Abstract
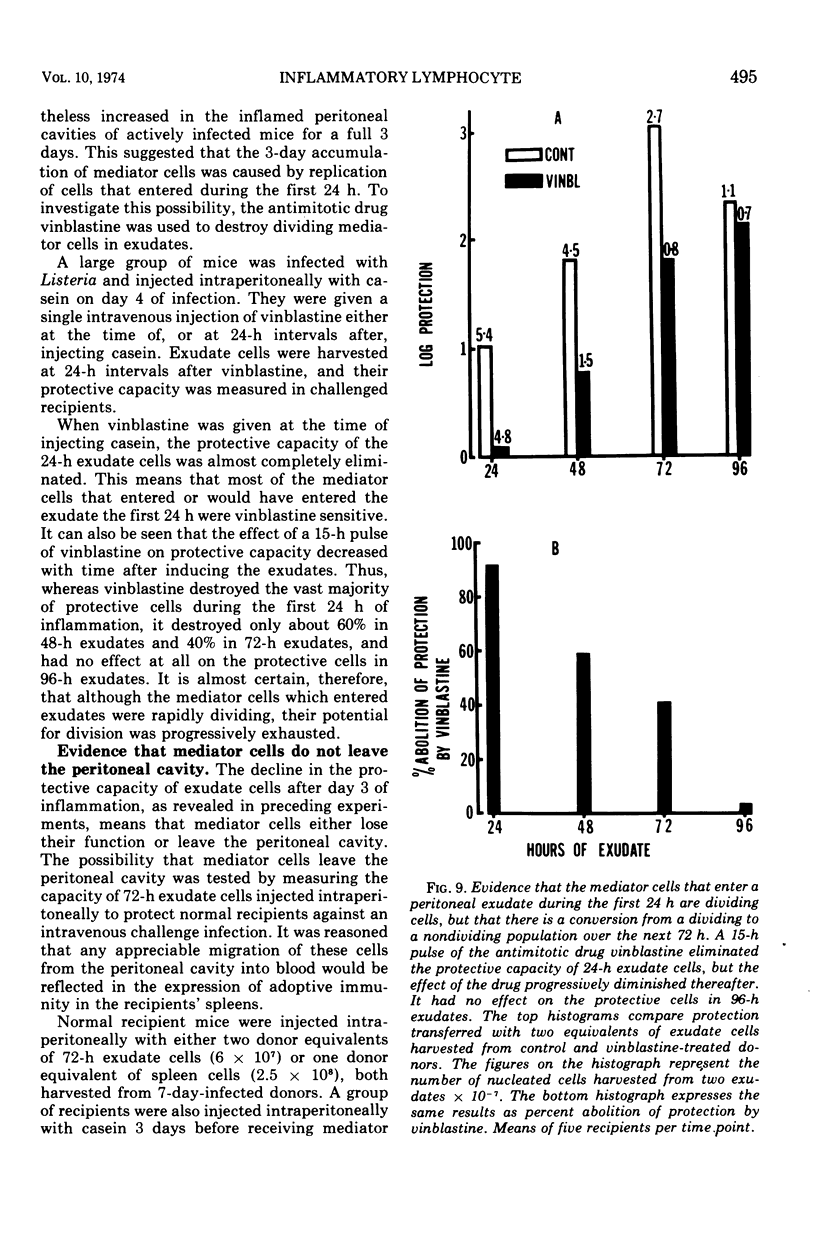
The lymphocytes which mediate immunity to infection with Listeria monocytogenes in the mouse accumulated in casein-induced peritoneal exudates. They were T cells, as evidenced by their susceptibility to anti-θ serum, but some were also destroyed by anti-immunoglobulin serum. For a given number of cells, exudate cells were at least six times more efficient than spleen cells in protecting normal recipients against lethal challenge. The extent to which mediator cells accumulated in exudates was found to be governed by the level of their production in responding lymphoid tissue and by the time available for them to migrate into an exudate. An intraperitoneal injection of casein at any stage of infection resulted in a progressive accumulation of mediator cells that continued for 3 days. The accumulation was not caused by continuous entry of cells during the whole of this period, but resulted from division of a limited number of cells that entered during the first 24 h. Accumulation of mediator cells in an exudate was associated with the conversion of a population of dividing cells into a population of nondividing T cells with a relatively short life-span.
Full text
PDF

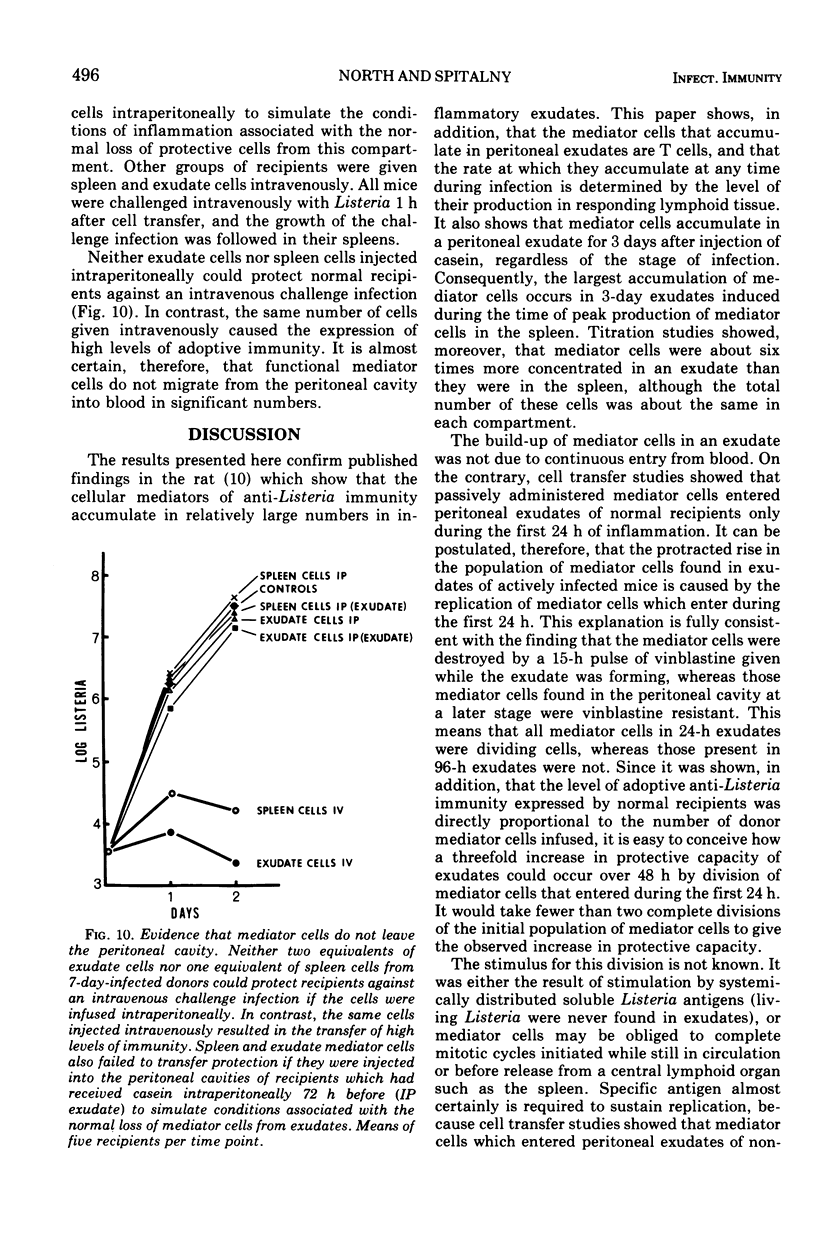


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Allwood G. G. Inflammatory lymphoid cells. Cells in immunized lymph nodes that move to sites of inflammation. Immunology. 1972 Mar;22(3):493–502. [PMC free article] [PubMed] [Google Scholar]
- Biesecker J. L. Cellular and humoral immunity after allogeneic transplantation in the rat. I. Cellular and humoral immunity of measured by a 51 Cr cytotoxicity assay after allogeneic tumor and renal transplantation. Transplantation. 1973 Mar;15(3):298–307. doi: 10.1097/00007890-197303000-00006. [DOI] [PubMed] [Google Scholar]
- Canty T. G., Wunderlich J. R. Quantitative assessment of cellular and humoral responses to skin and tumor allografts. Transplantation. 1971 Feb;11(2):111–116. doi: 10.1097/00007890-197102000-00001. [DOI] [PubMed] [Google Scholar]
- Cheers C., Sprent J., Miller J. F. Interaction of thymus lymphocytes with histoincompatible cells. IV. Mixed lymphocyte reactions of activated thymus lymphocytes. Cell Immunol. 1974 Jan;10(1):57–67. doi: 10.1016/0008-8749(74)90151-8. [DOI] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Cone R. E., Marchalonis J. J. Antigen binding specificity of cell surface immunoglobulin isolated from T (helper) cells. Aust J Exp Biol Med Sci. 1973 Oct;51(5):689–700. doi: 10.1038/icb.1973.64. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., McGREGOR D. D., COWEN D. M. Initiation of immune responses by small lymphocytes. Nature. 1962 Nov 17;196:651–655. doi: 10.1038/196651a0. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Cogen R. B. Immunoglobulin molecules on the surface of activated T lymphocytes in the rat. J Exp Med. 1973 Jul 1;138(1):163–175. doi: 10.1084/jem.138.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. G. Studies of the cells in the afferent and efferent lymph of lymph nodes draining the site of skin homografts. J Exp Med. 1967 May 1;125(5):737–754. doi: 10.1084/jem.125.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance E. M., Cooper S. Homing of specifically sensitized lymphocytes to allografts of skin. Cell Immunol. 1972 Sep;5(1):66–73. doi: 10.1016/0008-8749(72)90084-6. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHISON N. A. Passive transfer of transplantation immunity. Proc R Soc Lond B Biol Sci. 1954 Feb 18;142(906):72–87. doi: 10.1098/rspb.1954.0007. [DOI] [PubMed] [Google Scholar]
- McGregor D. D., Logie P. S. The mediator of cellular immunity. VI. Effect of the antimitotic drug vinblastine on the mediator of cellular resistance to infection. J Exp Med. 1973 Mar 1;137(3):660–674. doi: 10.1084/jem.137.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Mackaness G. B. Immunological control of macrophage proliferation in vivo. Infect Immun. 1973 Jul;8(1):68–73. doi: 10.1128/iai.8.1.68-73.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER K. A., CALNE R. Y. Origin of the infiltrating cells in skin and kidney homografts. Transplant Bull. 1960 Oct;26:458–464. doi: 10.1097/00006534-196010000-00041. [DOI] [PubMed] [Google Scholar]
- PRENDERGAST R. A. CELLULAR SPECIFICITY IN THE HOMOGRAFT REACTION. J Exp Med. 1964 Mar 1;119:377–388. doi: 10.1084/jem.119.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelants G. E., Rydén A., Hägg L. B., Loor F. Active synthesis of immunoglobulin receptors for antigen by T lymphocytes. Nature. 1974 Jan 11;247(5436):106–108. doi: 10.1038/247106a0. [DOI] [PubMed] [Google Scholar]
- Sprent J., Miller J. F. Interaction of thymus lymphocytes with histoincompatible cells. 3. Immunological characteristics of recirculating lymphocytes derived from activated thymus cells. Cell Immunol. 1972 Feb;3(2):213–230. doi: 10.1016/0008-8749(72)90161-x. [DOI] [PubMed] [Google Scholar]
- Tilney N. L., Ford W. L. The migration of rat lymphoid cells into skin grafts. Some sensitised cells localise preferentially in specific allografts. Transplantation. 1974 Jan 1;17(1):12–21. doi: 10.1097/00007890-197401000-00004. [DOI] [PubMed] [Google Scholar]
- Valeriote F. A., Bruce W. R. An in vitro assay for growth-inhibiting activity of vinblastine. J Natl Cancer Inst. 1965 Nov;35(5):851–856. [PubMed] [Google Scholar]
- Werdelin O., McCluskey R. T. The nature and the specificity of mononuclear cells in experimental autoimmune inflammations and the mechanisms leading to their accumulation. J Exp Med. 1971 Jun 1;133(6):1242–1263. doi: 10.1084/jem.133.6.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]


