Abstract
AIM: To investigate the role of nuclear factor κB (NF-κB) in the regulation of Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) in EBV transformed cells.
METHODS: LMP1 expression was examined in EBV transformed human B lymphocytes with modulation of NF-κB activity.
RESULTS: EBV infection is associated with several human cancers. EBV LMP1 is required for efficient transformation of adult primary B cells in vitro, and is expressed in several pathogenic stages of EBV-associated cancers. Regulation of EBV LMP1 involves both viral and cellular factors. LMP1 activates NF-κB signaling pathway that is a part of the EBV transformation program. However, the relation between NF-κB and LMP1 expression is not well established yet. In this report, we found that blocking the NF-κB activity by Inhibitor of κB stimulated LMP1 expression, while the overexpression of NF-κB repressed LMP1 expression in EBV-transformed IB4 cells. In addition, LMP1 repressed its own promoter activities in reporter assays, and the repression was associated with the activation of NF-κB. Moreover, NF-κB alone is sufficient to repress LMP1 promoter activities.
CONCLUSION: Our data suggest LMP1 may repress its own expression through NF-κB in EBV transformed cells and shed a light on LMP1 regulation during EBV transformation.
Keywords: Nuclear factor κB, Epstein-Barr virus, Latent membrane protein 1, Latency, Transformation
Core tip: We find a classical feedback inhibition of Epstein Barr virus (EBV) Latent membrane protein 1 (LMP1) and nuclear factor κB (NF-κB): LMP1 activates NF-κB, and NF-κB inhibits LMP1 expression. The regulatory loop may benefit EBV transformation processes.
INTRODUCTION
Epstein-Barr virus (EBV) is a human-herpesvirus that infects most humans without causing an obvious disease. However, EBV is associated with nasopharyngeal carcinoma, Hodgkin’s lymphoma, Burkitt’s lymphoma, post-transplantation lymphoproliferative diseases, and central nervous system lymphoma in certain healthy and immune-compromised hosts[1-3].
EBV transforms adult primary B cells into continually growing lymphoblastoid cell lines (LCLs) and concomitantly establishes type III latency in vitro[1]. Nine viral proteins are expressed, including six nuclear proteins (EBNA1, -2, -3A, -3B, -3C, and -LP) and three integral latent membrane proteins (LMP1, -2A, and -2B) plus EBV-encoded RNAs[3,4].
EBV LMP1 is an integral membrane protein and acts as a constitutively active, receptor-like molecule[5]. LMP1 is required for the efficient transformation of primary B cells in vitro[6-8]. Also, LMP1 is able to transform rodent fibroblasts[9-11]. In addition, LMP1 seems to be a central effector of altered cell growth, survival, adhesive, invasive, and antiviral potential in EBV transformed cells[12-18].
The nuclear factor κB (NF-κB) molecule plays a pivotal role in regulating a variety of biological processes, such as immunity, cell survival, and proliferation[19,20]. The NF-κB pathway can be regulated by many stimuli, and its activity is tightly controlled to ensure a transient response to infection or other stimuli. NF-κB transcription factors are homodimers or heterodimers of REL homology domain proteins p50, p52, RelA, RelB, or cREL[19,20]. LMP1 activates both canonical and noncanonical NF-κB pathways by the use of cellular signaling proteins, such as tumor necrosis factor-receptor-associated factors[21]. The functional importance of NF-κB is exemplified by the fact that the blockade of NF-κB triggers apoptosis of EBV-transformed lymphoblastoid cells[22].
Previously, there are several conflicting reports about the relation between NF-κB and LMP1[23-25]. During our study on NF-κB and LMP1, we found that NF-κB is likely to be the negative regulator for the LMP1 expression in EBV-transformed cells. LMP1 may negatively regulate its own expression through NF-κB, which is a classical feedback loop. Our results may provide an insight in NF-κB’s role in viral transformation and add the complexity of viral gene regulation and their relation to transformation.
MATERIALS AND METHODS
Plasmids, antibodies, and reporter assays
Expression plasmids of LMP1 and its signaling defective mutant, LMP-DM, were described previously[26]. The NF-κB expression plasmids (p65 and p50), and NF-κB reporter construct with two consensus recognition sites were gifts from Albert Baldwin. pCMV-beta-gal and renilla luciferase expression plasmids were purchased from Clontech. CD4 expression plasmid was provided by Dr. Jenney Ting. LMP1 promoter reporter construct, LMP-ISRE-luc and LMP-GAS-luc were described previously[27]. LMP1 (CS1-4) antibody was purchased from Dako. Glyceraldehyde-3-phosphate dehydrogenase (0411) and inhibitor of κB (IκB) (SC-371) antibodies were purchased from Santa Cruz Biotechnology. Tubulin antibody was purchased from Sigma (T6557). The luciferase and beta-galactosidase assays were performed by standard methods[27,28].
Cell culture, transfection, and inducible expression of IκBα
The IB4 cell line was an EBV-transformed B cell line with type III latency and were maintained in RPMI 1640 plus 10% fetal bovine serum (FBS). 293T cells were human fibroblast cell line. The cells were maintained in Dulbecco’s Modified Eagle’s Medium plus 10% FBS. The inducible IκB expression IB4 line was the gift from Cahir-McFarland et al[22] and were maintained in RPMI160 + 10% FBS plus 1 μg/mL tetracycline. For the induction of IκBα, cells were washed three times with RPMI without tetracycline and suspended in the media with or without tetracycline at a concentration of 105 cells per milliliter as described[22]. Cells were analyzed within 24 h after the initial inductions. The Electroporation (320 V; 925 μF) was used for the transfection of IB4 cells as described previously[11,29-31]. For transfection of 293T cells, the attractene transfection reagent (Qiagen) was used following manufacturers recommendations.
Isolation of CD4 positive cells
Enrichment for CD-4-positive cells was performed with the use of anti-CD-4-antibody conjugated to magnetic beads according to the manufacturer’s recommendation (Dynal, Inc.). IB4 cells were transfected with CD-4 expression and other plasmids. One day after the transfection, the cells were used for isolation of CD-4-positive cells with the use of Dynabeads CD4 (Dynal, Inc.) The transfected cells were incubated with Dynabeads CD4 at 72 μL of beads/107 cells for 15-30 min at room temperature with gentle rotation. CD4-positive cells were isolated by placing the test tube in a magnetic separation device (Dynal magnet). The supernatant were discarded while the CD4-positive cells were attached to the beads. The CD4-cells-attached beads were washed 3 times in phosphate-buffered saline plus 2% FBS. The isolated cells were used to prepare cell lysates immediately. Total time for isolation was approximately 30-40 min. No tetracycline was used in the process.
Western blot analysis with enhanced chemiluminescence
Separation of proteins on sodium dodecyl sulfate-polyacrylamide gel electrophoresis was carried out following standard protocol. After the proteins were transferred to a nitrocellulose or Immobilon membrane, the membrane was blocked with 5% nonfat dry milk in TBST (50 mmol/L Tris-HCl pH 7.5, 200 mmol/L NaCl, 0.05% Tween-20) at room temperature for 10 min. It was then washed briefly with TBST, and incubated with the primary antibody in 5% milk in TBST for 1 h at room temperature, or overnight at 4 °C. After washing with TBST three times (10 min each), the membrane was incubated with the secondary antibody at room temperature for 1 h. It was then washed three times with TBST, treated with enhanced chemiluminescence detection reagents, and exposed to Kodak XAR-5 film.
Statistical analysis
Student t tests between groups of data were carried out with the use of Excell 2013. The statistically significant differences between the indicated samples were assessed by P values.
RESULTS
Inhibition of NF-κB increased the expression of LMP1
To determine the role of NF-κB in LMP1 expression in EBV-transformed cells, we used an EBV-transformed LCL (IB4 cell line) in which expression of a degradation-resistant mutant IκBa that was regulated by tetracycline (Tet)[22]. We found that LMP1 was induced upon Tet removal (Figure 1). Moreover, the potential correlation between the induction of IκB and the expression of LMP1 was examined. Within six hours after culture in media lacking tetracycline (Tet-media), IκBa was induced to stable levels (Figure 1; Panel B), however at three hours post removal of Tet, the IκBa induction was not consistently detected (data not shown). Increase in IκBa expression was also detected during 6-24 h after induction. The endogenous LMP1 was associated with Tet-removal and IκBα inductions (Figure 1). Therefore, LMP1 is increased upon IκBa induction in IB4 cells.
Figure 1.
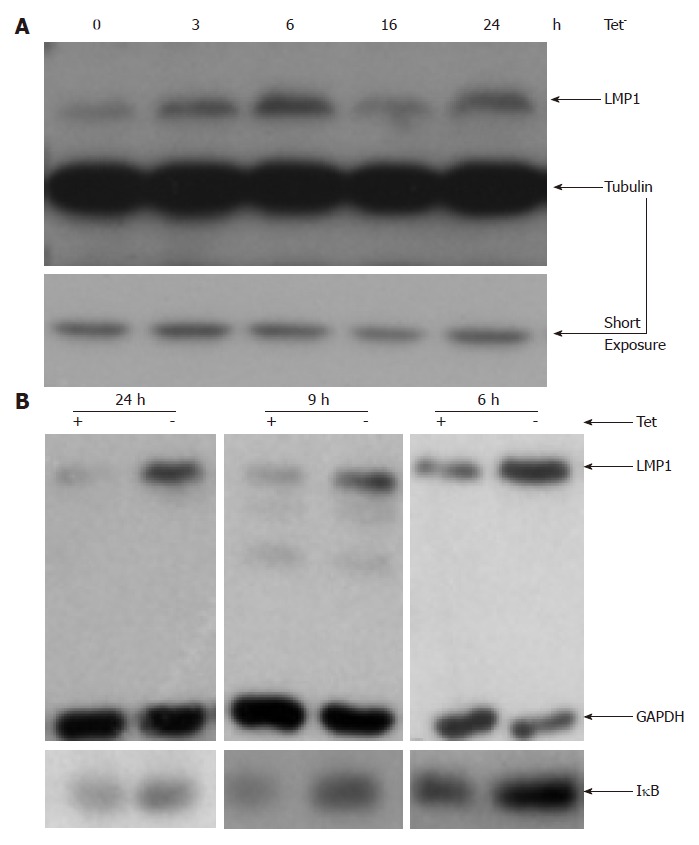
Blockage of nuclear factor-κB increases the expression of latent membrane protein 1 in Epstein-Barr virus-transformed cells. Inducible IκB-expression IB4 line were washed three times with fresh RPMI1640 medium, and re-suspended in tetracycline (Tet) plus or minus medium. Cells were isolated at indicated time, and used for Western blot analysis with indicated antibodies. The expression of latent membrane protein 1 (LMP1) was shown in (A) and the expression of IκB was shown in (B). GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Overexpression of NF-κB decreased the expression of LMP1
Next, we tested whether the activation of NF-κB itself would affect the expression of endogenous LMP1 in EBV transformed cells. NF-κB can be activated by many stimuli, however, the specificity of the treatment might vary significantly. Therefore, we chose to use the ectopic expression of NF-κB, or p65 and p50 simultaneous, in IB4 cells. The reason to choose IB4 as it is a parental line for the inducible IκB line. IB4 cells were transfected with the expression plasmids p65 and p50 at 1:1 ratio, and the transfected cells were enriched by CD4 selection (see “Materials and Methods” for detail). No tetracycline was involved as plain IB4 cells are used. Western blot analyses were used for detection of the expression of LMP1 in enriched transfected cells. The NF-κB activity was increased and LMP1 was reduced in the NF-κB-transfected IB4 cells (Figure 2). Therefore, the endogenous LMP1 is reduced upon NF-κB activation in EBV-transformed cells.
Figure 2.
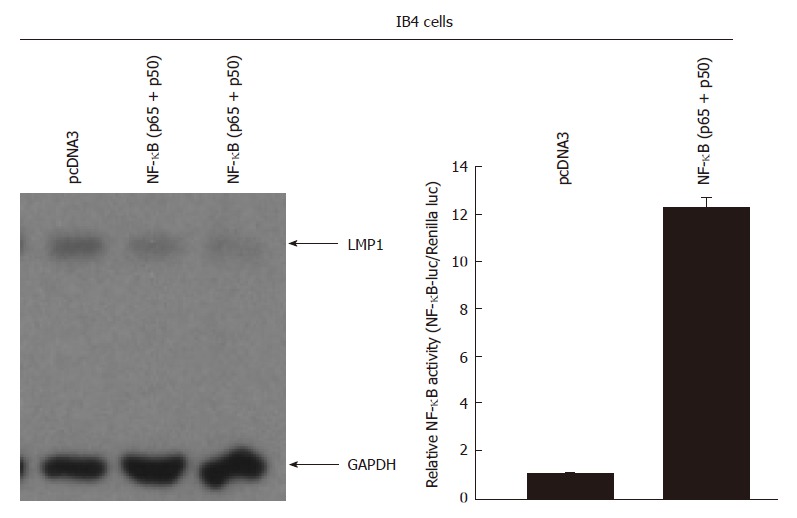
Overexpression of nuclear factor-κB decreases the expression of latent membrane protein 1 in Epstein-Barr virus-transformed cells. IB4 cells were transfected with CD4 expression plasmid along with pcDNA3 (vector) or nuclear factor κB (NF-κB) expression plasmids (p50 and p65 expression plasmids at 1:1 ratio). One day after the transfection, the transfected cells were enriched using the CD4 magnetic beads, and the cell lysates were used for Western blot analysis. The identity of the proteins is as shown. The right panel: IB4 cells were transfected with the indicated plasmid as shown at the top along with NF-κB specific reporter construct and Renilla luciferase reporter plasmid. One day later, luciferase and Renilla luciferase activities were measured. Relative promoter reporter activities are shown. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; LMP1: Latent membrane protein-1.
LMP1 represses its own promoter activity
It is well established that there is one functional NF-κB recognition site in LMP1 promoter and NF-κB binds to the site in the LMP1 promoter[23-25]. In addition, LMP1 activates NF-κB pathway through at least two independent domains[32]. We examined if LMP1 could repress the LMP1 promoter reporter constructs (Figure 3A). LMP-DM is an expression plasmid that has mutations in two functional domains of LMP1 for NF-κB activation[26]. The promoter reporter constructs and LMP1 or LMP-DM expression plasmid were co-transfected into 293T cells and the reporter activities were measured. As shown in Figure 3B, LMP1 was able to repress the LMP1 promoter reporter constructs. However, LMP-DM failed to repress the same reporter constructs, which correlated with that data that LMP-DM failed to activate NF-κB pathway (Figure 3C). Therefore, LMP1 represses its own promoter reporter constructs.
Figure 3.
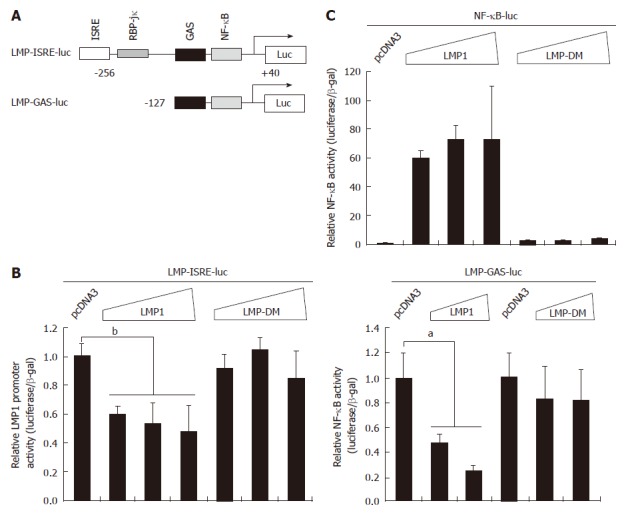
Latent membrane protein 1 negatively regulates its own promoter activity. A: Schematic diagram of Epstein-Barr virus (EBV) latent membrane protein-1 (LMP1) promoter reporter constructs. RNA start site is shown. The drawing is not to scale; B: 293T cells were transfected with LMP1 promoter reporter construct and expression plasmid (0.01, 0.05, and 0.1 μg) as shown at the top. The LMP-DM has mutations in the critical domains in LMP1 for signaling. Cell lysates were used for the luciferase and β-galactosidase assays. Relative promoter reporter activities (luciferase/β-galactosidase) are shown. The results represented an average of triplicate transfections; Standard error bars are shown. Results from one representative experiment are of shown. The statistically significant difference between the indicated samples is denoted as aP < 0.05; bP < 0.01; C: 293T cells were transfected with the indicated plasmid as shown at the top. Nuclear factor κB (NF-κB) specific reporter construct was used. One day later, luciferase and β-galactosidase activities were measured. Relative promoter reporter activities are shown.
NF-κB represses LMP1 promoter reporter construct
Next, we tested whether the activation of NF-κB alone would affect activities of the LMP1 promoter reporters. The promoter reporter construct and NF-κB (p65 + p50) expression plasmids were co-transfected into 293T cells and the reporter activities were measured. As shown in Figure 4, NF-κB activation alone was able to repress the LMP1 promoter reporter construct. The NF-κB-specific reporter construct was activated by the co-transfection of p65 and p50 expression plasmids, suggesting the NF-κB was functional (Figure 4B).
Figure 4.
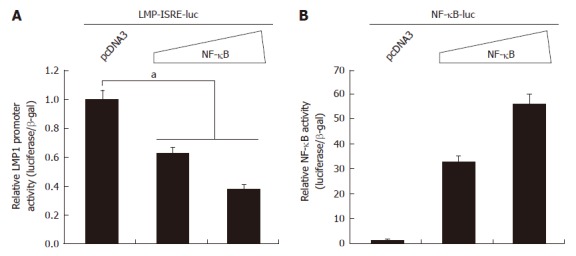
Nuclear factor κB represses latent membrane protein 1 promoter activity. A: 293T cells were transfected with latent membrane protein-1 (LMP1) promoter reporter construct and nuclear factor κB (NF-κB) expression plasmid (0.05 and 0.1 μg; p65 and p50 at 1:1 ratio) as shown at the top. Cell lysates were used for the luciferase and β-galactosidase assays one day later. Relative promoter reporter activities (luciferase/β-galactosidase) are shown. Standard error bars are shown. The statistically significant difference between the indicated samples is denoted as aP < 0.05; B: 293T cells were transfected with the indicated plasmid as shown at the top. NF-κB specific reporter construct was used. One day later, luciferase and β-galactosidase activities were measured. Relative promoter reporter activities are shown.
DISCUSSION
Both viral, such as EBNA2 and EBNA-LP, and cellular factors, such as IRF7, RBP-jκ, PU.1, and STAT are involved in the regulation of LMP1 in various EBV latencies and transformation processes[33-36]. LMP1 needs to be tightly regulated during viral transformation processes because LMP1 itself is a perplex protein with multiple functions, such as proliferative and anti-proliferative activities. The higher amounts of LMP1 may convert itself from a proliferative function to an anti-proliferative one[37].
Activation of NF-κB and the consequence of the action during viral transformation have been established clearly. NF-κB is required for the maintaining of the growth phenotypes of the transformed cells, and NF-κB seems to be responsible for most of the cellular changes during the transformation[38]. However, the relation between the NF-κB and LMP1 expression is somewhat unclear. While it is clear that there is a NF-κB recognition site in LMP1 promoter and NF-κB is able to physically bind to the site, the exact function of NF-κB on LMP1 expression is in debate[23-25]. During our research on LMP1 and other cellular factor interactions, we find that: (1) inhibition of NF-κB enhances the endogenous LMP1 expression, and activation of NF-κB leads to reduced expression of endogenous LMP1 in EBV transformed cells (Figures 1 and 2); (2) LMP1 repressed the LMP1-promoter reporter construct activities, and the repression was correlated with NF-κB activities (Figure 3); and finally (3) NF-κB itself repressed the LMP1-promoter activities (Figure 4). From all the results, it seems that NF-κB is a negative regulator of LMP1 in type III latency or EBV transformed cells. Interestingly, when we used NF-κB inhibiting drug (BAY11-7082) for the LCL and found that the effects on LMP1 expression were not obvious (unpublished observations). We reasoned the side effects of chemical NF-κB inhibitor might influence the end results. Because our experimental approaches were based “solely” on NF-κB activation/inactivation, the results about NF-κB activation and LMP1 expression might be more reliable than a chemical activator and inhibitor of the NF-κB. Moreover, IB4 is a cell line transformed by EBV in vitro and has been used extensively in EBV research[22,39-43]. The results based on the IB4 cells might be more comparable to others researches. Of note that induction of IκB in terms of time course and LMP1 expression seems to be slightly different from the previous report[22]. We think the discrepancies might be due to the growth condition for the cells in various laboratories and passages numbers of the cells might be slightly different. Interestingly, we have found that the detection of the IκB induction by IκB antibody was more sensitive than the FLAG antibody (data not shown).
As we mentioned above, there are several conflicting reports about LMP1 and NF-κB[23-25]. It is hard to reconcile with all the conflicting reports. We think the endogenous LMP1 levels, genetic differences in cell line used, the presence of other viral factors, type of assays, and promoter construct differences may all collectively caused the two quite different conclusions. We have used lines with high endogenous or ectopic LMP1 expression, therefore, the results may be most suitably extrapolated to type III latency or EBV transformed cells in vitro. Because high LMP1 is detrimental for growth, the observed negative effects might make sense in the LMP1-high situations. Furthermore, NF-κB might have dual roles in various backgrounds. Our results are in line with one previous report[24].
Because the response activated by NF-κB is so potent, tight regulation of the NF-κB activity is needed. There are many mechanisms for NF-κB signaling to be terminated to prevent potential tissue pathology due to prolonged expression of inflammatory mediators[44]. Fortunately, many of the NF-κB target genes encode inhibitors of the signaling pathways, which allow the inflamed tissues to reset to normal function once the danger has passed[44]. LMP1-mediated NF-κB activation seems to be the major mediator affecting cell expression programs in EBV-infected cells. The negative regulation of LMP1 expression by NF-κB would offer EBV a feedback inhibition to fine adjust NF-κB activity.
As NF-κB, LMP1 molecule per se is also need to be tightly regulated as it has both pro- and anti-proliferative effects[37]. The negative roles of NF-κB in regulation of LMP1 may offer EBV a feedback inhibition for its own LMP1 expression. The feedback loop between NF-κB and LMP1 might be important for the control of NF-κB as well as LMP1 activities, and eventually EBV transformation as a whole.
ACKNOWLEDGMENTS
We thank Drs. Elliot Kieff and Bo Zhao for providing inducible IκB expression IB4 cell line. Dr. Albert Baldwin for NF-κB expression plasmids (p65 and p50) plus NF-κB reporter constructs. Dr. Jenny Ting for CD4 expression plasmid.
COMMENTS
Background
Epstein-Barr virus (EBV) is a human herpesvirus with increasing medical significances. EBV is associated with many cancers and able to transform adult primary B cells into continually growing lymphoblastoid cell lines. EBV Latent membrane protein-1 (LMP1) is an integral membrane protein and acts as a constitutively active, receptor-like molecule. LMP1 is required for the efficient transformation of human primary B cells in vitro and possibly in vivo. In addition, LMP1 seems to be a central effector of altered cell growth, survival, adhesive, invasive, and antiviral potential in EBV transformed cells. The nuclear factor κB (NF-κB) molecule plays a pivotal role in regulating a variety of biological processes, such as immunity, cell survival, and proliferation. The NF-κB pathway can be regulated by many stimuli, and its activity is tightly controlled to ensure a transient response to infection or other stimuli. The functional importance of NF-κB is exemplified by the fact that the blockade of NF-κB triggers apoptosis of EBV-transformed cells.
Research frontiers
The relation between EBV and NF-κB, especially LMP1 and NF-κB, has been extensively studied. Other than the involvement in the EBV transformation, it is apparent that NF-κB regulates LMP1 expression via a well conserved binding site in LMP1 promoter region, however how the end results of the regulation is confusing. There are several conflicting reports about the relation between NF-κB and LMP1.
Innovations and breakthroughs
During the authors study on NF-κB and LMP1, The authors found that NF-κB is likely to be the negative regulator for the LMP1 expression at least in EBV-transformed cells. LMP1 may negatively regulate its own expression through NF-κB, which is a classical feedback loop.
Applications
The authors results provide an insight in NF-κB’s role in viral transformation and add the complexity of viral gene regulation and their relation to transformation. This will be useful to determine the therapeutic potential and benefit of drugs that targeting NF-κB and/or LMP1 in EBV-associated cancers.
Terminology
Both EBV LMP1 and NF-κB are proteins involved in the process called viral transformation in which normal cells are converted into cancerous ones by a virus. Such a mechanism is crucial in viral pathogenesis. Non-surprisingly, EBV LMP1 and NF-κB has a classical regulatory loop.
Peer review
The study design and paper writing are OK. The methods provided in this study are correct.
Footnotes
Supported by Grants from the NIH CA138213, RR15635, and Department of Defense W81XWH-12-1-0225 (Luwen Zhang); Qianli Wang was partially supported by Undergraduate Creative Activities and Research Experiences and Beckman Scholars Program
P- Reviewer: Cao GW, Martinez-Costa OH S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
References
- 1.Pagano JS. Epstein-Barr virus: the first human tumor virus and its role in cancer. Proc Assoc Am Physicians. 1999;111:573–580. doi: 10.1046/j.1525-1381.1999.t01-1-99220.x. [DOI] [PubMed] [Google Scholar]
- 2.Raab-Traub N. Pathogenesis of Epstein-Barr virus and its associated malignancies. Semin Virol. 1996;7:315–323. [Google Scholar]
- 3.Rickinso AB, Kieff E. Epstein-Barr Virus in Virology, 3rd ed. In Fields BN, Knipe DM, Howley PM, eds. Philadelphia, PA: Lippinscott-Raven Publishers; 1996. pp. 2397–2446. [Google Scholar]
- 4.Kieff E. Epstein-Barr virus and its replication in Virology, 3rd Edition. In Fields BN, Knipe DM, Howley PM, eds. Philadelphia, PA: Lippinscott-Raven Publishers; 1996. pp. 2343–2396. [Google Scholar]
- 5.Gires O, Zimber-Strobl U, Gonnella R, Ueffing M, Marschall G, Zeidler R, Pich D, Hammerschmidt W. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 1997;16:6131–6140. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaye KM, Izumi KM, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izumi KM, Kaye KM, Kieff ED. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc Natl Acad Sci USA. 1997;94:1447–1452. doi: 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The gatekeepers. Nat Struct Biol. 1998;5:165–166. doi: 10.1038/nsb0398-165. [DOI] [PubMed] [Google Scholar]
- 9.Baichwal VR, Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988;2:461–467. [PubMed] [Google Scholar]
- 10.Baichwal VR, Sugden B. The multiple membrane-spanning segments of the BNLF-1 oncogene from Epstein-Barr virus are required for transformation. Oncogene. 1989;4:67–74. [PubMed] [Google Scholar]
- 11.Zhang L, Zhang J, Lambert Q, Der CJ, Del Valle L, Miklossy J, Khalili K, Zhou Y, Pagano JS. Interferon regulatory factor 7 is associated with Epstein-Barr virus-transformed central nervous system lymphoma and has oncogenic properties. J Virol. 2004;78:12987–12995. doi: 10.1128/JVI.78.23.12987-12995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller WE, Earp HS, Raab-Traub N. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J Virol. 1995;69:4390–4398. doi: 10.1128/jvi.69.7.4390-4398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fries KL, Miller WE, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J Virol. 1996;70:8653–8659. doi: 10.1128/jvi.70.12.8653-8659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 15.Wang F, Gregory C, Sample C, Rowe M, Liebowitz D, Murray R, Rickinson A, Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990;64:2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshizaki T, Sato H, Furukawa M, Pagano JS. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc Natl Acad Sci USA. 1998;95:3621–3626. doi: 10.1073/pnas.95.7.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu D, Brumm K, Zhang L. The latent membrane protein 1 of Epstein-Barr virus (EBV) primes EBV latency cells for type I interferon production. J Biol Chem. 2006;281:9163–9169. doi: 10.1074/jbc.M511884200. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Das SC, Kotalik C, Pattnaik AK, Zhang L. The latent membrane protein 1 of Epstein-Barr virus establishes an antiviral state via induction of interferon-stimulated genes. J Biol Chem. 2004;279:46335–46342. doi: 10.1074/jbc.M403966200. [DOI] [PubMed] [Google Scholar]
- 19.Smale ST. Hierarchies of NF-κB target-gene regulation. Nat Immunol. 2011;12:689–694. doi: 10.1038/ni.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 21.Soni V, Cahir-McFarland E, Kieff E. LMP1 TRAFficking activates growth and survival pathways. Adv Exp Med Biol. 2007;597:173–187. doi: 10.1007/978-0-387-70630-6_14. [DOI] [PubMed] [Google Scholar]
- 22.Cahir-McFarland ED, Davidson DM, Schauer SL, Duong J, Kieff E. NF-kappa B inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc Natl Acad Sci USA. 2000;97:6055–6060. doi: 10.1073/pnas.100119497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson P, Jansson A, Rüetschi U, Rymo L. Nuclear factor-kappaB binds to the Epstein-Barr Virus LMP1 promoter and upregulates its expression. J Virol. 2009;83:1393–1401. doi: 10.1128/JVI.01637-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goormachtigh G, Ouk TS, Mougel A, Tranchand-Bunel D, Masy E, Le Clorennec C, Feuillard J, Bornkamm GW, Auriault C, Manet E, et al. Autoactivation of the Epstein-Barr virus oncogenic protein LMP1 during type II latency through opposite roles of the NF-kappaB and JNK signaling pathways. J Virol. 2006;80:7382–7393. doi: 10.1128/JVI.02052-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demetriades C, Mosialos G. The LMP1 promoter can be transactivated directly by NF-kappaB. J Virol. 2009;83:5269–5277. doi: 10.1128/JVI.00097-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Pagano JS. Interferon regulatory factor 7 is induced by Epstein-Barr virus latent membrane protein 1. J Virol. 2000;74:1061–1068. doi: 10.1128/jvi.74.3.1061-1068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu D, Coleman T, Zhang J, Fagot A, Kotalik C, Zhao L, Trivedi P, Jones C, Zhang L. Epstein-Barr virus inhibits Kaposi’s sarcoma-associated herpesvirus lytic replication in primary effusion lymphomas. J Virol. 2007;81:6068–6078. doi: 10.1128/JVI.02743-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad H, Gubbels R, Ehlers E, Meyer F, Waterbury T, Lin R, Zhang L. Kaposi sarcoma-associated herpesvirus degrades cellular Toll-interleukin-1 receptor domain-containing adaptor-inducing beta-interferon (TRIF) J Biol Chem. 2011;286:7865–7872. doi: 10.1074/jbc.M110.191452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu D, Zhao L, Del Valle L, Miklossy J, Zhang L. Interferon regulatory factor 4 is involved in Epstein-Barr virus-mediated transformation of human B lymphocytes. J Virol. 2008;82:6251–6258. doi: 10.1128/JVI.00163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Pagano JS. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol Cell Biol. 1997;17:5748–5757. doi: 10.1128/mcb.17.10.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Pagano JS. Interferon regulatory factor 2 represses the Epstein-Barr virus BamHI Q latency promoter in type III latency. Mol Cell Biol. 1999;19:3216–3223. doi: 10.1128/mcb.19.4.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gewurz BE, Mar JC, Padi M, Zhao B, Shinners NP, Takasaki K, Bedoya E, Zou JY, Cahir-McFarland E, Quackenbush J, et al. Canonical NF-kappaB activation is essential for Epstein-Barr virus latent membrane protein 1 TES2/CTAR2 gene regulation. J Virol. 2011;85:6764–6773. doi: 10.1128/JVI.00422-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ning S, Hahn AM, Huye LE, Pagano JS. Interferon regulatory factor 7 regulates expression of Epstein-Barr virus latent membrane protein 1: a regulatory circuit. J Virol. 2003;77:9359–9368. doi: 10.1128/JVI.77.17.9359-9368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F, Tsang SF, Kurilla MG, Cohen JI, Kieff E. Epstein-Barr virus nuclear antigen 2 transactivates latent membrane protein LMP1. J Virol. 1990;64:3407–3416. doi: 10.1128/jvi.64.7.3407-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johannsen E, Koh E, Mosialos G, Tong X, Kieff E, Grossman SR. Epstein-Barr virus nuclear protein 2 transactivation of the latent membrane protein 1 promoter is mediated by J kappa and PU.1. J Virol. 1995;69:253–262. doi: 10.1128/jvi.69.1.253-262.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H, Hutt-Fletcher L, Cao L, Hayward SD. A positive autoregulatory loop of LMP1 expression and STAT activation in epithelial cells latently infected with Epstein-Barr virus. J Virol. 2003;77:4139–4148. doi: 10.1128/JVI.77.7.4139-4148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam N, Sandberg ML, Sugden B. High physiological levels of LMP1 result in phosphorylation of eIF2 alpha in Epstein-Barr virus-infected cells. J Virol. 2004;78:1657–1664. doi: 10.1128/JVI.78.4.1657-1664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cahir-McFarland ED, Carter K, Rosenwald A, Giltnane JM, Henrickson SE, Staudt LM, Kieff E. Role of NF-kappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J Virol. 2004;78:4108–4119. doi: 10.1128/JVI.78.8.4108-4119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter KL, Cahir-McFarland E, Kieff E. Epstein-barr virus-induced changes in B-lymphocyte gene expression. J Virol. 2002;76:10427–10436. doi: 10.1128/JVI.76.20.10427-10436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson A, Ripley S, Heller M, Kieff E. Chromosome site for Epstein-Barr virus DNA in a Burkitt tumor cell line and in lymphocytes growth-transformed in vitro. Proc Natl Acad Sci USA. 1983;80:1987–1991. doi: 10.1073/pnas.80.7.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurley EA, Klaman LD, Agger S, Lawrence JB, Thorley-Lawson DA. The prototypical Epstein-Barr virus-transformed lymphoblastoid cell line IB4 is an unusual variant containing integrated but no episomal viral DNA. J Virol. 1991;65:3958–3963. doi: 10.1128/jvi.65.7.3958-3963.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cahir McFarland ED, Izumi KM, Mosialos G. Epstein-barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-kappaB. Oncogene. 1999;18:6959–6964. doi: 10.1038/sj.onc.1203217. [DOI] [PubMed] [Google Scholar]
- 43.Frost V, Delikat S, Al-Mehairi S, Sinclair AJ. Regulation of p27KIP1 in Epstein-Barr virus-immortalized lymphoblastoid cell lines involves non-apoptotic caspase cleavage. J Gen Virol. 2001;82:3057–3066. doi: 10.1099/0022-1317-82-12-3057. [DOI] [PubMed] [Google Scholar]
- 44.Ruland J. Return to homeostasis: downregulation of NF-κB responses. Nat Immunol. 2011;12:709–714. doi: 10.1038/ni.2055. [DOI] [PubMed] [Google Scholar]


