Abstract
The oviducts contain high grade serous cancer (HGSC) precursors (serous tubal intraepithelial neoplasia or STINs), which are γ-H2AXp- and TP53 mutation-positive. Although they express wild type p53, secretory cell outgrowths (SCOUTs) are associated with older age and serous cancer; moreover both STINs and SCOUTs share a loss of PAX2 expression (PAX2n). We evaluated PAX2 expression in proliferating adult and embryonic oviductal cells, normal mucosa, SCOUTs, Walthard cell nests (WCNs), STINs and HGSCs, and the expression of genes chosen empirically or from SCOUT expression arrays. Clones generated in vitro from embryonic gynecologic tract and adult fallopian tube were Krt7p/PAX2n/EZH2p and underwent ciliated (PAX2n/EZH2n/FOXJ1p) and basal (Krt7n/EZH2n/Krt5p) differentiation. Similarly non-ciliated cells in normal mucosa were PAX2p but became PAX2n in multilayered epithelium undergoing ciliated or basal (Walthard cell nests or WCN) cell differentiation. PAX2n SCOUTs fell into two groups; Type I were secretory or secretory/ciliated with a “tubal” phenotype and were ALDH1n and β-cateninmem (membraneous only). Type II displayed a columnar to pseudostratified (endometrioid) phenotype, with an EZH2p, ALDH1p, β-cateninnc (nuclear and cytoplasmic), stathminp, LEF1p, RCN1p and RUNX2p expression signature. STINs and HGSCs shared the Type I immunophenotype of PAX2n, ALDH1n, β-cateninmem, but highly expressed EZH2p, LEF1p, RCN1p, and stathminp. This study, for the first time, links PAX2n with proliferating fetal and adult oviductal cells undergoing basal and ciliated differentiation and shows that this expression state is maintained in SCOUTs, STINs and HGSCs. All three entities can demonstrate a consistent perturbation of genes involved in potential tumor suppressor gene silencing (EZH2), transcriptional regulation (LEF1), regulation of differentiation (RUNX2), calcium binding (RCN1) and oncogenesis (stathmin). This shared expression signature between benign and neoplastic entities links normal progenitor cell expansion to abnormal and neoplastic outgrowth in the oviduct and exposes a common pathway that could be a target for early prevention.
Keywords: fallopian tube, serous carcinoma, stem cell, PAX2, ALDH1
Introduction
Recent discoveries have strengthened the relationship between the distal fallopian tube and epithelial malignancies traditionally attributed to the ovary, specifically high-grade serous carcinomas (HGSC), the most lethal of ovarian cancers.1, 2, 3 With these discoveries has emerged a collective effort to resolve the sequence of histologic and molecular events giving rise to these tumors in the fallopian tube. The serous carcinogenic sequence involves not only frank malignancies with metastatic spread, but serous cancer precursors, including latent precursors—the p53 signature—and serous tubal intraepithelial neoplasms (STINs). The latter include intramucosal carcinomas (STICs) and lesser but immunophenotypically similar atypias that are considered premalignant intraepithelial lesions (STILs). 4, 5 Virtually all serous cancer precursors contain mutations in TP53, evidence of a DNA damage response (γ-H2AXp) and predominate in the distal fallopian tube.4 Contiguous benign (p53 signatures) and malignant (STICs) epithelia have been documented with shared mutations in specific codons of TP53.4, 6 In addition, further studies have unearthed other benign epithelial alterations, termed secretory cell outgrowths—SCOUTs—that do not contain TP53 mutations or evidence of a DNA damage response, yet share with precursors and carcinomas loss of PAX2 expression. 7, 8, 9 SCOUTs do not appear directly linked to HGSC, but have been documented at higher frequency in the normal tubes of postmenopausal women and those with HGSC.8, 9 Based on these properties we have designated SCOUTs as “surrogate precursors” and hypothesize that both SCOUTs and serous cancer precursors share properties or similar mechanisms in their pathogenesis albeit with different potential outcomes.
The shared loss of PAX2 expression in both SCOUTs and many “true” serous cancer precursors suggests that inactivation of this gene, while integral to neoplasia, has a wider range of associations and may signify a generic pathway common to epithelial cell expansion. The goals of this study were to first determine the breadth of the PAX2n immunophenotype in the fallopian tube by examining “normal” cell growth and differentiation in vitro and in vivo. Secondly, we wanted to characterize more fully the alterations in expression that typified SCOUTs by array analysis and employ a biomarker profile to determine whether the SCOUT signature was recapitulated in STINs and HGSCs.
Methods
Case material
This study was approved by the Brigham and Women’s human investigation committee and involved the use of discarded fresh and archived tissues. Case material for antibody staining consisted of the following epithelia/lesions: 1) normal salpingeal epithelium (n = 15), 2) SCOUTs (n = 44) and other outgrowths such as transitional-like metaplasia (Walthard cell nests, n = 5), 3) serous tubal intraepithelial neoplasms (STINs) (n = 18) and 4) metastatic or invasive serous carcinomas (n = 39). In addition, cultured clonogenic cells from normal fallopian tubes were examined for selected marker expression. Cases for immunohistochemistry were selected by one of us (CPC) using previously described criteria (Figure 1).10
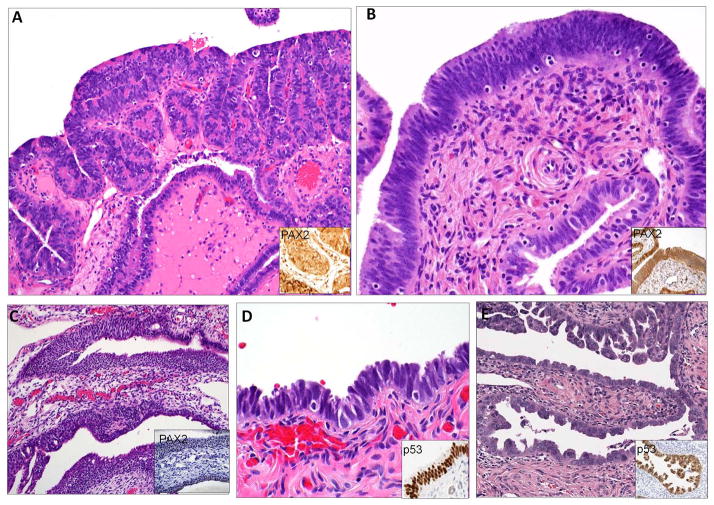
Figure 1.
Entities associated with PAX2n immunophenotype included (A) Type 1 secretory cell outgrowths (SCOUT), (B) Type 2 SCOUTs, (C) Walthard cell nests and (D) low- and (E) high-(serous tubal intraepithelial carcinoma) grade tubal intraepithelial neoplasia.
Cell culture
Fimbrial tissue was obtained from discarded surgical specimens of women undergoing benign procedures. Discarded fetal oviductal tissues were obtained by parental consent under an approved IRB protocol. Disaggregated cells were cultivated onto a feeder layer of lethally irradiated 3T3-J2 cells in stem cell culturing media (Jackson Laboratory, scm003). Clonal analysis and in vitro 3D differentiation were based on previously described methods for lung epithelial stem cells.11
Microarray and bioinformatics
In order to identify genes expressed in PAX2n epithelium, expression arrays were generated from formalin-fixed, laser-capture-micro-dissected (LCM) PAX2n SCOUTs and benign control oviductal epithelium. RNAs obtained from the LCM procedure were amplified using the Ovation FFPE WTA System, WT-Ovation Exon Module and Encore Biotin Module (NuGEN Technologies) and hybridized onto GeneChip® Human Exon 1.0 ST Arrays. GeneChip operating software was used to process all the Cel files and calculate probe intensity values. To validate sample quality, hybridization ratios were calculated using Affymetrix Expression Console software. The intensity values were log2-transformed and imported into the Partek Genomics Suite. Exons were summarized to genes and a 1-way ANOVA was performed to identify differentially expressed genes. P values and fold-change were calculated for each analysis. Heatmaps were generated using Pearson’s correlation and Ward’s method with selected genes based on p value. Pathway analyses were performed using Gene Set Enrichment Analysis (GSEA) software. Candidate biomarkers were culled from these arrays and are summarized in Table 1.
Immunohistochemistry
Immunostaining was performed with attention to the biomarkers in Supplementary Table 1, in which product information and dilutions are included. When normal-appearing epithelia were scanned for putative PAX2n secretory cells, sections were immunostained with two antibodies concurrently; PAX2, which stains non-ciliated cells, and FOXJ1, a ciliated cell marker. Antibodies to leukocyte common antigen (LCM) for CD3, as well as FASCIN, were also used to track intraepithelial lymphocytes and dendritic cells, which are normally PAX2n. Detection was completed with the Vectastain ABC kit (Cat. No. PK-6102; Vector Laboratories, Inc) with a liquid DAB-plus substrate kit (Cat. No. 00-2020). Slides were counterstained with Hematoxylin Stain 3 (Cat. No. CS402-1D). Antibody information is summarized in Supplementary Table 1. Reaction to antibody staining is indicated by superscripted “p” or “n” for positive or negative (PAX2, ALDH1, FOXJ1 etc), “m” or “wt” for mutated or wild type (p53) and “nc” or “mem” for nuclear and cytoplasmic vs. membrane localization (β-catenin). Immunohistochemistry, immunofluorescence staining and image acquisition were performed as previously described.9,11 Proliferating clones were identified and immunostained for PAX2, PAX8, FOXJ1, Krt7, Krt5, p63, EZH2 and Ki67. Evidence of ciliated cell differentiation was identified by immunostaining for FOXJ1 and acetylated alpha-tubulin. Basal cells were identified by Krt5 or p63 immunostaining.
Results
Histologic sub-classification of SCOUTs and STINs
Lesions under study are illustrated in Figure 1. Based on previous studies, SCOUTs were subdivided into two general histologic categories.8, 12 The first, designated as Type 1 SCOUTs, consisted of a typical mono or biphasic tubal epithelial composition with either single layers of tubal non-ciliated cells or (more commonly) a combination of non-ciliated and ciliated cells. The second, arbitrarily labeled Type 2 SCOUTs, consisted of proliferations with mildly pseudostratified and closely arranged elongated fusiform nuclei, similar to endometrial epithelium, and also termed “endometrioid” SCOUTs. Cells with ciliated differentiation (FOXJ1p) were present, but were typically less than 30% of the cells and scattered throughout the epithelium. Walthard cell nests (WCNs), consisting of basal cell outgrowth with a squamo-transitional phenotype were also studied because they signify another form of outgrowth derived from columnar epithelial cells, albeit metaplastic. STINs were sub-classified as previously described and contained strong p53 immuno-staining and evidence of DNA damage by H2AX staining.5 Those with mild or moderate atypia and preserved epithelial polarity were classified as low grade and are identical to lesions classified as “STILs”, “TILTs” and atypical hyperplasia in other reports. 13, 14, 15 Those with conspicuous loss of epithelial polarity were classified as high grade, synonymous with serous tubal intraepithelial carcinoma (STIC). The latter have a 0–11% outcome risk of HGSC, based on recent studies. 16, 17, 18 The HGSC outcome risk of lower grade STINs is unknown but presumed to be less than that of high grade STINs.
In vitro and in vivo expression of PAX2 in the fallopian tube mucosa
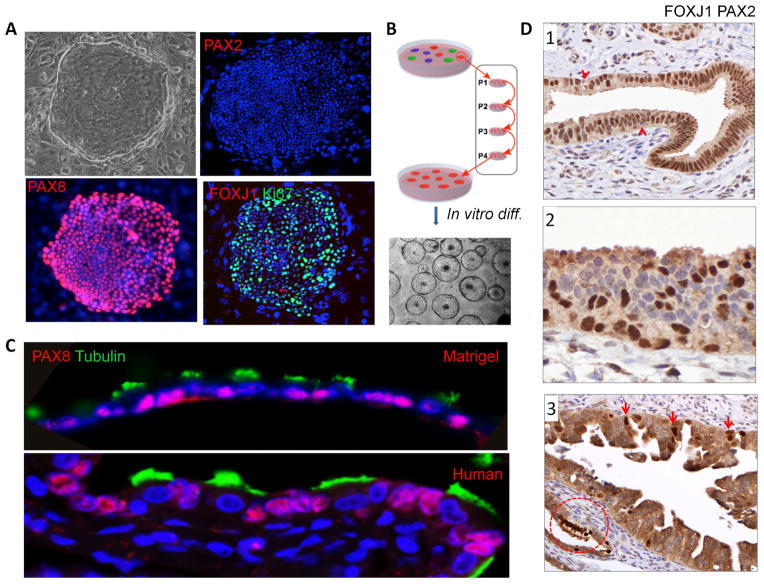
Cultured epithelial cells from the gynecologic tract, both in adults and at 20 weeks gestation, were plated and colonies of clonogenic cells were characterized. The dominant immunophenotype associated with highly-proliferative clonogenic cell outgrowth was Krt7p/PAX8p/EZH2p/PAX2n/Krt5n/p63n (Figure 2A, Figure 3A and Supplementary Figure 2A). FOXJ1 expression indicating ciliated cell differentiation was also seen occasionally in the non-proliferative cells that were not stained positively with Ki67 (Figure 2A). To examine the differentiation ability of these cloned cells at the single-cell level, we established single-cell pedigree lines by subsequent rounds of plating and clone selection (Figure 2B). Pedigree lines of these cloned oviductal progenitor cells were differentiated in either air-liquid interface (ALI) cell culture system or 3D Matrigel cultures for 10–20 days. In 3D Matrigel cultures, PAX8p oviductal progenitor cells differentiated into columnar epithelium comprised of acetylated tubulinp/FOXJ1p/PAX8n ciliated cells and PAX8p non-ciliated cells, which resembles the human oviduct histology (Figure 2C). In the ALI culture system, a series of images of acetylated tubulin expression were taken at different time points during the differentiation and showed that the oviductal progenitor cells started to differentiate into ciliated cells at day 3 and became maturely differentiated at day 10 (Supplementary Figure 2B). At day 10 in the ALI culture system, the cloned oviductal progenitor cells formed a simple epithelium with ciliated cells marked by FOXJ1 and acetylated tubulin and non-ciliated cells marked by PAX2 (Supplementary Figure 2C). It is noteworthy that while the proliferating population is PAX2n (Figure 2A), PAX2 expression was reclaimed in some non-ciliated (secretory) cells. This further indicates that the progeny of a single oviductal progenitor cell can give rise to all epithelial lineages typically found in the oviduct, including not only mature ciliated cells but non-ciliated (secretory) cells.
Figure 2.
In vitro propagation and differentiation of oviductal progenitor cells. (A) The cells cloned from fetal or adult oviduct are PAX2n, PAX8p and occasionally express differentiation marker (FOXJ1) in non-proliferative cells (Ki67n). (B) Schematic diagram of pedigree cell line establishment. (C) Upper panel, representative image of fetal (20-week) oviductal progenitor cells differentiated in 3D Matrigel culture system. Lower panel, immunofluoresence image of human adult oviduct epithelium. Acetyl-alpha-tubulin (green) indicates ciliated cell differentiation. PAX8 (red) indicates non-ciliated cells. DAPI stains nuclei (blue). (D1) Combined staining of histologic sections of normal tube with both PAX2 and FOXJ1 reveals widespread nuclear staining, except occasional lymphocytes (arrows). (D2) Occasional foci of multilayered epithelium undergoing ciliated cell differentiation (positive nuclei) consist of some cells negative for PAX2. (D3) Tubal intraepithelial carcinoma with focal FOXJ1 staining (arrowheads) indicating ciliated cell differentiation. Circled focus of normal ciliated cells is an internal positive control.
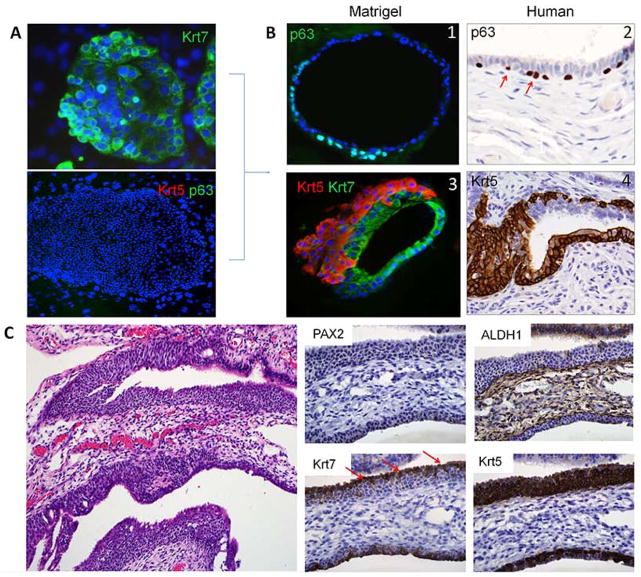
Figure 3.
In vitro and in vivo basal cell differentiation in the oviduct. (A) Colonies of Krt7p/Krt5n/p63n cells from a 20 week old fetal oviduct. (B1, B3) single (p63, green) and multilayered (Krt5, red) basal cell outgrowth seen in matrigel cultures. (B2, B4) similar basal cell growth highlighted by p63 and Krt5 in the adult fimbria. (C) Walthard cell nest in the adult tube is typically PAX2 and ALDH1 negative. Residual Krt7-positive cells (arrows) are displaced from beneath by an expanding Krt5 population.
Immunostaining of both fetal and adult fallopian tubes was performed to ascertain the distribution of PAX2-expressing cells and address the possibility that the PAX2n immunophenotype was programmed earlier in development. Histologic sections of fetal (at 21 weeks) and adult fallopian tubes were examined. Fetal tubes contained an abundance of PAX2p cells, with occasional interspersed ciliated cells (Supplemental Figure 1A). Expression of PAX8 was similar in distribution (Supplemental Figure 1B). Similarly, in normal adult tubes, PAX2 staining was extensive in cells that were not undergoing ciliated (tubulinp) differentiation (Supplemental Figure 1C). A summary of immunophenotypes for progenitor and adult cells is displayed in Supplementary Table 2.
In the adult tubes, sections were also stained with FOXJ1, and/or LCA to account for other PAX2n cells that were either undergoing ciliated differentiation or were non-epithelial. Mono-layered or mildly pseudostratified normal fallopian tube mucosa typically contained cells either expressing PAX2 or FOXJ1 (Figure 2D1). In occasional foci of prominent multilayered epithelium with some cells staining positive with FOXJ1, loss of PAX2 nuclear staining could be seen (Figure 2D2) giving the impression that loss of PAX2 expression in non-ciliated cells was coordinated with cell growth in multilayered epithelium. Albeit less so, FOXJ1 staining was also seen in STINs, supporting ciliated differentiation in PAX2n neoplastic growth (Figure 2D3).
Metaplastic (Walthard cell nests) differentiation of PAX2n columnar cells in vitro and in vivo
Walthard cell nests are foci of transitional-like metaplasia in the fimbria or adjacent peritoneal surface and are emblematic of basal cell outgrowth that can develop near the junctions of disparate epithelial types.19 Other sites include the gastro-esophageal and cervical squamo-columnar junctions. Both have been designated as sites harboring residual embryonic cells and studies of the latter have suggested that basal or reserve cells emerge from the overlying columnar cells and then undergo squamous metaplasia. 20, 21 This process has been termed “top down” differentiation, i.e. the progeny (basal cells) emerge from beneath the progenitor population. However, no study has ever displayed this sequence in vitro. Fetal tubal cells propagated in vitro were strongly positive for both Krt7 and PAX8 was seen, in keeping with Mullerian epithelium (Figure 2A and Figure 3A). Moreover, these progenitor cells did not express Krt5 or p63 (Figure 3A). Interestingly, when pedigree lines of these cloned oviductal progenitor cells were differentiated in 3D Matrigel cultures for 10–20 days, in addition to the typical ciliated cell differentiation (Figure 2C), subjacent p63/Krt5p basal cells emerged (Figure 3B1) and expanded (Figure 3B3) in a pattern similar to that seen in p63/Krt5p cells in Walthard cell nests (WCNs) in the adult tube (Figures 3B2 and 3B4). In vitro, the Krt5 and p63 immunopositive cells were superimposed, although the Krt5 staining index was higher (Supplementary Figure 2E ). Analysis of WCNs in tissue sections (Figure 3C) revealed a strikingly similar pattern of growth and differentiation, arising from either beneath Krt7p epithelial cells or in continuity with columnar epithelium typical of Type I SCOUTs. The result was a PAX2n/ALDH1n transitional-like outgrowth that was strongly Krt5p but stathminn (not shown). Taken in the context of the in vitro findings, this observation further linked the PAX2n immunophenotype to cell outgrowth and a Krt7p progenitor cell to the development of not only terminal (FOXJ1+) but also metaplastic (Krt5+) differentiation in the fallopian tube.
Altered gene expression in PAX2n proliferations (SCOUTs, STINs and HGSC)
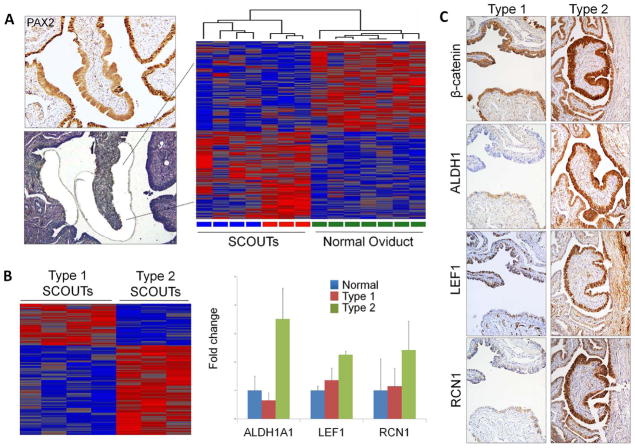
Supplementary Table 3 is a list of genes selected for analysis and found to be differentially expressed in SCOUTs relative to normal appearing epithelium. Arrays generated from RNA extracted from formalin-fixed laser-capture micro-dissected SCOUTs yielded differentially expressed genes, illustrated in the representative heat map (Figure 4A and B, Supplemental Figures 4&5). When stained with selected markers, Type 1 SCOUTs varied from strictly secretory to mixed secretory and ciliated and were ALDH1n, β-cateninmem and stained weakly or negative for LEF1, RCN1, RUNX2 and EZH2 (Figures 4C, Figure 5, and Supplemental Figure 3). Type 2 SCOUTs stained variably for ciliated cell differentiation and were β-cateninnc and ALDH1, LEF1, RCN1, EZH2, RUNX2 (not shown) and stathmin positive (Figure 4C and Figure 5 and Supplemental Figure 3). Basal cell differentiation, signifying WCN development, was associated with PAX2n columnar epithelium, suggesting this pathway of differentiation might initiate within Type I PAX2n SCOUTs.
Figure 4.
(A) Laser-captured microdissected SCOUTs (left) and a Heatmap comparison of SCOUTs and normal oviduct (right). (B) Arrays generated from PAX2n SCOUTs revealed genes differentially expressed across Type 1 and Type 2 SCOUTs, including ALDH1, LEF1 and RCN1 (right). (C) Coordinated expression of the above genes distinguish Type 1 SCOUTs, which show membraneous β-catenin localization and absent ALDH1 staining plus negative or weak staining for LEF1 and RCN1 staining (right) from Type 2 SCOUTs, with nuclear and cytoplasmic β-catenin, strong ALDH1, LEF1 and RCN1 staining.
Figure 5.
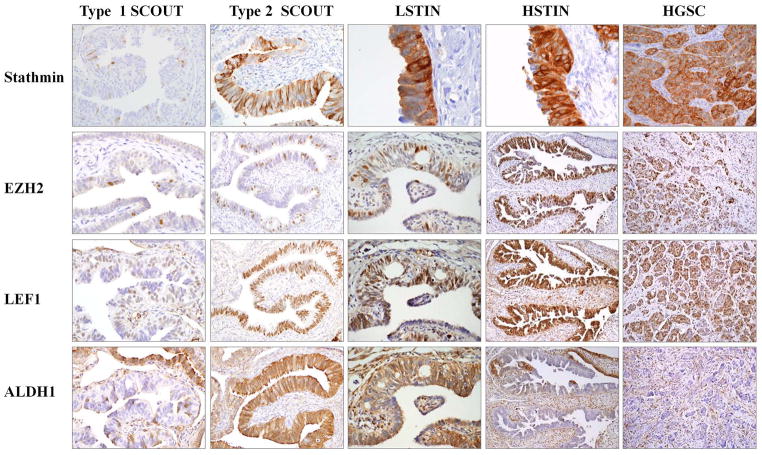
Shared expression of SCOUT markers with low (LSTIN) and high (HSTIN or STIC) grade serous tubal intraepithelial neoplasia and high-grade serous carcinoma (HGSC). Neoplasms (STINs, HGSCs) share with Type 1 SCOUTS loss of PAX2, ALDH1 and with Type 2 SCOUTs, increased LEF1, EZH2 and Stathmin and other markers (see text).
Figure 5 and Supplemental Figure 6 summarize the staining patterns observed in the different lesions. STINs and HGSCs shared expression of several markers with SCOUTs. Expression patterns for ALDH and β-catenin were identical to Type 1 SCOUTs (ALDHn and β-cateninmem). In addition, like Type 2 SCOUTs, there was increased staining for EZH2, Stathmin, LEF1, RCN, Krt5, RUNX2 (not shown). Not surprisingly, no marker in this group separated STINs or HGSCs from SCOUTs. This is in contrast to other published markers such as Ki67, Cyclin E, p16 and others, which are significantly more commonly expressed in STINS and HGSCs relative to benign fallopian tube mucosa. 4, 5, 15, 22
Discussion
Analysis of arrays generated from high-grade serous cancer has confirmed a transcriptome that parallels oviductal epithelium.23 Given that these tumors are strongly positive for biomarkers (such as PAX8) typically assigned to non-ciliated (so-called secretory) cells, the assumption has been that the secretory cell is the cell of origin.1 Levanon et al. showed that PAX8-expressing (secretory) cells of the tube were uniquely susceptible to DNA damage imposed by irradiation, a finding that parallels similar observations in latent precursors (p53 signatures) and STINs that contain p53 mutations.5, 24 However, with the discovery of PAX2n SCOUTs and a similar PAX2n expression pattern in many STINs, it became clear that there may be a relationship between the two entities, despite the fact that SCOUTs are more ubiquitous in the fallopian tube and do not arise in the setting of a DNA damage response and loss of p53 function. Although altered PAX2 expression has been focused on in neoplasia, we hypothesized that the PAX2n immunophenotype typified a “generic” series of molecular events that were the underpinning of stem cell expansion common to many proliferations.
We addressed PAX2 expression or loss in the fallopian tube from three perspectives. The first was by analyzing expression and differentiation in proliferating normal adult and fetal cells propagated in vitro. The second was by comparing the in vitro findings to expression in tissue sections from fetal and adult tubes. The third was to look for shared expression across PAX2n cells in cell proliferation and expansion (SCOUTs, STINs and HGSCs). We discovered that the PAX2n immunophenotype was particularly linked to in vitro and in vivo cell growth, not infrequently with an increase in EZH2 expression. Moreover, in highly clonogenic Krt7p/FOXj1n oviductal progenitor cells grown in vitro, we demonstrated for the first time that PAX2n expanding populations were capable of both ciliated (FOXJ1) and basal cell (Krt5) differentiation. This sequence of cell growth and differentiation was recapitulated in SCOUTs, STINs and HGSCs, with progressively reduced ciliated differentiation in the Type 2 SCOUTs, STINs and HGSCs. We thus concluded that all of these entities were related to a similar progenitor cell.
The next goal was to determine if the cells involved in benign and neoplastic outgrowth shared common expression patterns and we chose to use the least proliferative lesions (SCOUTs) as the reference. One advantage of this approach is to identify events that occur prior to the more dramatic molecular changes that characterize malignancy that may have profound influences on expression. The study delineated two general groups of SCOUTs; the first (Type 1) closely resembled normal tubal epithelium, histologically and in their expression profile (Figure 1D). The second (Type 2) was composed of proliferations with less pronounced ciliated differentiation, many noticeably “endometrial” like (Figure 1E). Accordingly, there was minimal difference in expression between Type 1 SCOUTs and control epithelium, although they were consistently ALDH1n. In contrast, type 2 SCOUTs demonstrated nuclear and cytoplasmic β-catenin staining plus increased BCL2 (see ref 7), ALDH1, and Krt5 staining. This diversity in phenotype underscores the complexity of cell growth and differentiation that can occur in the fallopian tubes with age. Type I SCOUTs appear to signify very minor genomic changes as evidenced by the similarities in transcription to normal controls. Thus, the alterations in transcription are limited to absence of ALDH1 expression. In contrast, Type 2 SCOUTs, which exhibit a more divergent histology, have a common biomarker signature—stathmin, EZH2, LEF1, RCN1 and RUNX2—that is more similar to premalignant (STIN) and malignant (HGSCs) entities in the tube (Figure 5).
A fundamental question stemming from the above observation is the relevance of the gene signature found in SCOUTs, STINs and HGSCs to both stem cell biology and neoplasia. ALDH1 has been identified as a marker of epithelial stem cells. Its expression can be both increased or absent, the latter more typical of STINs and HGSCs.25, 26 EZH2 is a polycomb suppressor that is implicated in stem cell maintenance and regulation of differentiation. It is noteworthy that EZH2 expression typically increased in areas of cell expansion, in keeping with the coordinated suppression of PAX2 expression. 27 EZH2 is also a potential suppressor of tumor suppressor genes.28 LEF1 is likewise expressed during lineage differentiation.29 The function of RCN1 is less clear but this gene product is a calcium binder that is weakly expressed in renal tubular cells and up-regulated in renal cell carcinomas.30 RUNX2 is a gene involved in morphogenesis and osteoblastic differentiation.31 Functions attributed to stathmin are multiple. It is a marker of P13 kinase activation that has been linked to serous neoplasia in some studies, tumor progression and metastases in others, and regulates p53 stability in still others.32, 33, 34 Its range of expression, including normal epithelium, SCOUTs and STINs, is similar to that of these other markers, several of which (ALDH1, PAX2, EZH2) have also been linked to not only stem cells but outcome or resistance to chemotherapy. 35, 36, 37 The significance of the unique β-catenin staining in Type 2 SCOUTs, with a shift in distribution from the membrane to cytoplasm and nucleus is unclear, but it is emblematic of Wnt pathway activation, and mutations in β-catenin are commonly found in endometrial and colon carcinomas.38
Walthard cell nests are a common benign condition seen in the distal fallopian tube mucosa or the adjacent peritoneal reflection.19 They bear a close resemblance to the cervical squamo-columnar junction where columnar cells are undermined by p63-positive basal cells. These cells could be envisioned to either originate from the columnar epithelium or give rise to the overlying Krt7-positive epithelial cells. This study has made two novel observations. First, based on the matrigel cell culture data, the basal cells emerge from the Krt7-positive columnar cells. Second, this process is marked by not only loss of PAX2 and but also ALDH1 expression, similar to that seen in Type 1 SCOUTs. The initiating cell, the Krt7p non-ciliated epithelial cell, is remarkably similar to the cells seen in the SC junction of the cervix from which squamous metaplasia is derived and this process is similar to so-called “top-down” differentiation reported in the squamocolumnar junction.21 The fact that WCNs are not considered direct precursors to malignancy is not surprising, in as much as they are terminally differentiated relative to their progenitors. This is similar to the cervix, where the progenitor cells in the SC junction are considered more vulnerable to neoplastic transformation than their metaplastic progeny.21 What is interesting is the fact that WCNs underscore the existence of multi-potential cells in the distal fallopian tube.12 Given that 40–60 percent of HGSCs do not have a documented source (or STIN) in the fallopian tube mucosa, coupled with the fact that a subset of HGSCs are strongly Krt5 positive, the possibility that cells involved in alternate differentiation pathways might contribute to a subset of these malignancies deserves further study (Hanamornroongruang S, Howitt BE, Crum CP, unpublished).5, 25
Epithelia in virtually every organ (breast being a prime example) display a wide range of clonal expansions, some of which may be direct precursors to malignancy and others of which serve as risk factors for a malignant outcome. The model depicted in Figure 6 reflects a similar but novel scenario in the oviduct, with multiple categories of putative monoclonal cell outgrowth and striking similarities in expression across multiple genes between surrogate precursors and lesions that are considered premalignant or pre-metastatic. These findings emphasize the complexity of molecular and phenotypic perturbations that can take place in the fallopian tubes during and following menopause. This complexity invites caution when considering the role (or diagnostic value) of newly discovered biomarkers as specific indicators of neoplasia. More importantly, it reveals a consistent disturbance in progenitor cell biology in keeping with a common pathway that is triggered by more than one initiating event. Thus, it introduces two approaches to cancer prevention, one directed at the initiating event and the other at the early perturbations in the pathway.
Figure 6.
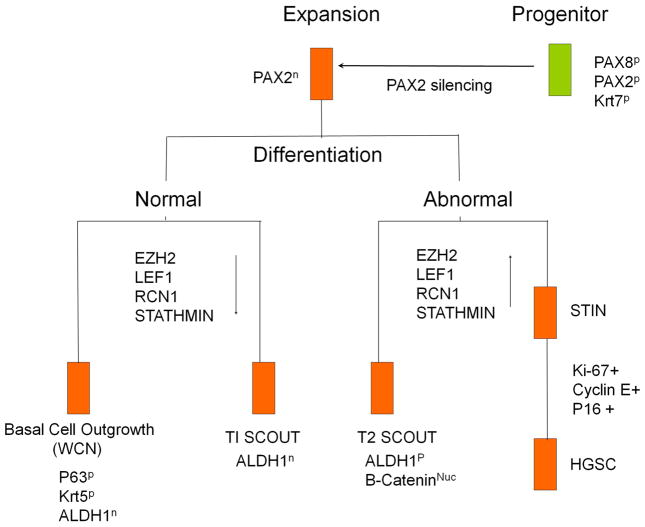
A progenitor cell model for the fallopian tube in which Krt7 identifies the progenitor cell and PAX2n defines progenitor cell expansion. Expanding PAX2n cells can differentiate into basal or ciliated cells in WCNs or Type 1 SCOUTs, both of which approximate normal differentiation pathways, with loss of ALDH1 and normal or minimally increased expression of LEF1, RCN1, Stathmin and EZH2. In contrast, Type 2 SCOUTs and STINs (right) share a different expression signature characterized by multiple genes, including EZH2, LEF1, RCN1, and stathmin and others involved in a divergent pathway of progenitor cell growth.
Supplementary Material
Supplemental Figure 1. Pax2 and Aceltyl-alpha-Tubulin (cilia) expression in fetal and adult fallopian tube. Aceltyl-alpha-Tubulin (green) indicates ciliated cell differentiation. PAX2 (red) indicates non-ciliated cells. Dapi stains nuclei (blue).
Supplemental Figure 2. In vitro differentiation of oviduct progenitor cells. (A) Proliferating oviduct progenitor cells expressed EZH2 (Green). (B) Time course images of fetal (20 week) oviductal progenitor cells differentiated in an air-liquid interface culture system. Aceltyl-alpha-Tubulin (green) indicates ciliated cell differentiation. Dapi stains nuclei (blue). (C) Representative image of differentiated structures at day 10 in ALI culture show the presence of ciliated cells that are stained positively with FOXJ1 (red), acetyl-alpha-tubulin (green) (lower panel) and non-ciliated cells labeled with PAX2 (red, upper panel). (D, E) Ciliated and basal (transitional) differentiation in Matrigel in vitro. Acetyl-alpha-tubulin (green) and FOXJ1 (red) positive ciliated cells and Krt5 (red)/p63 (green) positive metaplastic cells co-existed in the 3D Matrigel culture system.
Supplemental Figure 3. Two types of PAX2n SCOUTs are distinguished in these panels with β-Catenin, keratin 5 and LEF1 staining. PAX8 and tubulin staining highlight non-ciliated and ciliated cells respectively. Type 1 SCOUTs are β-cateninmem, LEF1n and Krt5 n. and are similarly non-ciliated (A) and ciliated (B). Type 2 are β-catenin nc and LEF1p and express Krt5, with few (A) or abundant (B) ciliated cells.
Supplemental Figure 4. Heat-map comparing Type 1 and Type 2 SCOUTs with normal tubal epithelium and high-grade serous cancer. ALDH1A1, RCN1 and LEF1, expressed in Type 2 SCOUTs, are highlighted.
Supplemental Figure 5. A depiction, in tabular (A) and graphical format (B) of genes up-regulated with the two-fold change in Type 2 relative to Type 1 SCOUTs, several of which have been linked to STIN and HGSC.
Supplemental Figure 6. H&E, p53 and PAX2 staining of cases under study (see Figure 5).
Acknowledgments
This work was supported by grants from the Department of Defense and National Cancer Institute (W81XWH-10-1-0289 and 5R21CA173190-02 to C. Crum). The authors thank the Division of Gynecologic Oncology at Brigham and Women’s Hospital and Dana Farber Cancer Institute for their contribution to the study, and Mei Zheng for assistance with the immunohistochemistry. We are also grateful for the support of this work by the Genome Institute of Singapore of the Agency for Science, Technology and Research and Bedside and Bench Grant from Singapore National Medical Research Council.
Abbreviations
- STIN
serous tubal intraepithelial neoplasia
- SCOUT
secretory cell outgrowth
- ALI
air-liquid interface culture
- WCN
Walthard cell nest
Footnotes
The authors declare that they have no conflicts of interest.
Author contributions
Contributions of the coauthors to Design (1), Data collection (2), Data analysis (3), Data interpretation (4), Literature search (5), Figures (6) and Manuscript writing (7) were as follows: Ning (1,2,3,4,6,7); Bijron (1,2,3,4,6,7); Yamamoto (1,2,3,6); Wang (2,3); Howitt (1,2,4,5); Herfs (1,2,3,4); Yang (1,2,3,5); Hong (2,3,4); Cornille (2,3,4); Wu(2,3,4); Hanamor (2,3,4); McKeon (1,4,7); Crum (1,4,7); Xian (1,3,4,7).
References
- 1.Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes nprophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–6. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 2.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serousc arcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 3.Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58–64. doi: 10.1016/j.ygyno.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. Erratum in: J Pathol 2007, 213, 116. [DOI] [PubMed] [Google Scholar]
- 5.Crum CP, Herfs M, Ning G, et al. Through the glass darkly: intraepithelial neoplasia, top-down differentiation and the road to ovarian cancer. J Pathol. 2013;231:402–12. doi: 10.1002/path.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson JW, Miron A, Jarboe EA, et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol. 2008;26:4160–5. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen EY, Mehra K, Mehrad M, et al. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the Fallopian tube. J Pathol. 2010;222:110–6. doi: 10.1002/path.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quick CM, Ning G, Bijron J, et al. PAX2-null secretory cell outgrowths in the oviduct and their relationship to pelvic serous cancer. Mod Pathol. 2012;25:449–55. doi: 10.1038/modpathol.2011.175. [DOI] [PubMed] [Google Scholar]
- 9.Bijron JG, Ning G, Laury AR, et al. Digital quantification of precursor frequency in the fallopian tube and its significance. Mod Pathol. 2012;25:1654–61. doi: 10.1038/modpathol.2012.100. [DOI] [PubMed] [Google Scholar]
- 10.Mehrad M, Ning G, Chen EY, et al. A pathologist’s road map to benign, precancerous, and malignant intraepithelial proliferations in the fallopian tube. Adv Anat Pathol. 2010;17:293–302. doi: 10.1097/PAP.0b013e3181ecdee1. [DOI] [PubMed] [Google Scholar]
- 11.Kumar PA1, Hu Y, Yamamoto Y, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–38. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laury AR, Ning G, Quick CM, et al. Fallopian tube correlates of ovarian serous borderline tumors. Am J Surg Pathol. 2011;35:1759–65. doi: 10.1097/PAS.0b013e318233b0f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vang R, Visvanathan K, Gross A, et al. Validation of an algorithm for the diagnosis of serous tubal intraepithelial carcinoma. Int J Gynecol Pathol. 2012;31:243–53. doi: 10.1097/PGP.0b013e31823b8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ning G, Bijron JG, Yuan J, et al. Differential expression of p-ERM, a marker of cell polarity, in benign and neoplastic oviductal epithelium. Int J Gynecol Pathol. 2013;32:345–52. doi: 10.1097/PGP.0b013e31826feee2. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Nelson G, Duan Q, et al. Precursor lesions and prognostic factors in primary peritoneal serous carcinoma. Int J Gynecol Pathol. 2013;32:547–55. doi: 10.1097/PGP.0b013e31827f3fa8. [DOI] [PubMed] [Google Scholar]
- 16.Wethington SL, Park KJ, Soslow RA, et al. Clinical outcome of isolated serous tubal intraepithelial carcinomas (STIC) Int J Gynecol Cancer. 2013;23:1603–11. doi: 10.1097/IGC.0b013e3182a80ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowell CB, Swisher EM, Cass I, et al. Long term follow up of BRCA1 and BRCA2 mutation carriers with unsuspected neoplasia identified at risk reducing salpingo-oophorectomy. Gynecol Oncol. 2013;129:364–71. doi: 10.1016/j.ygyno.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conner JR, Meserve E, Pizer E, et al. Outcome of unexpected adnexal neoplasia discovered during risk reduction salpingo-oophorectomy in women with germ-line BRCA1 or BRCA2 mutations. Gynecol Oncol. 2014;132:280–6. doi: 10.1016/j.ygyno.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seidman JD, Yemelyanova A, Zaino RJ, et al. The fallopian tube-peritoneal junction: a potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30:4–11. doi: 10.1097/PGP.0b013e3181f29d2a. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Ouyang H, Yamamoto Y, et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell. 2011;145:1023–35. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herfs M, Yamamoto Y, Laury A, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. 2012;109:10516–21. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sehdev AS, Kurman RJ, Kuhn E, et al. Serous tubal intraepithelial carcinoma upregulates markers associated with high-grade serous carcinomas including Rsf-1 (HBXAP), cyclin E and fatty acid synthase. Mod Pathol. 2010;23:844–55. doi: 10.1038/modpathol.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marquez RT, Baggerly KA, Patterson AP, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. KH Clin Cancer Res. 2005;11:6116–26. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]
- 24.Levanon K, Ng V, Piao HY, Zhang Y, et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–13. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng S, Yang X, Lassus H, et al. Distinct Expression Levels and Patterns of Stem Cell Marker, Aldehyde Dehydrogenase Isoform 1 (ALDH1), in Human Epithelial Cancers. PLoS One. 2010;5:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flesken-Nikitin A, Hwang CI, et al. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495:241–5. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou Ruey-Hwang, Yu Yung-Luen, Hung Mien-Chie. The roles of EZH2 in cell lineage commitment. Am J Transl Res. 2011;3:243–250. [PMC free article] [PubMed] [Google Scholar]
- 28.Deb G, Singh AK, Gupta S. EZH2: Not EZHY (Easy) to Dea. Mol Cancer Res. 2014;12:639–53. doi: 10.1158/1541-7786.MCR-13-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merrill BJ1, Gat U, DasGupta R, et al. cf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giribaldi G1, Barbero G, Mandili G, et al. Proteomics. Proteomic identification of Reticulocalbin 1 as potential tumor marker in renal cell carcinoma. J Proteomics. 2013;91:385–92. doi: 10.1016/j.jprot.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida T, Kanegane H, Osato M, et al. Functional analysis of RUNX2 mutations in Japanese patients with cleidocranial dysplasia demonstrates novel genotype-phenotype correlations. Am J Hum Genet. 2002;71:724–38. doi: 10.1086/342717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karst AM, Levanon K, Duraisamy S, et al. Stathmin 1, a marker of PI3K pathway activation and regulator of microtubule dynamics, is expressed in early pelvic serous carcinomas. Gynecol Oncol. 2011;123:5–12. doi: 10.1016/j.ygyno.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng P1, Liu YX, Chen L, et al. Stathmin, a new target of PRL-3 identified by proteomic methods, plays a key role in progression and metastasis of colorectal cancer. J Proteome Res. 2010;9:4897–905. doi: 10.1021/pr100712t. [DOI] [PubMed] [Google Scholar]
- 34.Sonego M, Schiappacassi M, Lovisa S, et al. Stathmin regulates mutant p53 stability and transcriptional activity in ovarian cancer. EMBO Mol Med. 2014;6:295. doi: 10.1002/emmm.201201504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hueber PA1, Waters P, Clark P, et al. PAX2 inactivation enhances cisplatin-induced apoptosis in renal carcinoma cells. Kidney Int. 2006;69:1139–45. doi: 10.1038/sj.ki.5000136. [DOI] [PubMed] [Google Scholar]
- 36.Han X, DUF, Jiang L, Zhu Y, et al. A2780 human ovarian cancer cells with acquired paclitaxel resistance display cancer stem cell properties. Oncol Lett. 2013;6:1295–1298. doi: 10.3892/ol.2013.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzo S, Hersey JM, Mellor P, et al. Ovarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2. Mol Cancer Ther. 2011;10:325–35. doi: 10.1158/1535-7163.MCT-10-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Zee M, Jia Y, Wang Y, et al. Alterations in Wnt-β-catenin and Pten signalling play distinct roles in endometrial cancer initiation and progression. J Pathol. 2013;230:48–58. doi: 10.1002/path.4160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Pax2 and Aceltyl-alpha-Tubulin (cilia) expression in fetal and adult fallopian tube. Aceltyl-alpha-Tubulin (green) indicates ciliated cell differentiation. PAX2 (red) indicates non-ciliated cells. Dapi stains nuclei (blue).
Supplemental Figure 2. In vitro differentiation of oviduct progenitor cells. (A) Proliferating oviduct progenitor cells expressed EZH2 (Green). (B) Time course images of fetal (20 week) oviductal progenitor cells differentiated in an air-liquid interface culture system. Aceltyl-alpha-Tubulin (green) indicates ciliated cell differentiation. Dapi stains nuclei (blue). (C) Representative image of differentiated structures at day 10 in ALI culture show the presence of ciliated cells that are stained positively with FOXJ1 (red), acetyl-alpha-tubulin (green) (lower panel) and non-ciliated cells labeled with PAX2 (red, upper panel). (D, E) Ciliated and basal (transitional) differentiation in Matrigel in vitro. Acetyl-alpha-tubulin (green) and FOXJ1 (red) positive ciliated cells and Krt5 (red)/p63 (green) positive metaplastic cells co-existed in the 3D Matrigel culture system.
Supplemental Figure 3. Two types of PAX2n SCOUTs are distinguished in these panels with β-Catenin, keratin 5 and LEF1 staining. PAX8 and tubulin staining highlight non-ciliated and ciliated cells respectively. Type 1 SCOUTs are β-cateninmem, LEF1n and Krt5 n. and are similarly non-ciliated (A) and ciliated (B). Type 2 are β-catenin nc and LEF1p and express Krt5, with few (A) or abundant (B) ciliated cells.
Supplemental Figure 4. Heat-map comparing Type 1 and Type 2 SCOUTs with normal tubal epithelium and high-grade serous cancer. ALDH1A1, RCN1 and LEF1, expressed in Type 2 SCOUTs, are highlighted.
Supplemental Figure 5. A depiction, in tabular (A) and graphical format (B) of genes up-regulated with the two-fold change in Type 2 relative to Type 1 SCOUTs, several of which have been linked to STIN and HGSC.
Supplemental Figure 6. H&E, p53 and PAX2 staining of cases under study (see Figure 5).