Abstract
The stage of development and age were found to effect the responsiveness of dog T-lymphocytes to phytohemagglutinin. T-lymphocytes from beagles 0 to 4 weeks of age showed significantly less response to phytohemagglutinin (P < 0.001) than T-lymphocytes from these same dogs at 6 to 12 weeks of age. Peak response to phytohemagglutinin occurred between 6 weeks to 6 months of age, after which there was a significant correlation (P < 0.02) between increase in age and decrease in phytohemagglutinin responsiveness.
Full text
PDF
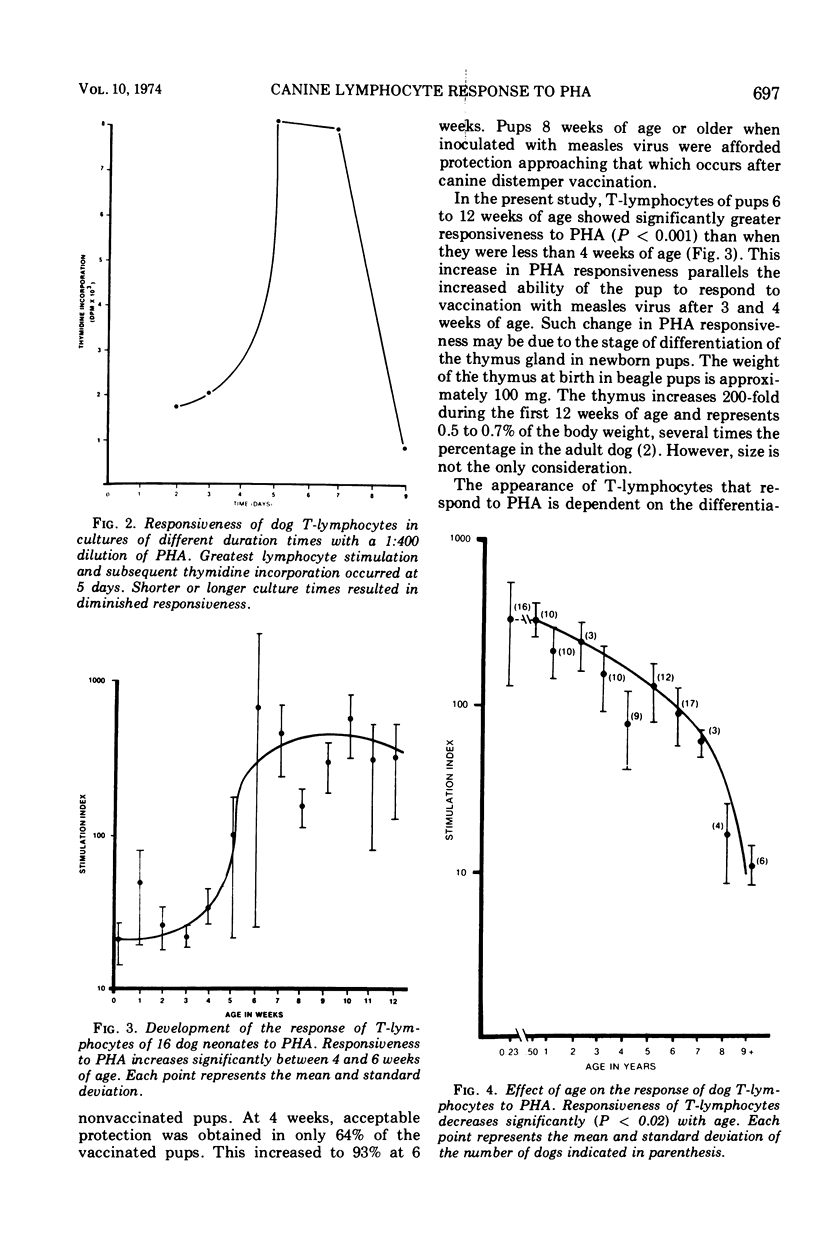


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Cell-mediated immune responses to virus infections and virus-induced tumours. Br Med Bull. 1967 Jan;23(1):60–65. doi: 10.1093/oxfordjournals.bmb.a070518. [DOI] [PubMed] [Google Scholar]
- BAKER J. A., ROBSON D. S., GILLESPIE J. H., BURGHER J. A., DOUGHTY M. F. A nomograph that predicts the age to vaccinate puppies against distemper. Cornell Vet. 1959 Jan;49(1):158–167. [PubMed] [Google Scholar]
- Biesecker J. L., Fitch F. W., Rowley D. A., Stuart F. P. Passive antibody delays development of cell-mediated immunity in rats receiving renal allografts. Transplant Proc. 1973 Mar;5(1):667–669. [PubMed] [Google Scholar]
- Blomgren H., Svedmyr E. Evidence for thymic dependence of PHA-reactive cells in spleen and lymph nodes and independence in bone marrow. J Immunol. 1971 Mar;106(3):835–841. [PubMed] [Google Scholar]
- Boulter E. A. Protection against poxviruses. Proc R Soc Med. 1969 Mar 3;62(3):295–297. [PMC free article] [PubMed] [Google Scholar]
- Brown A. L., McCarthy R. E. Relationship between measles and canine distemper viruses determined by delayed type hypersensitivity reactions in dogs. Nature. 1974 Mar 22;248(446):344–345. doi: 10.1038/248344a0. [DOI] [PubMed] [Google Scholar]
- Brown A. L., Vitamvas J. A., Merry D. L., Jr, Beckenhauer W. H. Immune response of pups to modified live-virus canine distemper-measles vaccine. Am J Vet Res. 1972 Jul;33(7):1447–1456. [PubMed] [Google Scholar]
- Bryant B. J., Shifrine M. Histogenesis of lymph nodes during development of the dog. J Reticuloendothel Soc. 1972 Jul;12(1):96–107. [PubMed] [Google Scholar]
- Bryant B. J., Shifrine M., McNeil C. Cell-mediated immune response in the developing dog. Int Arch Allergy Appl Immunol. 1973;45(6):937–942. doi: 10.1159/000231092. [DOI] [PubMed] [Google Scholar]
- Burnet F. M. Measles as an index of immunological function. Lancet. 1968 Sep 14;2(7568):610–613. doi: 10.1016/s0140-6736(68)90701-0. [DOI] [PubMed] [Google Scholar]
- Chase P. S. The effects of human serum fractions on phytohemagglutinin- and concanavalin A-stimulated human lymphocyte cultures. Cell Immunol. 1972 Dec;5(4):544–554. doi: 10.1016/0008-8749(72)90104-9. [DOI] [PubMed] [Google Scholar]
- Chilgren R. A., Meuwissen H. J., Quie P. G., Good R. A., Hong R. The cellular immune defect in chronic mucocutaneous candidiasis. Lancet. 1969 Jun 28;1(7609):1286–1288. doi: 10.1016/s0140-6736(69)92223-5. [DOI] [PubMed] [Google Scholar]
- Cooperband S. R., Davis R. C., Schmid K., Mannick J. A. Competitive blockade of lymphocyte stimulation by a serum immuno-regulatory alpha globulin (IRA). Transplant Proc. 1969 Mar;1(1):516–523. [PubMed] [Google Scholar]
- Dennis R. A., Jacoby R. O., Griesemer R. A. Development of immunity in fetal dogs: effects of thymectomy. Am J Vet Res. 1969 Sep;30(9):1517–1522. [PubMed] [Google Scholar]
- Dennis R. A., Jacoby R. O., Griesemer R. A. Development of immunity in fetal dogs: skin allograft rejection. Am J Vet Res. 1969 Sep;30(9):1511–1516. [PubMed] [Google Scholar]
- GOOD R. A., ZAK S. J. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics. 1956 Jul;18(1):109–149. [PubMed] [Google Scholar]
- Gelfand M. C., Steinberg A. D. Mechanism of allograft rejection in New Zealand mice. I. Cell synergy and its age-dependent loss. J Immunol. 1973 Jun;110(6):1652–1662. [PubMed] [Google Scholar]
- Gillespie J. H. The significance of passive immunity and the biological tests used in the study of distemper. J Am Vet Med Assoc. 1966 Sep 1;149(5):623–632. [PubMed] [Google Scholar]
- Hallgren H. M., Buckley C. E., 3rd, Gilbertsen V. A., Yunis E. J. Lymphocyte phytohemagglutinin responsiveness, immunoglobulins and autoantibodies in aging humans. J Immunol. 1973 Oct;111(4):1101–1107. [PubMed] [Google Scholar]
- Hori Y., Perkins E. H., Halsall M. K. Decline in phytohemagglutinin responsiveness of spleen cells from aging mice. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):48–53. doi: 10.3181/00379727-144-37524. [DOI] [PubMed] [Google Scholar]
- Jacoby R. O., Dennis R. A., Griesemer R. A. Development of immunity in fetal dogs: humoral responses. Am J Vet Res. 1969 Sep;30(9):1503–1510. [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Kay H. E., Doe J., Hockley A. Response of human foetal thymocytes to phytohaemagglutinin (PHA). Immunology. 1970 Mar;18(3):393–396. [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Chandler J. W., Schimke R. N. Chronic mucocutaneous moniliasis with impaired delayed hypersensitivity. Clin Exp Immunol. 1970 Mar;6(3):375–385. [PMC free article] [PubMed] [Google Scholar]
- Knight S. C., Newey B., Ling N. R. Ontogeny of cellular immunity: size and turnover of rat thymocytes responsive to in vitro stimulation. Cell Immunol. 1973 Nov;9(2):273–281. doi: 10.1016/0008-8749(73)90078-6. [DOI] [PubMed] [Google Scholar]
- Lewis R. M., Smith C. A., Garfield L. Kinetics of antibody synthesis to particulate and soluble antigen in newborn pups and adult dogs. Am J Vet Res. 1973 Feb;34(2):235–240. [PubMed] [Google Scholar]
- Lopez D. M., Siegel M. M., Lee J. C. Phylogenetic studies on T cells. I. Lymphocytes of the shark with differential response to phytohemagglutinin and concanavalin A. Cell Immunol. 1974 Feb;10(2):287–293. doi: 10.1016/0008-8749(74)90120-8. [DOI] [PubMed] [Google Scholar]
- McCANCE R. A., HUTCHINSON A. O. The cholinesterase activity of the serum on newborn animals, and of colostrum. Biochem J. 1949;45(4):493–496. doi: 10.1042/bj0450493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen H. J., Van Alten P. A., Good R. A. Decreased lymphoid cell multiplication in the post-thymectomy state. Transplantation. 1969 Jan;7(1):1–11. doi: 10.1097/00007890-196901000-00001. [DOI] [PubMed] [Google Scholar]
- Milton J. D. Effect of an immunosuppressive serum alpha-2-glycoprotein with ribonuclease activity on the proliferation of human lymphocytes in culture. Immunology. 1971 Feb;20(2):205–212. [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E. Ontogeny of mouse lymphocyte function. I. Paradoxical elevation of reactivity to allogeneic cells and phytohemagglutinin in BALB-c fetal thymocytes. J Immunol. 1974 Jan;112(1):305–310. [PubMed] [Google Scholar]
- OTT R. L., GORHAM J. R., GUTIERREZ J. C. Distemper in dogs. II. The response to vaccination. Am J Vet Res. 1957 Apr;18(67):375–381. [PubMed] [Google Scholar]
- Papiernik M. Correlation of lymphocyte transformation and morphology in the human fetal thymus. Blood. 1970 Oct;36(4):470–479. [PubMed] [Google Scholar]
- Park B. H., Good R. A. A new micromethod for evaluating lymphocyte responses to phytohemagglutinin: quantitative analysis of the function of thymus-dependent cells. Proc Natl Acad Sci U S A. 1972 Feb;69(2):371–373. doi: 10.1073/pnas.69.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisciotta A. V., Westring D. W., DePrey C., Walsh B. Mitogenic effect of phytohaemagglutinin at different ages. Nature. 1967 Jul 8;215(5097):193–194. doi: 10.1038/215193a0. [DOI] [PubMed] [Google Scholar]
- Prindull G. An in-vitro quantitative study of phytohaemagglutinin (PHA) induced transformation of lymphocytes from premature newborn infants, from older premature infants, and from full-term newborn infants. Blut. 1971 Jul;23(1):7–13. doi: 10.1007/BF01815128. [DOI] [PubMed] [Google Scholar]
- Riggio R. R., Schwartz G. H., Bull F. G., Stenzel K. H., Rubin A. L. Alpha-2-globulins in renal graft rejection. Effects of in vitro lymphocyte function. Transplantation. 1969 Nov;8(5):689–694. doi: 10.1097/00007890-196911000-00013. [DOI] [PubMed] [Google Scholar]
- Rodey G. E., Good R. A., Yunis E. J. Progressive loss in vitro of cellular immunity with ageing in strains of mice susceptible to autoimmune disease. Clin Exp Immunol. 1971 Sep;9(3):305–311. [PMC free article] [PubMed] [Google Scholar]
- Shifrine M., Smith J. B., Bulgin M. S., Bryant B. J., Zee Y. C., Osburn B. I. Response of canine fetuses and neonates to antigenic stimulation. J Immunol. 1971 Oct;107(4):965–970. [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. II. Acquisition of mitogen responsiveness and of theta antigen during the ontogeny of thymocytes and "T" lymphocytes. Cell Immunol. 1972 Aug;4(4):367–380. doi: 10.1016/0008-8749(72)90039-1. [DOI] [PubMed] [Google Scholar]
- Stutman O., Yunis E. J., Good R. A. Deficient immunologic functions of NZB mice. Proc Soc Exp Biol Med. 1968 Apr;127(4):1204–1207. doi: 10.3181/00379727-127-32910. [DOI] [PubMed] [Google Scholar]
- Tompkins W. A., Zarling J. M., Rawls W. E. In vitro assessment of cellular immunity to vaccinia virus: contribution of lymphocytes and macrophages. Infect Immun. 1970 Dec;2(6):783–790. doi: 10.1128/iai.2.6.783-790.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T. H., Santesson B., Skoog V. T. The activation of fetal lymphocytes. Scand J Haematol. 1973;11(3):177–183. doi: 10.1111/j.1600-0609.1973.tb00113.x. [DOI] [PubMed] [Google Scholar]
- Zeman G. O., Cohen G., Budrys M., Williams G. C., Javor H. The effect of plasma cortisol levels on the lymphocyte transformation test. J Allergy Clin Immunol. 1972 Jan;49(1):10–15. doi: 10.1016/0091-6749(72)90118-2. [DOI] [PubMed] [Google Scholar]


