Abstract
Objective
To evaluate relative accuracy of a newly developed Stroke Assessment of Fall Risk (SAFR) for classifying fallers and non-fallers, compared with a health system fall risk screening tool, the Fall Harm Risk Screen.
Design and setting
Prospective quality improvement study conducted at an inpatient stroke rehabilitation unit at a large urban university hospital.
Participants
Patients admitted for inpatient stroke rehabilitation (N = 419) with imaging or clinical evidence of ischemic or hemorrhagic stroke, between 1 August 2009 and 31 July 2010.
Interventions
Not applicable.
Main outcome measure(s)
Sensitivity, specificity, and area under the curve for Receiver Operating Characteristic Curves of both scales’ classifications, based on fall risk score completed upon admission to inpatient stroke rehabilitation.
Results
A total of 68 (16%) participants fell at least once. The SAFR was significantly more accurate than the Fall Harm Risk Screen (p < 0.001), with area under the curve of 0.73, positive predictive value of 0.29, and negative predictive value of 0.94. For the Fall Harm Risk Screen, area under the curve was 0.56, positive predictive value was 0.19, and negative predictive value was 0.86. Sensitivity and specificity of the SAFR (0.78 and 0.63, respectively) was higher than the Fall Harm Risk Screen (0.57 and 0.48, respectively).
Conclusions
An evidence-derived, population-specific fall risk assessment may more accurately predict fallers than a general fall risk screen for stroke rehabilitation patients. While the SAFR improves upon the accuracy of a general assessment tool, additional refinement may be warranted.
Keywords: Falls, prediction, rehabilitation, stroke
Introduction
A disproportionate number of stroke patients, as many as 48%,1 fall during inpatient rehabilitation; of those falls, nearly one-third lead to potentially serious injuries.2 Hospital-related falls are associated with long lengths of stay and poor outcomes,3 as well as reduced physical activity owing to fear of additional falls1 and diminished dignity.4 To prevent these negative outcomes, preventive strategies are needed for patients at high risk for falls.
To most effectively target preventive strategies, it is necessary to reliably identify patients at greatest risk of falls. Hospitals seek to identify high-risk patients using either published fall risk assessments developed for general hospital populations, or internally developed tools that have not been adequately validated in all the populations for which they are used. These measures, including the recently published and not well tested PREDICT-FIRST, often rely primarily upon demographic risk factors (e.g. age; gender) or general clinical characteristics (e.g. use of antihypertensive, antianxiety, or antidepressant medications; urinary incontinence; history of previous falls) to indicate fall risk. However, these characteristics may be less relevant to fall risk after stroke than are stroke-specific disabilities and impairments. As a result, existing fall risk tools may lack sensitivity and specificity for likely fallers in stroke rehabilitation.5 We developed and piloted a stroke-specific tool, the Stroke Assessment of Fall Risk (SAFR), as a quality improvement project,6 to improve fall prediction during the inpatient stroke rehabilitation stay. This study evaluated the accuracy of the SAFR in classifying fallers and non-fallers compared with an unpublished fall risk screening tool developed by our health system, the Fall Harm Risk Screen.
Methods
Participants were all patients admitted consecutively to two inpatient stroke rehabilitation units in the same academic health center between 1 August 2009 and 31 July 2010 who had imaging and/or clinical evidence of acute stroke (ischemic or hemorrhagic). Those with comorbid traumatic brain injury or degenerative neurological disorders (e.g. Parkinson’s disease and multiple sclerosis) were excluded.
This prospective quality improvement study was approved by our academic health center’s institutional review board. We retrospectively collected demographic (gender, age, race/ethnicity, and hospital unit) and clinical data (stroke etiology, hemisphere, location, and fall prevention interventions utilized during the stay) from each participant’s medical record. We also recorded hospital Fall Harm Risk Screen admission scores, and completed the SAFR based on the rehabilitation admission interdisciplinary clinical evaluation. Finally, we prospectively tracked and recorded fall occurrence during the rehabilitation stay.
The Fall Harm Risk Screen is a facility-developed, three-item scale that assesses three levels of fall risk (low, medium, and high) based on patient functional ability, history of falls, and the nurse’s clinical judgement of fall risk. Nursing staff completes the scale at rehabilitation admission, and periodically throughout the stay. The Fall Harm Risk Scale is used for all patients throughout the health system, regardless of diagnosis (the Fall Harm Risk Scale is provided in Appendix A, available online).
The SAFR is scored using clinical documentation from the first 72 hours of the inpatient rehabilitation admission. It assesses seven stroke-specific risk factors identified from the published literature1–3,7 and clinical audits. These comprise four impairments (impulsivity, hemi-neglect, static, and dynamic sitting balance) and three functional limitations (lowest score on three Functional Independence Measure items: transfers, problem solving, and memory). Impulsivity and hemi-neglect are scored dichotomously (0, absent; 7, present). The remaining items are scored using a 7-point scale similar to the Functional Independence Measure, but with zero indicating no impairment or deficit, and seven indicating the most severe impairment or deficit. The total score is a sum of item scores (0, low risk of falls; 49, highest risk of falls) (the SAFR is provided in Appendix B, available online.)
A fall was defined as unplanned contact with the floor. Fall occurrence was gleaned from hospital incident reports, and participants were coded accordingly (fall/no fall).
Analyses were performed using SAS Version 9.2 (SAS Institute, Cary, North Carolina) with a two-tailed significance level of α = 0.05 for all tests. We characterized the sample using descriptive statistics; we then compared fallers with non-fallers on key demographic and clinical attributes using chi-square tests and Mann–Whitney U-test or independent sample student’s t-tests, as appropriate. We constructed Receiver Operating Characteristic curves using logistic regression to determine the accuracy of fall identification of the SAFR and the Fall Harm Risk Screen. We then chose a clinically meaningful cut point for “at risk to fall” and calculated positive predictive value for each tool. Additionally, we conducted posthoc analyses of the SAFR items using Receiver Operating Characteristic curves to assess individual item performance.
Results
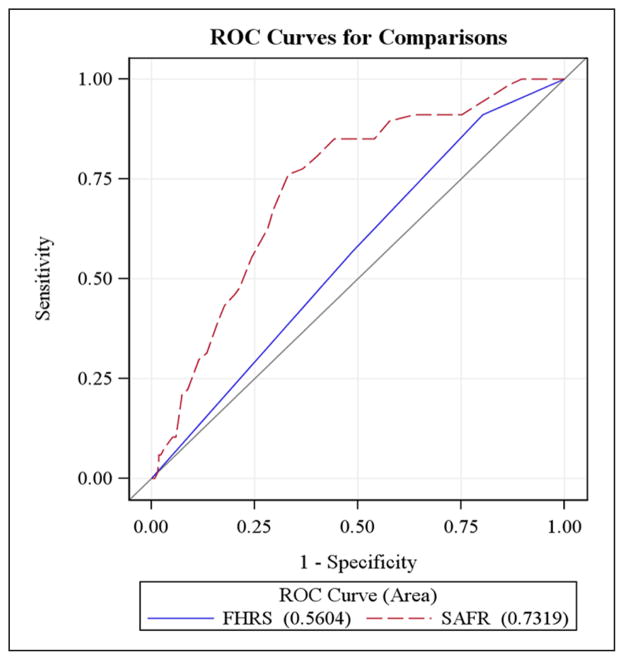
Of the 446 patients admitted for stroke rehabilitation during the study period, 27 (6%) were excluded owing to comorbid neurological conditions, for a final sample of 419 participants. A description of the sample is provided in Table 1. A total of 68 participants (16%) experienced at least one fall during their inpatient rehabilitation stay; of those participants who fell, 10 people (2% of the sample, 15% of the fallers) fell more than once. Fallers and non-fallers differed with respect to age, use of chair alarms, and use of restraints during inpatient rehabilitation. The median age of fallers was significantly younger than that of non-fallers (fallers M = 63.7 ± 13.5 years, non-fallers M = 68.2 ± 15.7 years, p = 0.026). Fallers were also significantly more likely to have a chair alarm (χ21 = 21.23, p < 0.001, odds ratio (OR) = 4.3, 95% confidence interval (CI) (2.2, 8.3)) or a restraint (χ21 = 23.98, p < 0.001, OR = 3.7, 95% CI (2.1, 8.3)) during their inpatient rehabilitation stay. The area under the curve was 0.56 (95% CI (0.50, 0.62)) for Fall Harm Risk Screen, and 0.73 (95% CI (0.67, 0.79)) for SAFR (Figure 1); it was significantly more accurate than the Fall Harm Risk Screen (χ21 = 17.28, p < 0.001). At a clinically meaningful cut point of 27, the positive predictive value for the SAFR was 0.29 and the negative predictive value was 0.94, yielding sensitivity and specificity of 0.78 and 0.63, respectively (Table 2). A Fall Harm Risk Screen score of two produced a positive predictive value of 0.19 and a negative predictive value of 0.86, yielding sensitivity and specificity of 0.57 and 0.48, respectively (Table 2). Posthoc analyses of the seven SAFR items revealed that the two dichotomous-scored items (impulsivity and hemi-neglect) were less predictive than the five ordinal-scored items (Table 3). Area under the curve values ranged from 0.55–0.69 for individual items, indicating the overall score (area under the curve = 0.73) provided a more accurate classification of fall risk than any one risk factor (Table 3).
Table 1.
Demographic and clinical characteristics of the sample.
| Variable | Entire sample (N = 419) | Falls occurrence
|
||
|---|---|---|---|---|
| Non-fallers (n = 351) | Fallers (n = 68) | Test statistic (p value) | ||
| Age in years, mean ±SD | 67.5 ± 15.5 | M= 68.2 ± 15.7 | 63.7 ± 13.5 |
t =2.23 p= 0.026 |
| Gender, n (%) female | 202 (48.2) | 176 (50.14) | 26 (38.24) | χ2(1) = 3.23 p= 0.07 |
| Race, n (%) white | 310 (73.99) | 260 (74.07) | 50 (73.53) | χ2(3) = 4.11 p= 0.25 |
| Stroke hemisphere, n (%) left | 188 (45.30) | 158 (45.53) | 30 (44.12) | χ2(3) = 0.47 p= 0.93 |
| Stroke etiology, n (%) ischemic | 333 (79.86) | 281 (80.52) | 52 (76.47) | χ2(1) = 0.58 p= 0.45 |
| Stroke type, n (%) | ||||
| Cortical | 73 (17.59) | 60 (17.29) | 13 (19.12) | χ2(3) = 0.61 p= 0.89 |
| Subcortical | 80 (19.28) | 67 (19.31) | 13 (19.12) | |
| Cortical/subcortical | 188 (45.30) | 156 (44.96) | 32 (47.06) | |
| Brainstem/cerebellar | 74 (17.83) | 64 (18.44) | 10 (14.71) | |
| Restraint use*, n (%) present | 155 (36.99) | 112 (31.91) | 43 (63.24) | χ2(1) = 23.98 p < 0.001 |
| Chair alarm use*, n (%) present | 239 (57.04) | 183 (52.14) | 56 (82.35) | χ2(1) = 21.23 p <0.001 |
Figure 1.
Predictive ability of Stroke Assessment of Fall Risk (SAFR) and Fall Harm Risk Screen (FHRS).
FHRS: Fall Harm Risk Screen; ROC: Receiver Operating Characteristic Curve; SAFR: Stroke Assessment of Fall Risk.
Table 2.
Predictive ability of Stroke Assessment of Fall Risk at cut point score of 27 vs. Fall Harm Risk Screen at cut point score of two.
| Predicted outcomes
|
||||
|---|---|---|---|---|
| Fall | No fall | |||
| Stroke Assessment of Fall Risk (cut point = 27) | Fall | TP, n = 52 | FN, n = 15 | Sensitivity = TP/(TP + FN) = 0.78 |
| No fall | FP, n = 128 | TN, n = 222 | Specificity = TN/(TN + FP) = 0.63 | |
| PPV = TP/(TP + FP) = 0.29 | NPV = TN/(TN + FN) = 0.94 | |||
| Predicted outcomes
|
||||
|---|---|---|---|---|
| Fall | No fall | |||
| Fall Harm Risk Screen (cut point = 2) | Fall | TP, n = 39 | FN, n = 29 | Sensitivity = TP/(TP + FN) = 0.57 |
| No fall | FP, n = 170 | TN, n = 181 | Specificity = TN/(TN + FP) = 0.48 | |
| PPV = TP/(TP + FP) = 0.19 | NPV = TN/(TN + FN) = 0.86 | |||
FN: false negative; FP: false positive; NPV: negative predictive value; PPV: positive predictive value; TN: true negative; TP: true positive.
Table 3.
Stroke Assessment of Fall Risk item performance.
| Item | AUC | Standard error | 95% Wald CI |
|---|---|---|---|
| Impulsivity | 0.60 | 0.0327 | 0.53–0.66 |
| Hemi-neglect | 0.55 | 0.0306 | 0.49–0.61 |
| Static | 0.69 | 0.0302 | 0.63–0.75 |
| Dynamic sitting balance | 0.69 | 0.0330 | 0.62–0.75 |
| Transfer | 0.69 | 0.0314 | 0.62–0.75 |
| Problem-solving | 0.67 | 0.0325 | 0.60–0.73 |
| Memory | 0.66 | 0.0333 | 0.59–0.72 |
AUC: area under the curve; CI: confidence interval.
Discussion
In our sample of 419 stroke patients, the Fall Harm Risk Screen identified inpatient post-stroke fallers no better than chance, while the SAFR accurately identified fallers nearly 75% of the time, representing a clinically important improvement in fall identification accuracy. Like many inpatient fall risk screens, such as the Morse scale,8 Hendrich II,9 and PREDICT_FIRST,10 the Fall Harm Risk Screen is based on general risk factors, such as medications, comorbidities, and gait disturbances, as well as on non-modifiable risk factors, such as age and gender. In stroke rehabilitation, every patient scores at high fall risk on these tools, yet not every patient will fall. Preventive strategies may be initiated for every patient, reducing the vigilance provided to those truly at risk. While the recently published PREDICT_FIRST’s predictive accuracy was similar to that of the SAFR (area under the curve = 0.73) in a sample of rehabilitation patients comprising a variety of diagnoses,10 it underestimated the rate of falls in a sample of stroke rehabilitation patients.5 In contrast, the SAFR was derived from stroke-specific indicators, and which may lead to more accurate prediction. Moreover, with its focus on modifiable risk factors, the SAFR may suggest patient-specific rehabilitative strategies to therapeutically modify each patient’s specific risk indicators, providing greater clinical value than that provided by a simple risk prediction tool.
The SAFR’s sensitivity (0.78) suggests that it will accurately identify 78% of fallers at the chosen cut point of 27. However, results also suggest that the SAFR will rate 37% of patients who do not fall as being “at risk” (based on calculating 1-specificity), resulting in application of unnecessary preventive interventions for some patients in stroke rehabilitation. The Fall Harm Risk Screen’s sensitivity will correctly identify 57% of fallers; yet, given its specificity, 48% of non-fallers will be incorrectly designated at risk to fall and will receive unnecessary fall prevention interventions. Implementing unneeded interventions wastes staff time (e.g. through increased surveillance of “at risk” patients) and uses costly resources (bed and chair alarms, enclosure beds). We believe that the SAFR, with its improved sensitivity and specificity, represents a clinically desirable improvement in fall risk designation over the currently used Fall Harm Risk Screen, although it may benefit from further development to improve precision.
There were unexpected differences in our sample between subjects who fell and those who did not fall. First, fallers were significantly younger than non-fallers. Traditionally, increasing age has been associated with greater fall occurrence among older adults,11 although this relationship may not extend to the inpatient stroke rehabilitation population.7 Inpatient stroke rehabilitation falls are frequently associated with impulsive behavior, poor judgment, or calculated risk-taking by patients.12 It is possible that the younger persons in our sample were more active, or were more prone to attempt ‘risky’ behaviors, such as standing or walking unassisted. This conclusion is supported by the fact that almost half (47.5%) of persons who fell scored as “impulsive” on the SAFR, while only 27.5% of persons who did not fall were scored as “impulsive”. Of note, persons scored as “impulsive” were slightly younger (M = 63.8) compared with persons scored as not impulsive (M = 69.1).
Second, subjects who fell were more likely to have chair alarms or restraints, which are intended to prevent falls. However, we do not know whether these strategies were initiated prior to the fall, or afterward, to prevent further falls. Future analyses should attempt to identify the temporal sequence of intervention initiation and fall occurrence.
Study limitations
A key limitation is that some SAFR items rely on narrative medical charting of clinician impression, instead of standardized assessment scales. Incorporating objective measures of impairments, such as impulsivity and neglect, into clinical care may improve overall scoring accuracy. Further, we used hospital incident reports to identify falls. Relying on incident reports may result in under-identification of falls.13 However, our health system actively encourages recording of all falls to identify trends and improve patient care processes, so the likelihood of under reporting is low. Further, we only evaluated the ability of the SAFR to predict the occurrence of any fall during rehabilitation, rather than examining its ability to identify “repeat fallers”. However, the proportion of repeat fallers was such a low percentage of the sample (2%) that meaningful analysis of repeat falls was precluded. Although we provided some preliminary evidence of item performance, further analyses should be performed to assess item-level characteristics. Future work using psychometric testing (i.e. Rasch analysis) could determine whether some items should be weighted based on importance, to increase their contribution to the SAFR score.
Finally, we did not exclude unanticipated physiologic falls from our study. Unanticipated physiologic falls are falls resulting from a medical event (e.g. syncopal episode, seizure), which are not directly related to post-stroke impairments or activity limitations. In her classic text, Morse14 notes that unanticipated physiologic falls occur unpredictably among all hospitalized patients, have little relationship to the factors that place patients at increased fall risk, and should not be a focus of fall risk assessment initiatives. The predictive accuracy of both the SAFR and the Fall Harm Risk Screen may have been slightly decreased because we did not exclude unanticipated physiologic falls.14 Current hospital policy requires reporting of all falls, including unanticipated physiologic falls, so some of these may have been included in our data. However, the rate of unanticipated physiologic falls on our unit is very low; thus, the likelihood that our results were significantly affected by unanticipated physiologic falls is minimal. A strength of this study is that we relied upon routinely collected clinical data to complete the SAFR, eliminating burdensome “double documentation” for nurses and therapists.
Preliminary results suggest that in stroke inpatient rehabilitation, an evidence-derived, population-specific fall risk assessment may more accurately predict fallers than a general fall risk screen. The SAFR shows promise as such an assessment. Increasing fall prediction accuracy may help to decrease incident fall rates in inpatient stroke rehabilitation, thereby minimizing the harmful consequences of falls.
Supplementary Material
Clinical messages.
A stroke-specific fall risk assessment, based on common post-stroke impairments and activity limitations, can improve fall prediction among inpatient stroke rehabilitation patients.
Persons of advanced age exhibiting impulsivity, neglect, impaired balance, memory impairment, impaired problem solving skills, and deficits in transfers, may be at particularly increased risk of falls.
Acknowledgments
The authors would like to acknowledge Kerri O’Rourke, MOT, OTR/L for her assistance with data collection and entry.
Funding
This research was supported by the University of Pittsburgh Medical Center Rehabilitation Institute Clinician Pilot Grant Program (TPB), the Comprehensive Opportunities for Rehabilitation Research Training Program K12 HD055931 (ERS), the John A. Hartford Foundation Building Academic Geriatric Nursing Capacity Predoctoral Scholarship (GBC), and the National Research Service Award Predoctoral Fellowship F31 NR011561 (GBC).
Footnotes
Reprints and permissions: sagepub.co.uk/journalsPermissions.nav
Contributors
TPB and GBC were responsible for the conceptualization and design of the Stroke Assessment of Fall Risk instrument. TPB, ERS, and GBC were responsible for study design, interpretation of results, and for writing and revising the manuscript. TPB was responsible for conducting the study and supervising data collection and data entry. CN and LT performed data analysis and assisted with interpretation of results, and with writing and revising the manuscript.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Suzuki T, Sonoda S, Misawa K, Saitoh E, Shimizu Y, Kotake T. Incidence and consequence of falls in inpatient rehabilitation stroke patients. Experimental Aging Res. 2005;31:457–469. doi: 10.1080/03610730500206881. [DOI] [PubMed] [Google Scholar]
- 2.Teasell R, McRae M, Foley N, Bhardwaj A. The incidence and consequences of falls in stroke patients during inpatient rehabilitation: factors associated with high risk. Arch Phys Med Rehabil. 2002;83:329–333. doi: 10.1053/apmr.2002.29623. [DOI] [PubMed] [Google Scholar]
- 3.Czernuszenko A, Czlonkowska A. Risk factors for falls in stroke patients during inpatient rehabilitation. Clin Rehabil. 2009;23:176–188. doi: 10.1177/0269215508098894. [DOI] [PubMed] [Google Scholar]
- 4.Rapport LJ, Hanks RA, Millis SR, Deshpande SA. Executive functioning and predictors of falls in the rehabilitation setting. Arch Phys Med Rehabil. 1998;79:629–633. doi: 10.1016/s0003-9993(98)90035-1. [DOI] [PubMed] [Google Scholar]
- 5.Nystrom A, Hellstrom K. Fall risk six weeks from onset of stroke and the ability of the Prediction of Falls in Rehabilitation Settings Tool and motor function to predict falls. Clin Rehabil. 2013;27:473–479. doi: 10.1177/0269215512464703. [DOI] [PubMed] [Google Scholar]
- 6.Breisinger TP, Campbell GB. Development and testing of the Stroke Assessment of Fall Risk (SAFR): A pilot study. Arch Phys Med Rehabil. 2011;92(10):1696. [Google Scholar]
- 7.Campbell GB, Matthews JT. An Integrative review of factors associated with falls during post-stroke rehabilitation. J Nursing Scholarship. 2010;42(4):404–413. doi: 10.1111/j.1547-5069.2010.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morse JM, Morse R, Tylko S. Development of a scale to identify the fall-prone patient. Can J Aging. 1989;8(4):366–377. [Google Scholar]
- 9.Hendrich A. Predicting patient falls: Using the Hendrich II Fall Risk Model in clinical practice. Am J Nursing. 2007;107(11):50–58. doi: 10.1097/01.NAJ.0000298062.27349.8e. [DOI] [PubMed] [Google Scholar]
- 10.Gillespie LD, Robertson MC, Gillesptie WJ, et al. Interventions for preventing falls ion older people living in the community (Review) The Cochrane Library. 2012;(9) doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubenstein LZ, Josephson KR. Falls and their prevention in elderly people: what does the evidence show? Medical Clinics N Am. 2006;90:807–824. doi: 10.1016/j.mcna.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Hanger HC, Wills KL, Wilkinson T. Classification of falls in stroke rehabilitation—not all falls are the same. Clin Rehabil. 2014;28(2):183–195. doi: 10.1177/0269215513496801. [DOI] [PubMed] [Google Scholar]
- 13.Hill AM, Hoffman T, Hill KD, et al. Measuring falls events in acute hospitals—A comparison of three reporting methods to identify missing data in the hospital reporting system. J Am Geriatrics Soc. 2010;58:1347–1352. doi: 10.1111/j.1532-5415.2010.02856.x. [DOI] [PubMed] [Google Scholar]
- 14.Morse JM. Preventing patient falls. Thousand Oaks, CA: SAGE Publications; 1997. p. 151. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.