Summary
Background
ERp57 is required for platelet function; however, whether ERp57 contributes to fibrin generation is unknown.
Methods and Results
Using an inhibitory anti-ERp57 antibody (Mab1), Pf4-Cre/ERp57fl/fl mice, Tie2-Cre/ERp57fl/fl mice, and mutants of ERp57, we analyzed the function of ERp57 in laser-induced thrombosis. Fibrin deposition was decreased in Pf4-Cre/ERp57fl/fl mice consistent with a role for platelet ERp57 in fibrin generation. Fibrin deposition was further decreased with infusion of Mab1 and in Tie2-Cre/ERp57fl/fl mice consistent with endothelial cells also contributing to fibrin deposition. Infusion of eptibifatide inhibited platelet and fibrin deposition, confirming a role for platelets in fibrin deposition. Infusion of recombinant ERp57 corrected the defect in fibrin deposition but not platelet accumulation suggesting a direct effect of ERp57 on coagulation. Mab1 inhibited thrombin generation in vitro consistent with a requirement for ERp57 in coagulation. Platelet accumulation was decreased to a similar extent in Pf4-Cre/ERp57fl/fl mice, Tie2-Cre/ERp57fl/fl mice and normal mice infused with Mab1. Infusion of completely inactivated ERp57 or ERp57 with a nonfunctional second active site inhibited fibrin deposition and platelet accumulation, indicating that the isomerase activity of the second active site is required for these processes.
Conclusion
ERp57 regulates thrombosis via multiple targets.
Keywords: Blood platelets, platelet aggregation, fibrin, blood coagulation, protein disulfide-isomerases
Introduction
Members of the protein disulfide isomerase family of enzymes have extracellular functions [1] and several of these enzymes have important roles in hemostasis and thrombosis [2–4]. The prototypic protein disulfide isomerase (PDI) and ERp5 were the first members of this family shown to be important in platelet function [5–7] and fibrin deposition [3, 4]. Recently, ERp57 was shown to have an important role in platelet aggregation, hemostasis and thrombosis [8–10]. Following platelet activation ERp57 is secreted and expressed on the platelet surface [10, 11]. In vivo, ERp57 accumulates early in the platelet thrombus after laser-induced arterial injury [8]. These observations indicate that ERp57 is a key mediator of thrombosis. However, it is not known if ERp57 contributes to fibrin deposition. In this study we use an inhibitory antibody to ERp57, ERp57-deficient mouse models and ERp57 mutant proteins to demonstrate a role for ERp57 in fibrin deposition in vivo.
Materials and Methods
Materials
The inhibitory monoclonal antibody to ERp57, Mab1, was previously characterized [9]. Isotype-specific control mouse IgG2a (Sigma) was used as control antibody. Anti-CD41 Ag-binding F(ab)2 fragments (clone MWReg30) (BD Bioscience) was conjugated with Alexa 488 (Invitrogen). Anti-fibrin antibody hybridoma (clone 59D8) was a generous gift from Dr. Hartmut Weiler [12]. Antibodies were labeled using Alexa-fluor monoclonal antibody labeling kits (Invitrogen).
Mice
Platelet-specific ERp57 knockout mice were generated by crossing ERp57 floxed mice [13] with Pf4-Cre mice (Jackson Labs) as previously described [10] These mice have normal platelet counts and platelet glycoprotein expression. Cre-negative littermate ERp57 loxp mice were used as controls. To study contributions of endothelial cell-derived ERp57 we used Tie2-Cre mice [14]. These mice were generated by crossing ERp57fl/fl mice [13] with Tie2-Cre mice (Jackson Labs) to produce Tie2-Cre/ ERp57fl/fl mice. C57BL/6 mice were from Jackson Laboratories. Experiments with mice were performed in accordance with the Soochow University and Temple University institutional guidelines and approval of the Institutional Animal Care and Use Committees.
Preparation of catalytically inactive first or second active sites of ERp57
Two different mutant ERp57 enzymes with the cysteine residues of one active site mutated to serine residues (C57S with C60S, or C406S with C409S) were prepared (QuikChange Site-Directed Mutagenesis Kit) as previously described [10]. The mutants are designated as ERp57 (oo-ss) (representing the Cys/SH change to Ser/OH in the first CGHC active site) and ERp57 (ss-oo) (representing the Cys/SH change to Ser/OH in the second CGHC active site). ERp57 (oo-oo) (completely inactivated ERp57) was also prepared [9]. DNA sequencing confirmed the correct base substitutions.
Aggregation of mouse platelets
Washed mouse platelets were prepared and adjusted to 200,000/µL using the Sysmex Coulter Counter and aggregation was performed as described [9, 10].
Thrombin generation assay
Human blood was collected from healthy donors into sodium citrate and mononuclear cells were prepared by density gradient centrifugation (Histopaques 1077, Sigma), as described [15]. After washing with PBS, mononuclear cells (20 µl) were added to citrated normal human plasma (80 µl) to give a final cell concentration of 3×106/ml. LPS (30 µg/ml) was added in the presence of Mab1 or control IgG for four hours. The thrombin generation assay was performed on the cells suspended in plasma according to the method described by Hemker et al [16], following the manufacturer’s protocol using the Thermo Electron Fluoroskan Ascent Analyzer (Diagnostica Stago). The final addition of the substrate with calcium initiated the reaction. Each sample and the standard calibrator were measured in quadruplicate.
Intravital microscopy of laser-induced thrombosis of the mouse cremaster muscle arterioles
Laser-induced injury of the cremaster muscle arteriole was performed as previously described [17, 18]. Alexa-647-labeled anti-fibrin antibody and Alexa-488-labeled anti-CD41 F(ab)2 fragments (BD Biosciences) were infused at 0.1 µg/g into the jugular vein followed by infusion of Mab 1 (450 µg/mouse) or mutants of ERp57 (300 µg/mouse). After 5 minutes, arterioles (30 to 45 µm diameter) were injured using a Laser Ablation system (Intelligent Imaging Innovations) through a Zeiss microscope (Axio Examiner D1) objective parfocal with the focal plane. The laser power was set to 55–65% and the laser fired at the vessel wall in 1 to 3 pulses until thrombi were induced. Approximately 10 thrombi were studied in a single mouse. Injuries in which puncture of the vessel occurred or injuries in which no thrombus formed were excluded. The average number of laser pulses/injury was equal for all conditions. Data was captured using a CCD camera (Cool SnapTM HQ2) using Slidebook 5.0 image acquisition and analysis software (Intelligent Imaging Innovations (I3)). Data were collected for 5 minutes after vessel wall injury. Image analysis was performed using Slidebook Version 5.0 (I3).
Statistics
Data analysis was performed using the statistical software GraphPad Prism 5. Statistical significance for the area under the curve (AUC) and bar graphs of the median fluorescence intensity of the laser injury was assessed by Wilcoxon-Mann Whitney test for nonparametric comparison, as previously described [19, 20]. The Student t-test for parametric comparison for the AUC in the thrombin generation assay was as described [21]. A P value less than 0.05 was considered significant.
Results
ERp57 is important in fibrin deposition and platelet accumulation in arteriole-injury
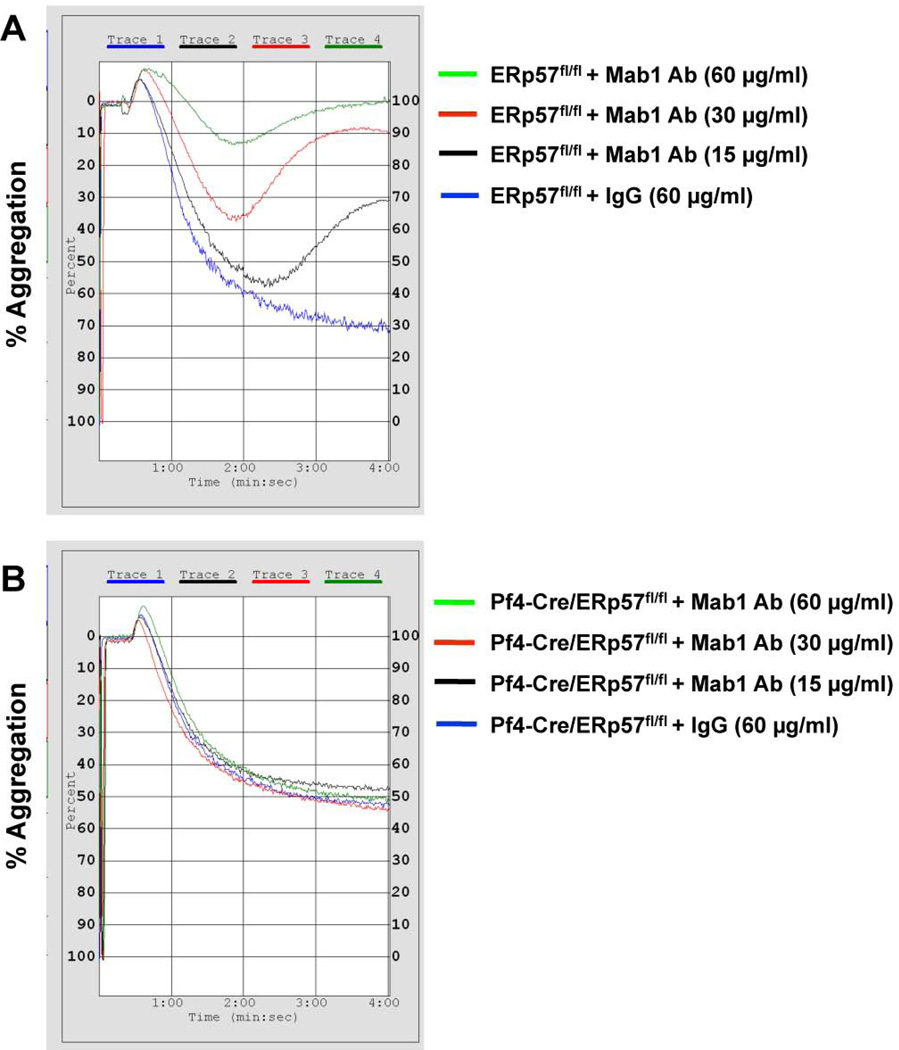
To determine whether ERp57 is involved in fibrin formation the inhibitory anti-ERp57 antibody Mab1 [9] was infused into littermate Cre-negative ERp57 loxp control mice that express normal levels of ERp57 [10]. Mab1 does not inhibit PDI (the closest homologue to ERp57 in platelets) or ERp5 [9] and does not bind to [10] or inhibit aggregation of ERp57-deficient platelets (Fig. 1). Therefore this antibody is specific for ERp57. We previously showed that ERp57- deficient platelets have ~50% of normal aggregation [22], with the amount of aggregation being dependent on the concentration of the agonist. In the current studies Mab1 inhibits aggregation of wild type platelets to a greater degree than the aggregation seen in the knockout platelets (Fig. 1). However, Mab1 does not sterically hinder binding of an αIIbβ3 activation dependent antibodies to αIIbβ3 in human or mouse platelets (Supplemental Fig. 1). This is consistent with the knockout platelets having compensatory mechanisms mitigating the loss of the ERp57 as described for other types of knockout mice [23, 24].
Fig. 1.
Anti-ERp57 antibody Mab1 does not inhibit aggregation of ERp57-null platelets. Mab1 at the indicated concentrations or control IgG was incubated with washed mouse platelets for 20 minutes. Platelets were prepared as described [10] from Cre-negative ERp57fl/fl littermate controls (A) or Pf4-Cre/ERp57fl/fl mice (B) and activated with thrombin (0.015 U/ml).
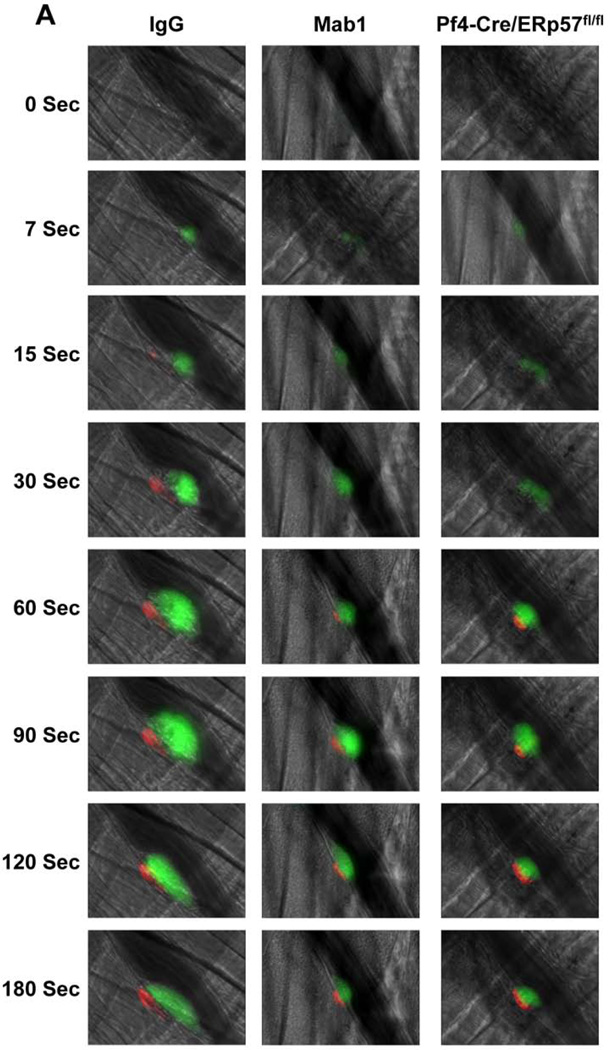
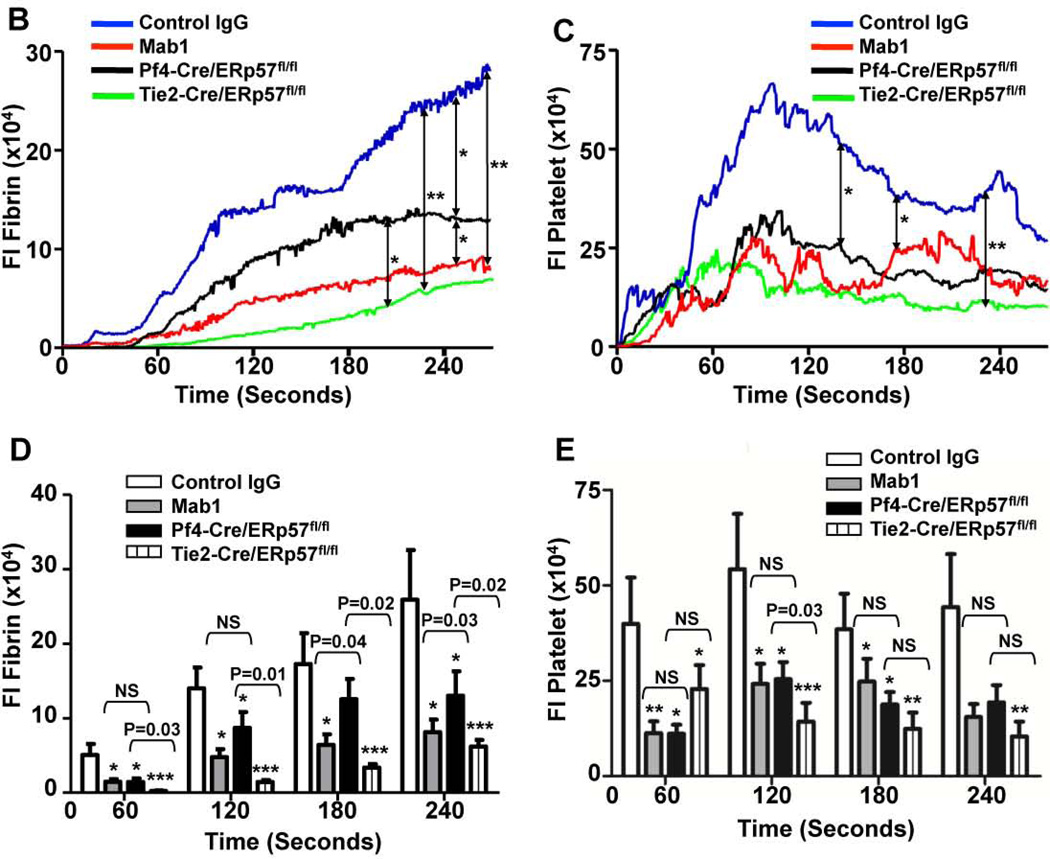
The effect of Mab1 on thrombosis was analyzed by fluorescence intravital microscopy. Following laser-induced cremaster arteriolar wall injury, fibrin generation and platelet accumulation were visualized by infusion of Alexa 647–conjugated anti-fibrin and Alexa 488–conjugated anti-CD41 Fab fragments, respectively. Mab1 decreased both fibrin formation and platelet accumulation compared with control IgG2a after laser-arteriolar injury (Fig. 2A). The median integrated fluorescence intensity obtained from quantitative data from multiple thrombi indicated that Mab1 inhibited fibrin formation and platelet accumulation as a function of time (Fig. 2B, C). These data are consistent with intravascular ERp57 having a role in both fibrin and platelet deposition in vivo.
Fig. 2.
Intravascular ERp57 is required for fibrin deposition and platelet accumulation in vivo. Cremaster arteriole injury was induced in Cre-negative ERp57fl/fl mice pretreated with control IgG or Mab1 (450 µg/mouse) and Pf4-Cre/ERp57fl/fl mice. Platelets and fibrin were detected using anti-CD41 F(ab)2 fragments (binds to platelet β3) conjugated to Alexa Fluor 488 and anti-fibrin antibody conjugated to Alexa Fluor 647. (A) Using intravital microscopy representative fluorescence images show platelet accumulation (green) and fibrin deposition (red) at selected time points up to 180 seconds after vascular injury. Platelet accumulation was visualized at 7 seconds, earlier then the appearance of fibrin, which was seen at 15 seconds. The median integrated fluorescence intensities (FI) of anti-fibrin (fibrin, B) and anti-CD41 (platelet, C) antibodies over 270 seconds were obtained from a total of 30 thrombi in 3 mice per group. Fluorescence signal was not observed using fluorescently labeled control IgG (not shown). The data was analyzed by area under curve (AUC) with a Mann-Whitney rank sum [19]. Only significant differences (*P < .05; **P < .01) are shown. The data was also analyzed by comparing the fluorescence intensity with control mice of anti-fibrin (D) and anti-CD41 (E) antibodies at 60, 120, 180, 240 seconds after vascular injury as described [37]. *P < .05; **P < .01, ***P < .001. P values between the Mab1 and Pf4-Cre/ERp57/fl/fl mice are as indicated.
Pf4-Cre/ERp57fl/fl mice with ERp57-deficient platelets had decreased fibrin formation compared with control ERp57-loxp mice (Fig. 2A, B) indicating the importance of platelet-derived ERp57 in fibrin generation. Mice infused with Mab1 had significantly greater inhibition of fibrin deposition then mice with a platelet-specific deficiency of ERp57 (Fig. 2B, D). This is consistent with ERp57 released from non-platelet sources such as the vessel wall also supporting fibrin formation during arterial thrombosis.
Endothelial cell-derived PDI contributes to fibrin deposition in vivo [25] and endothelial cells also secrete ERp57 that may be involved in fibrin deposition [26, 27]. To determine if ERp57 from the vascular endothelium contributes to fibrin deposition we generated Tie2-Cre/ERp57fl/fl mice to knockout endothelial cell ERp57. Tie2-Cre targeting effectively eliminates the protein in endothelial cells; however, protein expression in platelets is also affected [14]. We found that platelets of Tie2-Cre/ERp57fl/fl mice do not contain ERp57 (Supplemental Fig. 2A). Complete blood counts in Tie-Cre/ERp57fl/fl mice revealed no abnormalities and platelet counts (Supplemental Fig. 2B) and size (mean platelet volume 6.99 ± 0.08 fL) were comparable with those of Cre- negative littermates (7.14 ± 0.22, P = NS, n = 9). There were no differences in expression of the major platelet surface glycoproteins, αIIbβ3, GpIbα and GpVI (Supplemental Fig. 2C). Tie2-Cre/ERp57fl/fl mice showed a decrease in fibrin deposition relative to Pf4-Cre knockout mice (Fig. 2B) suggesting that endothelial cell-derived ERp57 contributes to fibrin deposition.
Since platelets themselves may contribute to fibrin generation [28, 29] the role of ERp57 in fibrin deposition could be through a direct effect of ERp57 on coagulation or secondary to inhibition of platelet accumulation. Pf4-Cre/ERp57fl/fl mice, Tie2-Cre/ERp57fl/fl mice and mice infused with Mab1 all have a comparable decrease in platelet accumulation (Fig. 2C, E). However, fibrin deposition in Tie2-Cre/ERp57fl/fl mice or the mice infused with Mab1 is substantially less than in Pf4-Cre/ERp57fl/fl mice (Fig. 2B, D). The decreased fibrin deposition in Tie2-Cre/ERp57fl/fl mice and mice infused with Mab1 cannot be accounted for by a decrease in platelet accumulation suggesting the direct involvement of ERp57 in coagulation.
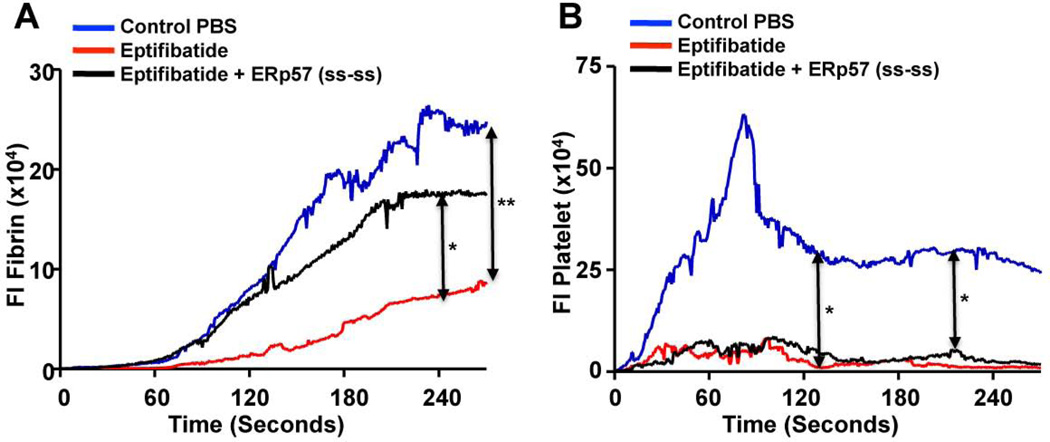
To determine whether fibrin generation is dependent on platelets in our injury model we infused eptibifatide (Shering Plough) to block platelet accumulation. A major decrease in fibrin generation was seen (Fig. 3A) indicating that platelets have a role in fibrin deposition. As expected, platelet accumulation was entirely inhibited by eptibifatide (Fig. 3B). The major decrease in fibrin deposition with eptibifatide may reflect both loss of a direct effect of platelet-derived ERp57 on coagulation and loss of platelet procoagulant activity. The decrease in fibrin deposition but not the decrease in platelet accumulation was largely reversed with infusion of wild type ERp57 (Fig. 3A, B). The increased fibrin deposition resulting from infusion of ERp57 further supports a direct role of intravascular ERp57 in fibrin generation in vivo.
Fig. 3.
Eptifibatide inhibits fibrin deposition and platelet accumulation. Eptifibatide (10 µg/g body weight) was infused into C57BL/6 wild type mice before injury and reinfused every 20 minutes for subsequent thrombi as described [25]. (A) Fibrin deposition; (B) platelet accumulation. In some experiments wild type ERp57 (ss-ss) was infused (200 µg/mouse) 10 minutes prior to the first dose of eptifibatide. The median integrated fluorescent intensities (FI) were obtained from a total of 30 thrombi in 3 mice per group.
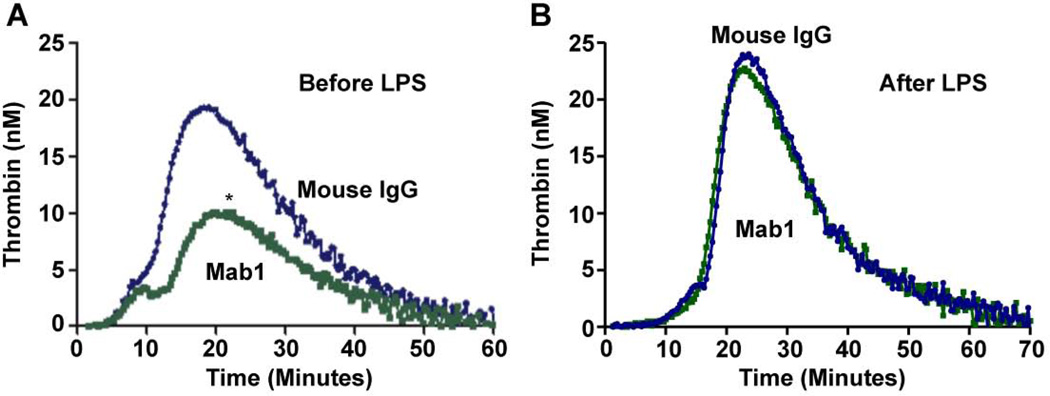
We further evaluated the role of ERp57 in coagulation using a thrombin generation assay. Mab1 substantially inhibited thrombin generation in a cell-based assay when added before but not after LPS stimulation of the cells (Fig. 4). These findings are consistent with a direct role for ERp57 in coagulation. Although we have not excluded a role for ERp57 in LPS-induced cell activation, a previous study that did not use LPS found involvement of ERp57 in tissue factor dependent factor Xa generation [30]. PDI catalyzes similar reactions as ERp57 and may directly modulate tissue factor activity [31, 32] raising the possibility that ERp57 also affects tissue factor activity.
Fig. 4. Anti-ERp57 inhibits thrombin generation when added before stimulation of human mononuclear cells with LPS.
The data was analyzed by AUC; *P < 0.02. Mab1 was added before (A) or after (B) LPS stimulation of the cells (n = 4).
The second active site of ERp57 mediates fibrin formation and platelet accumulation
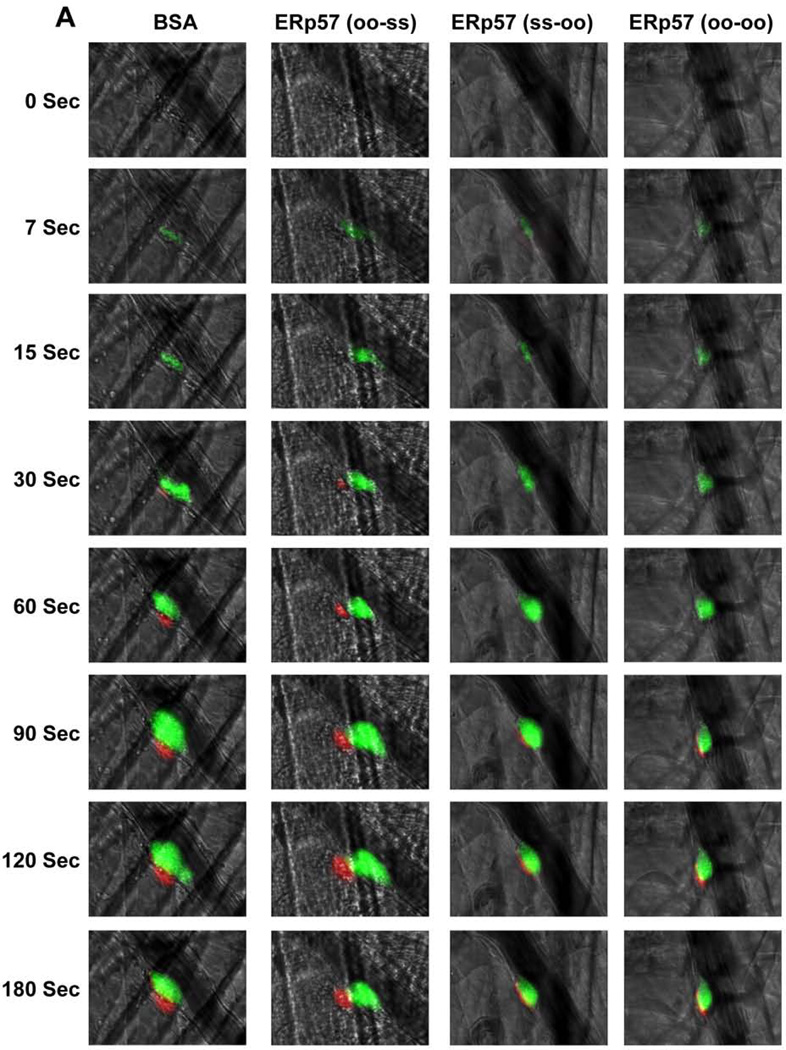
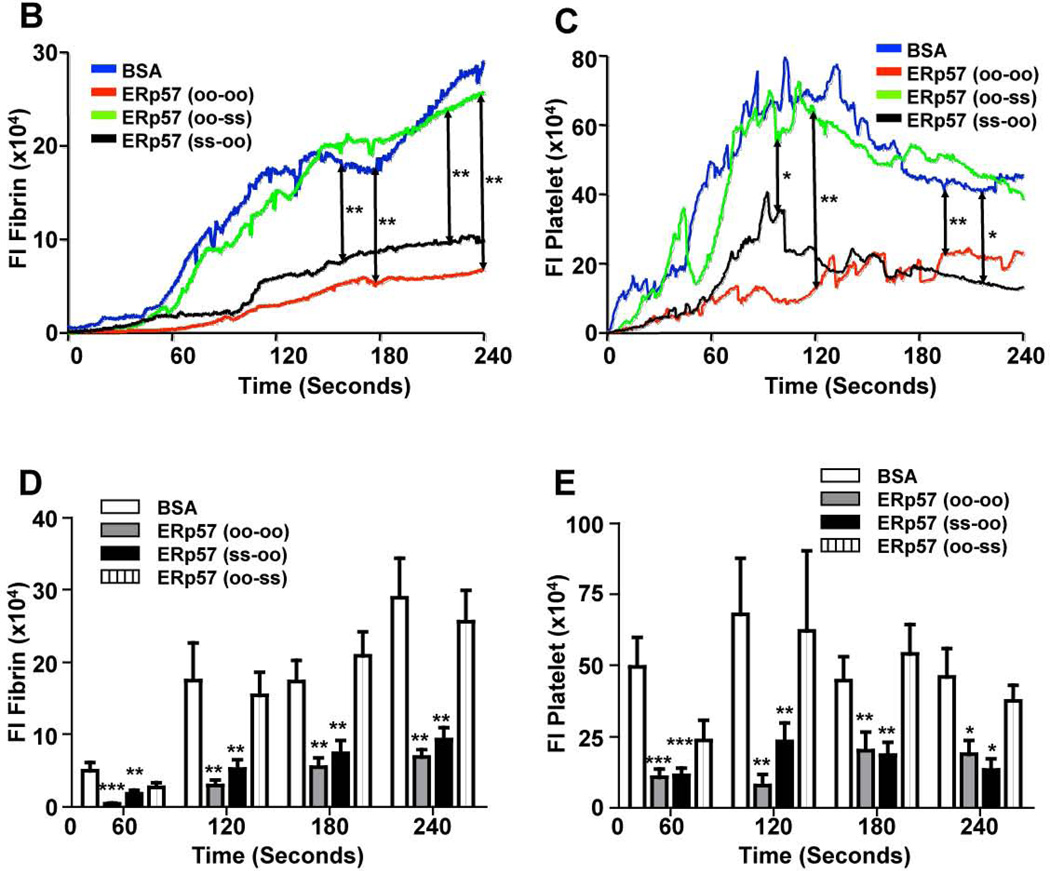
ERp57 has two CGHC thioredoxin motifs that are essential for oxidoreductase activity. Enzymatically inactivated ERp57 inhibits platelet aggregation implying that the catalytic activity of ERp57 is required for aggregation [10]. To determine whether the catalytic activity of ERp57 is required for fibrin formation and platelet accumulation in the laser model, completely inactive ERp57 (oo-oo) was infused into wild type C57BL/6 mice. Infusion of ERp57 (oo-oo) markedly reduced fibrin formation and platelet accumulation (Fig. 5A–E) implying that the catalytic activity of the active sites of ERp57 is required for these processes. Using ERp57 with catalytically inactive first or second active sites we previously showed that the second CGHC motif is critical for platelet aggregation [10]. To determine which active site of ERp57 catalyzes reactions in vivo, we examined the effect of infusing ERp57 mutants with only one functional active site. Infusion of ERp57 (ss-oo) (functional first active site, inactive second active site) inhibited fibrin deposition and platelet accumulation similar to ERp57 (oo-oo) (Fig. 5A–E). In contrast, infusion of ERp57 (oo-ss) (inactive first active site, functional second active site) gave no inhibition (Fig. 5A–E). These results indicate that the second active site of ERp57 is critical for fibrin formation and platelet accumulation.
Fig. 5. The second active site of ERp57 is required for fibrin deposition and platelet accumulation.
Wild-type C57BL/6 mice were infused with 300 µg of ERp57 (oo-oo), ERp57 (ss-oo), ERp57 (oo-ss) or as a control, bovine serum albumin (BSA). Using intravital microscopy the effect on thrombosis after laser-induced injury of cremaster arterioles was observed. (A) Representative images of platelet accumulation (green) and fibrin deposition (red) are shown. The median integrated fluorescence signal of anti-fibrin (B) and anti-CD41 (C) antibodies obtained from 30 thrombi in 3 mice per group is presented over time. The data was analyzed by AUC with only significant differences shown (*P < .05; **P < .01). Fluorescence intensity of anti-fibrin (D) and anti-CD41 (E) antibodies at 60, 120, 180 and 240 seconds after vascular injury. *P < 0.05; **P<0.01; ***P < .001 vs. control mice infused with BSA.
Discussion
In this study we demonstrate a role for intravascular ERp57 in fibrin generation in vivo. Since fibrin generation in vivo may depend on platelet accumulation [33], the effect of ERp57 on coagulation could be secondary to the role of ERp57 in platelet accumulation. However, several finding in our studies are consistent with a direct effect of ERp57 in coagulation. First, the decreased fibrin generation seen with infusion of Mab1 compared to Pf4-Cre/ERp57fl/fl mice suggests non-platelet sources of ERp57 contribute to coagulation. Second, the decreased fibrin generation in Tie2-Cre/ERp57fl/fl mice relative to Pf4-Cre/ERp57fl/fl suggests that endothelial cell-derived ERp57 contributes to fibrin generation. These findings with Mab1 and Pf4-Cre/ERp57fl/fl mice support a direct effect of ERp57 on coagulation because the decreased fibrin deposition is not readily explained by decreased platelet accumulation. Third, infusion of wild type ERp57 increases fibrin deposition even when there is no platelet accumulation (Fig. 3A). Finally, Mab1 decreased thrombin generation induced by mononuclear cells when added before stimulation of the cells with LPS.
Our observation that platelet accumulation supports fibrin deposition in laser injury is consistent with the observation of others. Fibrin formation was reduced in mice with a platelet specific deficiencies of STIM1 [28] or Rac1 [29] with the authors concluding that platelets are important in supporting fibrin generation in vivo.
Much remains to be elucidated about the roles of both PDI and ERp57 in coagulation. The role for PDI in coagulation depends on the specific conditions and redox environment of the study. For example, PDI may potentiate or inhibit coagulation [3, 4, 34, 35] or tissue factor activation [31, 32, 35]. PDI may also regulate binding of coagulation factors to the surface of activated platelets [36]. Mab1 does not inhibit PDI [9], and the platelets of Pf4-Cre/ERp57fl/fl mice have normal PDI levels [10]. Therefore, the decrease in fibrin deposition in mice infused with Mab1 or in Pf4-Cre/ERp57fl/fl mice (Fig. 2A, B, D) implies that PDI cannot compensate for the loss of ERp57. This suggests that PDI and ERp57 have distinct roles in coagulation.
Platelet-derived ERp57 has an important role in activation of the αIIbβ3 integrin and platelet accumulation in a ferric chloride arterial injury model [10]. We here confirm the critical role for platelet-derived ERp57 in platelet accumulation in a laser-induced injury model. We also demonstrate a role for the catalytic activity of the second active site of ERp57 in platelet function in vivo. However, the novel finding in this study is that ERp57 significantly potentiates fibrin formation likely through a direct effect on coagulation. While the principal target of ERp57 on the platelet surface appears to be the αIIbβ3 integrin [10], the mechanism by which platelet or endothelial cell-derived ERp57 triggers fibrin formation awaits further investigation. Since ERp57 functions in thrombosis mainly via the second CGHC domain, this active site could be a therapeutic target for anti-thrombotic treatment.
Supplementary Material
Acknowledgments
This work was supported by startup funds from Temple University School of Medicine; the Priority Academic Program Development of Jiangsu Higher Education Institutions, Natural Science Foundation of China (81270592, 31201058); a Grant in Aid from the American Heart Association (Great Rivers Affiliate) and an NIH grant (1R01HL118526).
L. Rauova and M. Poncz report grants from NIH, during the conduct of the study.
Footnotes
Addendum
Contribution: D. W. Essex and Y. Wu designed the project, supervised the research, analyzed the data and wrote the manuscript. J. Zhou, Y. Wu and L. Wang performed research and collected and analyzed data, which J. Zhou and Y. Wu contributed equally to. L. Rauova, V. M. Hayes and M. Poncz provided critical reagents, supervised and performed laser-injury experiments and helped revise the manuscript.
Disclosure of Conflict of Interests
Other authors state that they have no conflict of interests.
References
- 1.Turano C, Coppari S, Altieri F, Ferraro A. Proteins of the PDI family: unpredicted non-ER locations and functions. J Cell Physiol. 2002;193:154–163. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- 2.Essex DW. Redox control of platelet function. Antioxid Redox Signal. 2009;11:1191–1225. doi: 10.1089/ars.2008.2322. [DOI] [PubMed] [Google Scholar]
- 3.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Essex DW, Li M. Protein disulphide isomerase mediates platelet aggregation and secretion. Br J Haematol. 1999;104:448–454. doi: 10.1046/j.1365-2141.1999.01197.x. [DOI] [PubMed] [Google Scholar]
- 6.Lahav J, Jurk K, Hess O, Barnes MJ, Farndale RW, Luboshitz J, Kehrel BE. Sustained integrin ligation involves extracellular free sulfhydryls and enzymatically catalyzed disulfide exchange. Blood. 2002;100:2472–2478. doi: 10.1182/blood-2001-12-0339. [DOI] [PubMed] [Google Scholar]
- 7.Jordan PA, Stevens JM, Hubbard GP, Barrett NE, Sage T, Authi KS, Gibbins JM. A role for the thiol isomerase protein ERP5 in platelet function. Blood. 2005;105:1500–1507. doi: 10.1182/blood-2004-02-0608. [DOI] [PubMed] [Google Scholar]
- 8.Holbrook LM, Sasikumar P, Stanley RG, Simmonds AD, Bicknell AB, Gibbins JM. The platelet-surface thiol isomerase enzyme ERp57 modulates platelet function. J Thromb Haemost. 2012;10:278–288. doi: 10.1111/j.1538-7836.2011.04593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Ahmad SS, Zhou J, Wang L, Cully MP, Essex DW. The disulfide isomerase ERp57 mediates platelet aggregation, hemostasis, and thrombosis. Blood. 2012;119:1737–1746. doi: 10.1182/blood-2011-06-360685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Wu Y, Zhou J, Ahmad SS, Mutus B, Garbi N, Hammerling G, Liu J, Essex DW. Platelet-derived ERp57 mediates platelet incorporation into a growing thrombus by regulation of the alphaIIbbeta3 integrin. Blood. 2013;122:3642–3650. doi: 10.1182/blood-2013-06-506691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holbrook LM, Watkins NA, Simmonds AD, Jones CI, Ouwehand WH, Gibbins JM. Platelets release novel thiol isomerase enzymes which are recruited to the cell surface following activation. Br J Haematol. 2010;148:627–637. doi: 10.1111/j.1365-2141.2009.07994.x. [DOI] [PubMed] [Google Scholar]
- 12.Weiler-Guettler H, Christie PD, Beeler DL, Healy AM, Hancock WW, Rayburn H, Edelberg JM, Rosenberg RD. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest. 1998;101:1983–1991. doi: 10.1172/JCI2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garbi N, Tanaka S, Momburg F, Hammerling GJ. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat Immunol. 2006;7:93–102. doi: 10.1038/ni1288. [DOI] [PubMed] [Google Scholar]
- 14.Billon-Gales A, Fontaine C, Douin-Echinard V, Delpy L, Berges H, Calippe B, Lenfant F, Laurell H, Guery JC, Gourdy P, Arnal JF. Endothelial estrogen receptor-alpha plays a crucial role in the atheroprotective action of 17beta-estradiol in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:2567–2576. doi: 10.1161/CIRCULATIONAHA.109.898445. [DOI] [PubMed] [Google Scholar]
- 15.Dai J, Zhu X, Yoder MC, Wu Y, Colman RW. Cleaved high-molecular-weight kininogen accelerates the onset of endothelial progenitor cell senescence by induction of reactive oxygen species. Arterioscler Thromb Vasc Biol. 2011;31:883–889. doi: 10.1161/ATVBAHA.110.222430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Beguin S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 17.Greene TK, Wang C, Hirsch JD, Zhai L, Gewirtz J, Thornton MA, Miao HZ, Pipe SW, Kaufman RJ, Camire RM, Arruda VR, Kowalska MA, Poncz M. In vivo efficacy of platelet-delivered, high specific activity factor VIII variants. Blood. 2010;116:6114–6122. doi: 10.1182/blood-2010-06-293308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, Brass LF. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121:1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owens AP, 3rd, Passam FH, Antoniak S, Marshall SM, McDaniel AL, Rudel L, Williams JC, Hubbard BK, Dutton JA, Wang J, Tobias PS, Curtiss LK, Daugherty A, Kirchhofer D, Luyendyk JP, Moriarty PM, Nagarajan S, Furie BC, Furie B, Johns DG. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012;122:558–568. doi: 10.1172/JCI58969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivanciu L, Krishnaswamy S, Camire RM. New insights into the spatio-temporal localization of prothrombinase in vivo. Blood. 2014 doi: 10.1182/blood-2014-03-565010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandler WL, Roshal M. Optimization of plasma fluorogenic thrombin-generation assays. American journal of clinical pathology. 2009;132:169–179. doi: 10.1309/AJCP6AY4HTRAAJFQ. [DOI] [PubMed] [Google Scholar]
- 22.Wu HP, Yang WC, Wu YK, Zhao LL, Chen CY, Fu YC. Clinical significance of blood pressure ratios in hypertensive crisis in children. Archives of disease in childhood. 2012;97:200–205. doi: 10.1136/archdischild-2011-300373. [DOI] [PubMed] [Google Scholar]
- 23.Godecke A, Flogel U, Zanger K, Ding Z, Hirchenhain J, Decking UK, Schrader J. Disruption of myoglobin in mice induces multiple compensatory mechanisms. Proc Natl Acad Sci U S A. 1999;96:10495–10500. doi: 10.1073/pnas.96.18.10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene. 2009;28:2925–2939. doi: 10.1038/onc.2009.170. [DOI] [PubMed] [Google Scholar]
- 25.Jasuja R, Furie B, Furie BC. Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo. Blood. 2010;116:4665–4674. doi: 10.1182/blood-2010-04-278184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ioannou Y, Zhang JY, Passam FH, Rahgozar S, Qi JC, Giannakopoulos B, Qi M, Yu P, Yu DM, Hogg PJ, Krilis SA. Naturally occurring free thiols within beta 2-glycoprotein I in vivo: nitrosylation, redox modification by endothelial cells, and regulation of oxidative stress-induced cell injury. Blood. 2010;116:1961–1970. doi: 10.1182/blood-2009-04-215335. [DOI] [PubMed] [Google Scholar]
- 27.Jasuja R, Sharda AV, Kim SH, Furie B, Furie BC. Endothelium but not platelet derived thiol isomerase ERp57 is required for thrombosis in vivo. J Thromb Haemost. 2013;11(suppl 2) abstract:202. [Google Scholar]
- 28.Ahmad F, Boulaftali Y, Greene TK, Ouellette TD, Poncz M, Feske S, Bergmeier W. Relative contributions of stromal interaction molecule 1 and CalDAG-GEFI to calcium-dependent platelet activation and thrombosis. J Thromb Haemost. 2011;9:2077–2086. doi: 10.1111/j.1538-7836.2011.04474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delaney MK, Liu J, Kim K, Shen B, Stojanovic-Terpo A, Zheng Y, Cho J, Du X. Agonist-induced platelet procoagulant activity requires shear and a Rac1-dependent signaling mechanism. Blood. 2014 doi: 10.1182/blood-2014-03-560821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz C, Leuschen NV, Frohlich T, Lorenz M, Pfeiler S, Gleissner CA, Kremmer E, Kessler M, Khandoga AG, Engelmann B, Ley K, Massberg S, Arnold GJ. Identification of novel downstream targets of platelet glycoprotein VI activation by differential proteome analysis: implications for thrombus formation. Blood. 2010;115:4102–4110. doi: 10.1182/blood-2009-07-230268. [DOI] [PubMed] [Google Scholar]
- 31.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langer F, Spath B, Fischer C, Stolz M, Ayuk FA, Kroger N, Bokemeyer C, Ruf W. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood. 2013;121:2324–2335. doi: 10.1182/blood-2012-10-460493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lhermusier T, Chap H, Payrastre B. Platelet membrane phospholipid asymmetry: from the characterization of a scramblase activity to the identification of an essential protein mutated in Scott syndrome. J Thromb Haemost. 2011;9:1883–1891. doi: 10.1111/j.1538-7836.2011.04478.x. [DOI] [PubMed] [Google Scholar]
- 34.Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood. 2010;116:993–1001. doi: 10.1182/blood-2009-10-249607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langer F, Ruf W. Synergies of phosphatidylserine and protein disulfide isomerase in tissue factor activation. Thromb Haemost. 2014;111:590–597. doi: 10.1160/TH13-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurk K, Lahav J, H VANA, Brodde MF, Nofer JR, Kehrel BE. Extracellular protein disulfide isomerase regulates feedback activation of platelet thrombin generation via modulation of coagulation factor binding. J Thromb Haemost. 2011;9:2278–2290. doi: 10.1111/j.1538-7836.2011.04509.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim K, Hahm E, Li J, Holbrook LM, Sasikumar P, Stanley RG, Ushio-Fukai M, Gibbins JM, Cho J. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood. 2013;122:1052–1061. doi: 10.1182/blood-2013-03-492504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.