;13:R98. doi: 10.1186/cc7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coute RA, Mader TJ, Sherman LD. Outcomes by rescue shock number during the metabolic phase of porcine ventricular fibrillation resuscitation. Am J Emerg Med. 2014;32:586–91. doi: 10.1016/j.ajem.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 50.Krismer AC, Lindner KH, Wenzel V, Mayr VD, Voelckel WG, Lurie KG, Strohmenger HU. The effects of endogenous and exogenous vasopressin during experimental cardiopulmonary resuscitation. Anesth Analg. 2001;92:1499–504. doi: 10.1097/00000539-200106000-00029. [DOI] [PubMed] [Google Scholar]
- 51.Mayr VD, Wenzel V, Voelckel WG, Krismer AC, Mueller T, Lurie KG, Lindner KH. Developing a vasopressor combination in a pig model of adult asphyxial cardiac arrest. Circulation. 2001;104:1651–6. doi: 10.1161/hc3901.095896. [DOI] [PubMed] [Google Scholar]
- 52.Voelckel WG, Lurie KG, McKnite S, Zielinski T, Lindstrom P, Peterson C, Krismer AC, Lindner KH, Wenzel V. Comparison of epinephrine and vasopressin in a pediatric porcine model of asphyxial cardiac arrest. Crit Care Med. 2000;28:3777–83. doi: 10.1097/00003246-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Voelckel WG, Lurie KG, McKnite S, Zielinski T, Lindstrom P, Peterson C, Wenzel V, Lindner KH, Benditt D. Effects of epinephrine and vasopressin in a piglet model of prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit Care Med. 2002;30:957–62. doi: 10.1097/00003246-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Gueugniaud PY, David JS, Chanzy E, Hubert H, Dubien PY, Mauriaucourt P, Braganca C, Billeres X, Clotteau-Lambert MP, Fuster P, Thiercelin D, Debaty G, Ricard-Hibon A, Roux P, Espesson C, Querellou E, Ducros L, Ecollan P, Halbout L, Savary D, Guillaumee F, Maupoint R, Capelle P, Bracq C, Dreyfus P, Nouguier P, Gache A, Meurisse C, Boulanger B, Lae C, Metzger J, Raphael V, Beruben A, Wenzel V, Guinhouya C, Vilhelm C, Marret E. Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation. N Engl J Med. 2008;359:21–30. doi: 10.1056/NEJMoa0706873. [DOI] [PubMed] [Google Scholar]
- 55.Mentzelopoulos SD, Malachias S, Chamos C, Konstantopoulos D, Ntaidou T, Papastylianou A, Kolliantzaki I, Theodoridi M, Ischaki H, Makris D, Zakynthinos E, Zintzaras E, Sourlas S, Aloizos S, Zakynthinos SG. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA. 2013;310:270–9. doi: 10.1001/jama.2013.7832. [DOI] [PubMed] [Google Scholar]
- 56.Mentzelopoulos SD, Zakynthinos SG, Siempos I, Malachias S, Ulmer H, Wenzel V. Vasopressin for cardiac arrest: meta-analysis of randomized controlled trials. Resuscitation. 2012;83:32–9. doi: 10.1016/j.resuscitation.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 57.Ong ME, Tiah L, Leong BS, Tan EC, Ong VY, Tan EA, Poh BY, Pek PP, Chen Y. A randomised, double-blind, multi-centre trial comparing vasopressin and adrenaline in patients with cardiac arrest presenting to or in the Emergency Department. Resuscitation. 2012;83:953–60. doi: 10.1016/j.resuscitation.2012.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.