Abstract
Cells are able to navigate environments, communicate, and build complex patterns by initiating gene expression in response to specific signals. Engineers need to harness this capability to program cells to perform tasks or build chemicals and materials that match the complexity seen in nature. This review describes new tools that aid the construction of genetic circuits. We show how circuit dynamics can be influenced by the choice of regulators and changed with expression “tuning knobs.” We collate the failure modes encountered when assembling circuits, quantify their impact on performance, and review mitigation efforts. Finally, we discuss the constraints that arise from operating within a living cell. Collectively, better tools, well-characterized parts, and a comprehensive understanding of how to compose circuits are leading to a breakthrough in the ability to program living cells for advanced applications, from living therapeutics to the atomic manufacturing of functional materials.
Introduction
The ability to perform computation in a living cell will revolutionize biotechnology by improving existing products and enabling new applications. In the short term, the production of bio-based chemicals can be improved by timing gene expression at different stages of fermentation, or turning on an enzyme only under particular conditions (e.g., low oxygen)1–6. As circuits become more advanced, entire algorithms from control theory could be applied to improve biochemical production (Fig. 1a)7–16. Synthetic regulation is also an important tool for the discovery of natural products, including pharmaceuticals, insecticides, and entirely new classes of chemicals. Accessing these products may require the coordinated expression of genes identified in sequence databases, intractable organisms, or “silent” gene clusters17–22. Moving outside of the fermenter, living cells could be programmed to serve as therapeutic agents that correct genetic disease (Fig. 1b) or colonize niches in the human microbiome to perform a therapeutic function (Fig. 1c)23–35. Longer-term capabilities include “smart plants” that sense and adapt to environmental challenges (Fig. 1d) and bacteria that organize to weave functional materials with nanoscale features36–42.
Figure 1. Potential uses of synthetic genetic circuits.
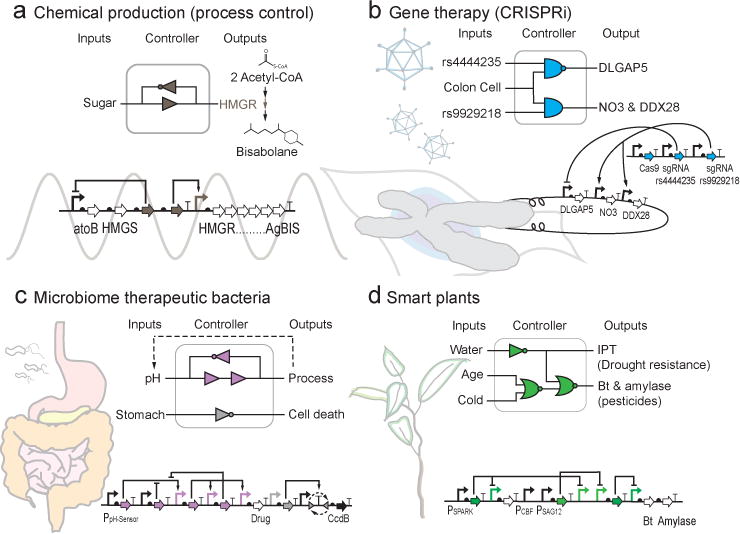
Here, we present several hypothetical uses of circuits in different application areas. (a) In industrial applications, most synthetic metabolic pathways are overexpressed at all times or are under simple inducible control. This could be improved by incorporating timing, feedback of metabolic intermediates, or dynamic control. Here, we show a circuit that is controlling the production of a diesel fuel alternative (bisobolane221) by regulating the accumulation of a toxic intermediate (HMG-CoA) by sensing sugar, which induces oscillations in the production of HMGR. This type of oscillatory control occurs in natural metabolic networks222. (b) Gene therapy circuits could be built based on CRISPRi technology by detecting SNPs and integrating this information with tissue-specific sensors. As a hypothetical example, we show a circuit that could detect two SNPs associated with colon cancer susceptibility (rs4444235 and rs9929218)223 and this is integrated with a promoter that is specific to colon cells (pAMUC2) to control the expression of misregulated genes (DLGAP5, NO3, and DDX28). (c) Bacteria could be programmed to colonize human microbiota and implement a therapeutic response. An example is envisioned where a commensal bacterium is used to stabilize pH to treat gastoesophageal acid reflux (GERD). A bacterium that is naturally commensal with the stomach could be programmed to maintain the pH using a circuit that enables set point control via a PI controller9,224 whose output is proton pump inhibitors (PPIs). The circuit also restricts acid regulation to the stomach by terminating the bacterium via an irreversible switch if it leaves this organ225. (d) Genetic circuits could also be used to build “smart plants” that are able to sense environmental stimuli and implement a response. Currently, traits are produced all the time whether or not they are needed by the plant. Here, we envision a circuit that would operate in the chloroplast integrate sensors for drought (pSpark), temperature (pCBF), and plant maturity (pSAG12) to control multiple traits. This could reduce the amount of recombinant protein that is produced and enters the food supply without reducing the effectiveness of the trait.
Despite its potential, genetic circuit design remains one of the most challenging aspects of genetic engineering43. The earlier fields of protein and metabolic engineering have yielded tools to optimize enzymes and fluxes through a metabolic network. These tools include computational methods that can predict the impact of an amino acid substitution on protein thermostability44 or the distribution of flux through modified metabolic networks45. Biotech companies often have research groups dedicated to protein and metabolic engineering that have specialized training in these tools. However, industrial groups dedicated to building synthetic regulation are rare and even simple tasks, like building a switch or inducible system, tend to be one-off projects performed by a non-specialist.
Several features of genetic circuits make them challenging to work with, as compared with other areas of genetic engineering. First, circuits require the precise balancing of their component regulators to generate the proper response46,47. Computational tools and part libraries that enable the tuning of expression levels have only been developed recently48–51. Before this, only course-grained control was achievable with small sets of parts46,47,52. Second, many circuits are difficult to screen in directed evolution experiments for correct performance. Digital logic has clear on and off states that can form the basis for a screen12,53–59. However, screening for dynamic circuits, such as oscillators, is significantly more complex60 and it is hard to imagine how screens would be established for more sophisticated functions, like a PID controller with proscribed response properties. Third, there are few tools to measure circuit performance. Typically, a fluorescent reporter is used to measure the output, but fluorescence detection requires artificially high expression levels and fluorescent protein degradation rates can limit the ability to measure dynamics. Fourth, synthetic circuits are very sensitive to environment, growth conditions, and genetic context in ways that are poorly understood61. Finally, the process of building a large genetic circuit requires the assembly of many DNA parts and this process has been both technically challenging (until recently) and fraught with its own sources of errors58,62–67.
The purpose of this review is to serve as a guide to designing a prokaryotic transcriptional circuit, where both the inputs and outputs are promoters53,55,68–71. Transcriptional circuits maintain a common signal carrier, which simplifies the connection of circuits to build up sophisticated operations72. Post-transcriptional circuits, including those based on protein and RNA interactions, are covered in other excellent reviews73–75. Although the majority of this guide is dedicated to bacterial circuits, many of the principles, albeit not the details, are relevant for eukaryotes, including human cells and plants76,77.
I. Genetic circuit design based on different regulator classes
Transcriptional circuits operate by affecting the flow of RNA polymerase (RNAP) on DNA. There are a number of molecules that impact this flux that have been used as the basis for building synthetic circuits (Fig. 2). For example, DNA-binding proteins can recruit or block RNAP to increase or decrease the flux, respectively. Analogously, the new CRISPRi system uses Cas9 protein to bind to the DNA and alter transcription78,79. RNAP flux can also be altered with invertases that change the orientation of promoters, terminators, or gene sequences. Additionally, RNA translational repressors, such as RNA-IN/OUT, can be converted to control RNAP flux80,81. In this section, we describe recent advances in these methods and analyze the impact that each regulator has on circuit response.
Figure 2. Logic gates built based on different regulator types.

All of the gates are transcriptional, where there are two input promoters (PIN1 and PIN2) and one output promoter (POUT). Two-input transcriptional logic gates have not yet been built for CRISPRi and RNA-IN/OUT so we hypothesize how these biochemistries could be used. The graphs at the right show how the gates will respond to inputs introduced at the same time (graphs at left) or sequentially (right). In all panels, the on state is assumed to generate ten fold higher than the off state. (a) A NOR gate is shown based on a repressor that binds DNA110. The lines are based on measured induction (τ1/2 ~36 min) and relaxation (τ1/2 ~35 min) half-lives226. (b) An AND gate based on an activator that binds DNA that requires a second protein to be active55. The lines are based on a measured induction (τ1/2 ~36 min)55 and approximate relaxation (τ1/2 ~35 min) half-life. (c) A NOR gate based on integrases that flip two terminators to turn off the output122,123. We assume a small readthrough probability, which leads to a change in the rate when only one terminator is flipped. Conceptually, a NOR gate could also be constructed by having two input promoters in series drive the expression of a single integrase. The lines are based on an on-rate of 1.8 hours119,121,122. (d) An AND gate based on integrases122. The same on- and off- rates are used as in part c. (e) A NOR gate could be built based on CRISPRi by setting a constitutive level of Cas9 expression and then having the two input promoters drive the expression of two guide RNAs. The lines are based on measured induction (τ1/2 = 35 min) and relaxation (τ1/2 = 47 min) half-lives79. (f) A NOR gate could be built based on the RNA-IN/RNA-OUT system developed by Arkin and co-workers80. RNA-OUT represses translation of tnaC, which allows Rho to bind the mRNA and repress transcription of the output. The CRISPR machinery needed to process RNA-IN mRNA for this circuit is not shown. The lines are based on theoretical induction (τ1/2 ~30 min) and relaxation (τ1/2 ~35 min) half-lives162,226.
DNA-binding Proteins
Many families of proteins can bind to specific DNA sequences (operators). The simplest way to use these proteins as regulators is to design promoters with operators that block the binding or progression of RNAP. Such repressors have been built out of zinc finger proteins (ZFPs)82, transcription activator-like effectors (TALEs)83,84, TetR homologues71, phage repressors85,86, and LacI homologues87. A core set of ~3 repressors were re-used in many of the first synthetic circuits (CI, TetR, LacI)47,53,88–91. However, recently there have been efforts to expand the number of DNA-binding proteins that are available for circuit design54,92–99. Expanding protein libraries can be challenging because each repressor has to be orthogonal; i.e., only interact with their operator and not the others in the set. Because of their simple function, repressors are relatively easy to move between species, including to eukaryotes92–97. DNA-binding proteins can also function as activators that increase the flux of RNAP on DNA. Activators either recruit host RNAP to a promoter or are alternative RNAPs that transcribe genes directly. Recent efforts have increased the number of such proteins that are available for constructing circuits54,98–100.
Many logic gates have been constructed with DNA binding proteins that recruit or block RNAP71,101–109. For example, NOT and NOR gates have been built with inducible promoters that drive expression of repressors (Fig. 2a)71,110,47,53,88. Additional transcriptional logic has been achieved with regulators that bind directly to the proteins to either inhibit or enhance their function. For example, AND gates have been built with activators that require chaperones (Fig. 2b)55,101 and with artificially split proteins111. Similarly, NAND gates can be built with proteins that block the activity of an activator, such as anti-σ factors, which inhibit σ factors100.
Of the regulators described in this review, DNA-binding proteins are the only class (so far) that has been used to build dynamic circuits. This includes pulse generators112, bistable switches47,53,113, counters69, feedback loops, and oscillators that have different periods and amplitudes70,88,114–116. Analog computing modules have also been built with DNA binding proteins, which highlights the diverse signal processing capabilities of these regulators55,71,101,103,110.
Invertases
Invertases are site-specific recombinase proteins that facilitate the inversion of DNA segments between binding sites117. All invertases mediate “cut-and-paste” recombination, during which DNA is looped, cleaved and re-ligated118. Two types of invertases have been used to build genetic circuits. The first are tyrosine recombinases, such as Cre, Flp, and FimBE, which require host-specific factors69,119–121. These recombinases can be reversible and flip the DNA in both directions, or irreversible and only flip in a single direction. The second class of invertases are serine integrases, which catalyze unidirectional reactions that rely on double stranded breaks to invert DNA. Serine integrases typically do not require host factors and often have cognate excisionases that can be expressed independently to return the DNA to its original orientation.
Invertases have been used to build switches119, memory circuits120,121, counters69, and logic gates122,123. These proteins are ideal for memory storage, because they flip DNA permanently and once flipped they do not require the continuous input of materials or energy to maintain their new orientation. In invertase logic gates, these discrete physical states of the DNA can correspond to on and off states (0 and 1). However, using invertases can be challenging because their reactions are slow (requiring 2–6 hours) and can generate mixed populations when targeting a multicopy plasmid121. Reversible invertases can also generate mixed populations, however this limitation was overcome recently by using a serine integrase to flip DNA in one direction and an integrase/exisionase pair to return it to the original state124.
All two-input gates, including AND and NOR logic, have been constructed using orthogonal serine integrases (Fig. 2c and 2d)122,123. The gates are organized such that two input promoters express a pair of orthogonal recombinases, which change RNAP flux by inverting unidirectional terminators, promoters, or entire genes. These gates are based on unidirectional serine integrases without excisionases, therefore they operate as memory circuits that remember exposure to two input signals. Once flipped, the circuits cannot be returned to their original state, therefore the gates do not distinguish the order they were exposed to the inputs or even if they occurred at the same time.
CRISPRi
CRISPR (Clustered Regularly Spaced Short Palindromic Repeat) arrays function as a bacterial “immune system” that targets specific DNA sequence motifs for degradation125. CRISPR systems utilize a Cas nuclease and guide RNA to introduce double strand breaks to specific DNA sequences126. Mutant Cas proteins (dCas979, Cas9N-127) that do not have nuclease activity have been developed and used as transcription factors that knock down gene expression by forming a DNA bubble that interferes with RNAP activity78,79. CRISPR can also activate transcription via fusion of an RNAP recruiting domain to catalytically inactive Cas978,127–131. Considering the needs of synthetic circuits, one advantage of CRISPRi is the designability of the RNA-DNA complex. It is possible to imagine creating a very large set of orthogonal guide sequences that target different promoters. This set would enable the construction of large genetic circuits, however it would need to be experimentally screened because predicting guide RNA orthogonality is complicated132–135.
CRISPRi is still relatively new and NOT gates are the most complex circuits built to date79. The NOT gates induce sgRNA and dCas9 expression simultaneously to repress transcription at an output promoter. In theory, a NOR gate could be created by introducing a second sgRNA that targets the same output promoter (Fig. 2e). In general, CRISPRi circuits will probably resemble DNA binding protein circuits. However, unlike the repressor based NOR gate (Fig. 2a), the CRISPRi NOR gate will need to have the sgRNAs expressed from separate input promoters because 5′ RNA extensions can reduce or eliminate activity (Fig. 2e)134. Circuits based on CRISPRi are expected to operate on similar timescales to protein-based circuits because of the stability of the regulatory dCas9/sgRNA/DNA duplex79.
A current challenge in implementing CRISPRi circuits is toxicity, which is difficult to control. Toxicity is most likely the result of Cas9 binding to the host genome at PAM sequences (NGG), forming bubbles that deleteriously impact host gene expression136,137. Another consideration for building CRISPRi circuits is retroactivity138, which could arise from using Cas9 as a shared resource (Section III). One way to circumvent retroactivity would be to express multiple orthogonal Cas9 homologues132,139.
Adapted RNA-IN / RNA-OUT
The RNA-IN/OUT system from E. coli represses translation of a target protein when a short noncoding RNA (RNA-OUT) is expressed. In the natural system, RNA-OUT binds to a specific sequence at the 5′ end of an mRNA (RNA-IN) to occlude ribosome binding and increase mRNA degradation140–142. Arkin and co-workers retooled this system to repress transcription, instead of translation, using a transcriptional adaptor from the tna operon80. The tna regulatory element is composed of a ribosome binding site (RBS), the coding sequence for a short peptide called tnaC, a Rho binding site and an RNAP pause site that facilitates Rho-mediated transcription termination. Translation of tnaC causes ribosomal stalling, which blocks Rho-factor binding and allows RNAP to transcribe genes downstream of tnaC. However, when translation of tnaC is prohibited, Rho binds the growing mRNA and knocks off RNAP thereby inhibiting transcription elongation. RNA-IN/OUT RNAs regulate transcription elongation by altering translation of tnaC. Like CRISPRi, the adapted RNA-IN/OUT system could be used to generate a large set of orthogonal regulators because it is based on designable RNA-RNA interactions. To date, more than 150 different families of at least seven orthogonal RNA-IN/OUT mutants have been designed using an RNA-IN/OUT model and all of the mutants tested experimentally have been functional and orthogonal81.
Adapted RNA-IN/OUT has been used to build two-, three-, and four-input NOR gates (Fig. 2f)80. In these systems, orthogonal RNA-IN variants were connected such that expression of any cognate RNA-OUT represses transcription of the output gene. Additional layers of regulation could be engineered into the adapted RNA-IN/OUT system with ligand-responsive aptamers that regulate RNA-OUT activity143 or tRNAs that control ribosomal pausing in tnaC144. A challenge in building larger RNA-IN/OUT circuits is that each transcriptional regulator requires the same tna regulatory element (~290bp). The re-use of this part in multiple circuits could lead to homologous recombination (Section III). Engineering TnaC to reduce the length of the repeated sequence80 or using homologs from other organisms and alternative Rho binding sites could potentially attenuate recombination.
II. Selecting Parts to Tune the Circuit Response
Genetic circuits need to be tuned to meet the specifications required for a particular application. For example, a large dynamic range may be required to strongly activate a pathway. Similarly, low off states are desirable when expressing toxic proteins145. When the first synthetic circuits were built, there were few options available for tuning circuits and only course-grained changes were possible46,47. New libraries of well-characterized parts and computational tools have made it easier to design and tune genetic circuits. Moreover, new classes of insulators improve the reliability of these parts when they are placed in the local genetic context of a circuit. Additional biochemical interactions, such as small RNA (sRNA), have been incorporated into circuits in order to provide additional tuning knobs. In a prior review, we detailed advances in part design and tools to obtain reliable expression levels146. Here, we show how the selection or modification of different parts impacts the response of a circuit.
Two circuits are used as model systems to demonstrate the effects of various tuning knobs. The first, a NOT gate, represents a simple logic operation (Fig. 3a)46,53. Logic gates are often characterized by their response function, which captures how the steady-state output changes as a function of input. The shape of this function is defined by: 1. the ON and OFF states, which define the circuit’s dynamic range, 2. the amount of input required to reach the half-maximum output (also referred to as the threshold), and 3. cooperativity of the switch147,148. An oscillator was selected as an example of a dynamic circuit (Fig. 3h). These types of circuits can be very difficult to tune because they need to be balanced in a narrow region of parameter space in order to function properly90,149,150. For an oscillator, tuning will affect the period, amplitude, and shape of the oscillations. Tuning can also force the system out of the oscillating parameter space and cause the circuit to fail90.
Figure 3. Methods of Modifying Circuit Behavior.
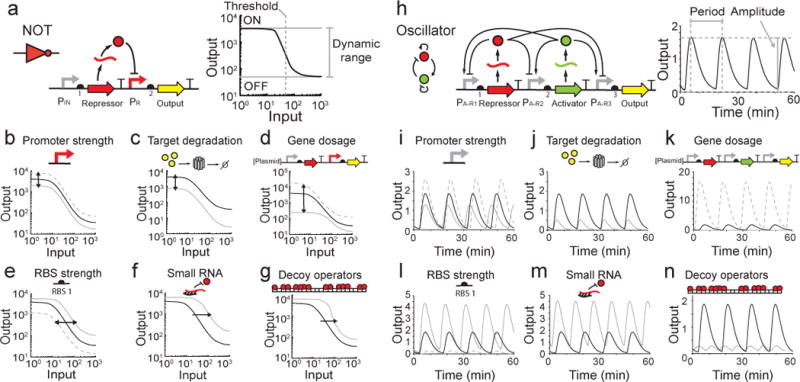
ODE models were built to simulate a NOT gate and an oscillator. Model equations and parameters are included in the SI in SMBL format205,227. Parameters were adjusted to demonstrate the effect of each tuning knob on circuit behavior. Every panel displays circuit outputs with original parameter values (black) or tuning knob variations (grey). Inputs in (a-f) are IPTG. (a/h) Architecture and ideal response functions for the NOT gate (a) and oscillator (h). (b/i) Promoter strength. In both circuits, promoter strength is increased (dashed grey line) or decreased (solid grey line) by a factor of two. (c/j) Enzymatic degradation of the reporter protein was modeled as a five fold increase in the protein degradation rate228. (d/k) Gene dosage. The circuits are moved between a high copy plasmids (dashed grey line) and the genome (solid grey line) to tune expression. The high copy plasmid is assumed to be ten times more abundant than the original circuit. (e/l) Ribosome binding site strength. Repressor RBSs (RBS1) are increased (dashed grey line) or decreased (solid grey line) by a factor of five. Altering the reporter RBS would shift the output of both circuits vertically (not pictured). (f/m) Small RNA designed to bind repressor mRNA are modeled with the introduction of a new species that binds repressor mRNA with the same affinity as a ribosome (this value was chosen arbitrarily and can be modulated to change circuit dynamics). In this model, small RNAs are produced constitutively and sRNA/mRNA duplexes are degraded faster than either RNA alone. (g/n) Decoy operators that bind repressor proteins. Decoy operators were modeled by introducing a new species that binds repressor protein with the same Kd as the repressible promoter. The oscillator model has 25 decoy operator sites, however circuits can be tuned with more or less as needed.
The response function of a digital logic gate can be shifted up or down by changing promoter strengths (Fig. 3b)151, ribosome binding sites (RBS), or the proteins’ degradation rates (Fig. 3c)152. Promoter strength can be altered with mutations in the promoter sequence153 or by selecting new promoters from a characterized library49,154. Increased degradation can be achieved with protease tags or N-terminal degrons152. Circuit components are often distributed between multiple plasmids at different copy numbers in order to synthesize each component at the necessary level. However, when entire circuits are expressed on one plasmid, copy number can be shifted to simultaneously alter the circuit’s dynamic range and threshold (Fig. 3d). Different origins of replication can generate complex and poorly understood effects on expression, for example, by changing localization and supercoiling. This can be minimized by using plasmid systems where the copy number can be controlled without changing the origin155.
The threshold of the gate can be changed via several methods. Selecting a stronger or weaker RBS, adding multiple operators, or changing operator positions within the repressible promoter can change the threshold (Fig. 3e)59,71,156,157. The threshold of a gate becomes steeper and more switch-like when small changes in the input have a large effect on the output158. This phenomenon, known as ultrasensitivity, can be important for controlling actuators where intermediate levels of expression are undesirable158. It can also make connecting gates easier by decreasing the range of input needed from an upstream circuit to span the induction threshold. One way to make a gate ultrasensitive is to change the cooperativity of repressor binding to the promoter or to introduce DNA looping159,160. Another approach is to express a sequestering molecule that binds a circuit component and prevents it from functioning. Sequestration has been achieved using sRNAs that bind to mRNA161,162 (Fig. 3f), proteins that bind to transcription factors113,163,164, and decoy DNA operators that titrate the transcription factor away from the output promoter165 (Fig. 3g).
In an oscillator, parts that impact the rate of gene expression change the amplitude of the response and can shift the period (Fig. 3i,l). Rapid protein degradation is critical for dynamic circuits to function correctly. If proteins are slow to degrade, then the circuit may slow down or stop functioning altogether (Fig. 3j)166. Protease tags can be used to decrease the degradation rate from several hours to ~20 minutes, which will increase the rate at which a gate switches152,70,89,112. Cooperativity is critical for obtaining robust oscillators because it increases the region of phase space that produces oscillations159. Therefore, sequestration approaches (e.g., sRNA or dummy operators) are predicted to have a large impact on the period and amplitude of oscillations (Fig. 3m–n)167.
III. Common Failure Modes from Connecting Circuits
Gates can be combined to build larger circuits that implement more sophisticated computational operations. To connect transcriptional gate, the output promoter of one circuit is used as the input promoter to the next. This method applies for all transcriptional circuits, including digital, analog and dynamic circuits or a combination of types. To connect circuits, they have to broken up into their component parts and then combined in a particular order (Fig. 4a). Reorganizing the parts places them in new local contexts that are different from those where they were characterized. This can be problematic because circuit components can behave differently in new genetic contexts and small circuits may have identical component parts (e.g., terminators) that interfere with each other in the larger circuit. In this section, we discuss failure modes that can arise when building larger circuits, show the impact that each failure has on circuit function, and discuss engineering approaches to mitigate these problems.
Figure 4. Common failure modes and their impact on circuit dynamics. (a).
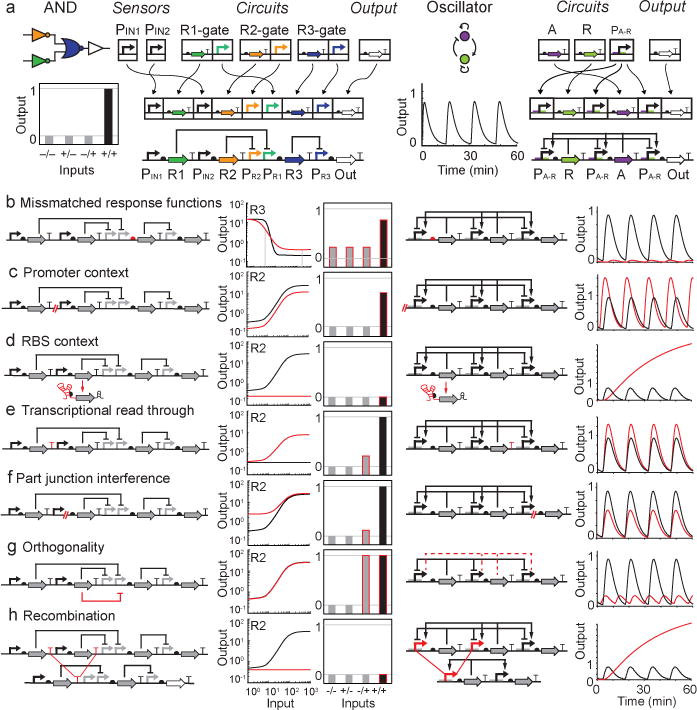
An AND gate71 and oscillator70 are used as model systems to demonstrate the assembly of parts to build more complex circuits. Repression is indicated with a blunt ended connector and activation is indicated with an arrow. For the AND gate, the input promoters are PIN1 and PIN2 and the output promoter is PR3. Promoters are named by the repressor to which it responds (e.g., PR1 is repressed by R1). The steady-state response to different combinations of inputs is shown as a bar graph, where the OFF states are grey and the ON state is black. For the oscillator, the promoters PA-R are repressed by R and activated by A. The impact of various failures (red lines) are shown for the AND gate (left) and oscillator (right) with expected dynamics shown in black. Models were used to simulate the R2 NOT gate, the AND gate71, and oscillator. Parameters and model equations are included in the SI in SBML format205,227. Unless indicated otherwise, Input 2 is the input to the NOT gate transfer function. (b) Mismatched response functions. In the AND gate, R3 was modeled as a different repressor: Bet1 (kd = 0.2, n = 2.4, max = 13, min = 0.4) instead of Orf2 (kd = 0.4, n = 6.1, max = 16, min = 0.2)71. R2 is the input for the R3 transfer function. In the oscillator, the R translation rate is increased ten-fold. (c) Promoter context. Strength of the indicated promoters is reduced by 50% in both circuits. (d) RBS context. The translation rates of R2 (AND gate) and R (oscillator) are set to zero. Input 1 is the input for the R2 transfer function (e) Transcriptional read-through. 30% read-through from upstream operons through the red terminator is simulated in both circuits. (f) Part-junction interference. A new constitutive promoter (AND gate) is simulated as approximately 20% of the strength of PIN2. New terminator (oscillator) decreases transcription 40%. (g) Orthogonality. R3max is set as R2min to simulate repression of PR3 by R2. Additional equations are added to the oscillator model to simulate repressor-activator complex formation. (h) Recombination. R2 and R were removed from the AND gate and oscillator models, respectively.
When connecting circuits, a common problem is that the upstream circuit’s output does not span the dynamic range required to stimulate next circuit in series (Fig. 4b). In digital logic, this ‘mismatch’ manifests as either a decrease in the dynamic range of the complete circuit or a loss of function. Connectivity mismatches can be corrected by selecting parts that shift the thresholds of individual gates. For example, RBSs can be mutated to force the threshold of a gate to fall within the dynamic range produced from an upstream circuit46,102. ‘Mismatches’ in an oscillator can dampen oscillations or force the system outside the functional parameter space (Fig. 4b). Mathematical models could be used to streamline circuit design by predicting the functional parameter space and selecting appropriate RBSs and promoters to achieve the required expression levels48,68,157.
Genetic parts are often context dependent, meaning their functions change when the DNA sequences on either side of the part are altered168,169. Context dependencies complicate part substitutions because part characterizations are often carried out in isolation and their activity in a new context may not match the measured strength. For example, promoters are that are defined as DNA sequences <50 bp may behave differently in new contexts because the α-domain of E. coli RNAP can contact the DNA ~100 bp upstream of the transcription start site153. In a digital circuit, reducing promoter efficiency attenuates the response of individual gates and reduces the output of the complete circuit (Fig. 4c). Promoter attenuation can increase the amplitude of an oscillator and elongate the period, by reducing repressor expression. Insulator sequences can relieve some compositional context effects by standardizing the DNA sequences flanking promoters169,170.
Context effects can also occur when promoters are fused to different RBSs. Promoters are sensitive to the DNA sequences near the transcription start site because that region can alter promoter melting and polymerase escape frequency154. Transcription start sites can also fluctuate based on the local sequence context171,172, which can impact RBS strength by altering the length of the 5′-UTR and changing mRNA secondary structure. Tandem promoters can generate especially long 5′-UTRs that exacerbate this effect by base pairing with the RBS or sequences in the open reading frame173–175. Circuits can fail completely when mutations in the 5′-UTRs cause hairpins completely occlude the RBSs and prohibit translation (Fig. 4d). To solve these problems, the 5′-UTR can be cleaved with ribozymes or CRISPR processing to standardize RBS accessibility170,176. Catalytic insulator elements serve dual functions by standardizing both the 5′ end of mRNA and the promoter region downstream of the transcription start site. RBSs can be further insulated from the local context using bicistronic designs, which prime the mRNA for translation with an upstream RBS that keeps the mRNA unfolded49.
Transcriptional read-through can be a problem in genetic circuits with monocistronic designs, where every gene has its own promoter and terminator. These designs require strong terminators to insulate against read-through from neighboring promoters. Failure to fully insulate each cistron can link the expression of genes that are supposed to be regulated independently (Fig. 4e) and can contribute to the leaky expression of uninduced genes. Strong, tandem terminators can be placed on either side of each gene to ensure isolated expression of individual operons177. Large libraries of rho-independent terminators were recently built and characterized to enable the construction of large circuits that are robust to read-through and homologous recombination (described below)50,177.
DNA sequences are information rich, therefore connecting two parts can create a new functional sequence at the junction178. New regulatory elements, such as promoters or terminators, can be generated at a part junction if the combination creates a sequence of DNA that resembles a regulatory element. For large circuits, many parts have to be combined in a new order and unexpected parts that interfere with gene expression can be generated (Fig. 4f). One way to scan for unintended functional sequences is to use computer algorithms that search for various regulatory elements48,177,179–185.
Crosstalk, which occurs when regulators interact with each other’s targets, can change the topology of a circuit and can lead to errors in the desired operation55. For example, crosstalk between a repressor and non-cognate promoter can inappropriately decrease expression of a gene and cause a circuit to fail (Fig. 4g). Avoiding crosstalk requires that parts be screened for orthogonality via combinatorial experiments that test every combination of promoter and regulatory element71,81,83,98,100,186.
Many of the circuits built to date re-use the same regulatory parts, which can lead to homologous recombination. Homologous recombination deletes DNA between repeated sequences and can result in the loss of circuit components and circuit failure (Fig. 4h)177. In general, the rate of recombination increases with circuit toxicity187 and homolgous DNA length, with the threshold occurring between 20–30bp188. Homologous recombination can be avoided with large libraries of parts with redundant functions that have enough sequence diversity to avoid recombination177,189.
IV. Interactions between Synthetic Circuits and the Host Organism
Genetic circuits are based on biochemical interactions within living cells. Most circuits use host resources to function, including transcription/translation machinery (e.g., ribosomes and RNAP), DNA replication equipment, and metabolites (e.g., amino acids). The availability of these resources and the details of the intracellular environment change significantly in different strain backgrounds, environmental conditions, media, growth rate, and cell density. When the first synthetic circuits were built, they were fragile and it was unclear why they would only work in specific conditions20,21. Now, there is a more precise understanding of the ways in which circuits break due to interactions with the host61. A better understanding these failure modes are and the methods natural systems use to overcome them will lead to new design rules for composing synthetic circuits.
A common observation is that some synthetic regulators can cause growth defects. Yet it remains unclear why certain regulators can be expressed at high levels with no noticeable impact whereas others in the same class are very toxic. This was evident in analyzing large libraries of TetR and σ factor homologues sourced from diverse organisms and transferred into E. coli71,100. Expression of some of regulators slowed E. coli growth, but the origin of this effect is unclear as it does not correlate with the number of predicted binding sites in the genome or off-target gene expression measured using RNA-seq. T7 RNAP is another part that can be very toxic when combined with a strong T7 promoter102. It is also unclear how this toxicity arises, but it could be due to the difficulty terminating T7 RNAP, which could cause circular transcription on a plasmid or expose mRNA by decoupling RNAP and ribosome progression. Circuits based on protein-protein interactions can also exhibit toxicity when the proteins bind to off-target partners. We observed this with anti-σ factors, which appear to bind and titrate native σ factors100. Small RNA with RBS-like sequences can also cause toxicity by titrate ribosomes, increasing expression variability, and reducing growth (Fig. 5a)145. Larger circuits are particularly sensitive to the toxicity that can arise from individual regulators because their effects are compounded when they are expressed together190.
Figure 5. Circuit performance within the context of a living cell. (a).
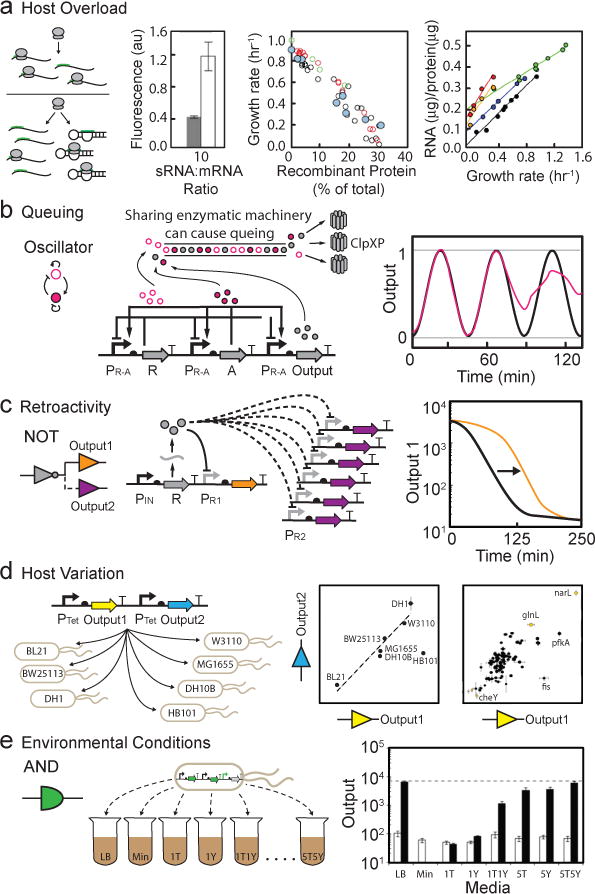
Recombinant protein expression can cause a growth defect by reducing the availability of host resources (e.g., RNAP and ribosomes). Here, synthetic sRNAs compete with mRNA for ribosomes to illustrate the impact of exogenous protein expression on host resource allocation. When sRNAs are produced (left graph, grey bars), ribosomes are titrated away from fluorescent protein mRNA and observed fluorescence is reduced relative to no sRNA (left graph, white bars)151. Center graph, colored circles represent the overexpression of different proteins in E. coli (blue: Pu promoter β-Galactosidase, red: T7 promoter β-Galactosidase, black: tac promoter ΔEF-Tu, green: bla promoter β-Lactamase)229. Right graph, colored circles represent growth of different bacterial strains as a function of rRNA supply (blue: E. coli 30°C, green: A. aerogenes 37°C, red: C. utilis 25°C, orange: C. utilis 30°C, black: N. crassa 30°C)229. (b) Queuing as a result of overloading the ClpXP protease machinery with proteins from a synthetic oscillator. The graph shows the difference between expected (black) and measured (red) dynamics for an oscillator affected by queuing166. (c) An additional output (PR2) on a high copy plasmid is added to the NOT gate. This causes retroactivity, which alters the activation dynamics of the original output (PR1) (black line: original dynamic response, orange line: retroactive effect)195. (d) One plasmid with two reporter proteins is transformed into different E. coli strains. The ratio of expression varies in some strains (left graph: wild type E. coli strains, right graph: KEIO collection knockouts)196. (e) Different media impact the performance of an AND gate based on T7 RNAP2,102. Data are shown for the circuit in the absence (white) and presence (black) of both inputs in different medias (LB: luria broth, Min: minimal media, #T and/or #L: minimal media supplemented with tryptone (#T = #g/L) or yeast extract (#Y = #g/L).
Circuits can also decrease growth rate by monopolizing host resources and slowing essential protein/RNA production (Fig. 5a)191. A small reduction in the growth rate can be a problem when using a circuit for industrial applications that rely on high product yields. A decrease in growth rate can reduce the dilution rate of circuit components and lead to unintended build up of proteins or RNA that can cause a circuit to fail. In fact, circuits can appear to function better when growth is impeded because slow dilution increases the observed concentration of transcription factors and reporters. Slow growth can also put pressure on the host organism to evolve away the burdensome circuit, either via homologous recombination, point mutations/deletions, or copy number reduction.
Circuits can diverge from their expected behavior when they use a limited resource that is shared with other cellular processes. Overburdening resources causes queuing, which results in a delay or reduction in circuit activity192. For example, when σ factors are overexpressed, they can occupy the entire pool of free core RNAP. When this happens, sigma factors must compete to bind to the core, which indirectly couples their activity and can disrupt host processes193. Native σ factors are able to avoid queuing by pulsing their expression such that they alternate the usage of core RNAP over time194. A similar coupling effect has been observed when the ClpXP protease is shared by regulators that have been modified to contain C-terminal tags for fast degradation. If too many proteins are targeted for degradation, the enzymatic machinery can become overwhelmed and force substrates to wait for processing166. The rapid degradation of regulators is important for dynamic circuits, such as oscillators, which will fail if the regulatory proteins accumulate (Fig. 5b).
Retroactivity can also interfere with circuit activity. Retroactivity is defined as the influence that a downstream genetic element can have on an upstream one and it describes the changes in circuit behavior that result from connecting new downstream modules to a circuit138. Downstream modules may affect the performance of upstream circuits by titrating regulators away from the original circuit. For example, connecting a second output to a NOT gate may cause retroactivity by titrating the repressor away from the original output promoter (Fig. 5c). Retroactivity will impact the NOT gate’s dynamics by increasing the time it takes to build up an adequate amount of protein to repress promoter activity195. Retroactivity that delays a circuit’s response to input stimulation can be alleviated by increasing expression of the problematic circuit component; however, increasing expression can lead to other trade-offs, including toxicity.
Strain variation can affect circuit performance in different ways. Differences in growth rate, ribosome concentration, and induction lag time have been identified as the main contributors to strain dependent variations in circuit performance196. In recent studies, these phenotypes have been correlated with specific genes by studying growth and circuit performance across single gene knockouts (Fig. 5d)196,197. Media and growth conditions can also impact circuit performance by altering promoter activity, protein stability, and regulator dilution198,199. These effects can be so severe that switching from LB to minimal media can cause circuits to fail (Fig. 5e)2.
One approach to reduce strain- and media-based variation is to use reference standards to report circuit performance. To this end, the Relative Expression Unit (REU) was introduced as a standard for reporting promoter measurements2,200. REUs report the promoter activity by normalizing measurements to a constitutive promoter standard in a strain that is treated identically and measured simultaneously. REU measurements have yielded reliable, reproducible data when compared across labs, strains, and media, which is important for transcriptional circuits that use promoters as inputs and outputs. In the future, this will facilitate the computer-aided design of large circuits.
Conclusions
The first circuits were built by repurposing a small number of regulators and genetic parts from other areas of genetic engineering. After early success47,88, these parts were put together in different combinations to explore the range of circuit functions that could be performed in the cell. We are now in a phase where there are >100 new regulators55,71,78–80,82,83,101,146,180 that are orthogonal and could theoretically be used to build synthetic regulatory networks at the scale of natural networks in bacteria201.
There are several key advances that have to happen before we can build and debug genetic circuits this large. First, computational tools have to be developed to aid the design process. These programs need to be able to simulate the dynamics of a circuit and convert the designs into a linear assembly of genetic parts68,202–205. Insulating DNA sequences will be critical in future circuits because the majority of parts will be in new contexts206,207. Second, new approaches to whole cell omics measurements have to be integrated into the debugging cycle. Currently, there is an over-reliance on fluorescent proteins as the output of circuits. However, transcriptomics is now sufficiently inexpensive such that it could be used to infer polymerase flux on many of the parts internal to a circuit208. Other single molecule approaches, such as ribosome and RNAP mapping, will become powerful when the experiments become more routine209,210. Third, new approaches need to be developed that can rapidly test circuits under conditions that are difficult to control in the cell. Circuits are sensitive to parameters like the number of ribosomes, RNAP, redox, temperature, and ATP all of which change in different cell types and conditions. However, these are difficult to measure in the cell without broadly impacting the host. To this end, the development of in vitro cell-free methods to debug circuits will be valuable for designing circuits that are robust to these changes211,211–220.
New biochemistries, tuning knobs, and troubleshooting methods are now converging for the sophisticated design and construction of genetic circuits. In these circuits, different classes of regulators can be used in a single circuit to fulfill specialized functions. In this vision, each regulator has found a niche within the larger circuit that exploits their strengths. For example, digital circuits can be used to integrate sensors and respond to a particular set of conditions, whereas analog circuitry can perform arithmetic function functions with a small number of regulators103. Integrases can store memory or cause an irreversible commitment. CRISPRi can regulate essentially any gene in the genome. A vision of this marriage is shown in Figure 6, which is an example of a commensal bacterium that has been engineered to produce a pharmaceutical while colonizing the gut. In it, repressor-based logic gates respond dynamically to environmental states and invertases record these observations. Analog circuits can be used to calculate a dosage rate and, if surpassed, CRISPRi knocks down specific host genes to arrest growth and avoid overmedication. Collectively, these new circuits and the tools and knowledge to connect and debug them will enable a new era of cellular programming and the applications that come with this capability.
Figure 6. Conceptual circuit for a therapeutic bacterium that colonizes a niche in the human microbiome and delivers a drug.
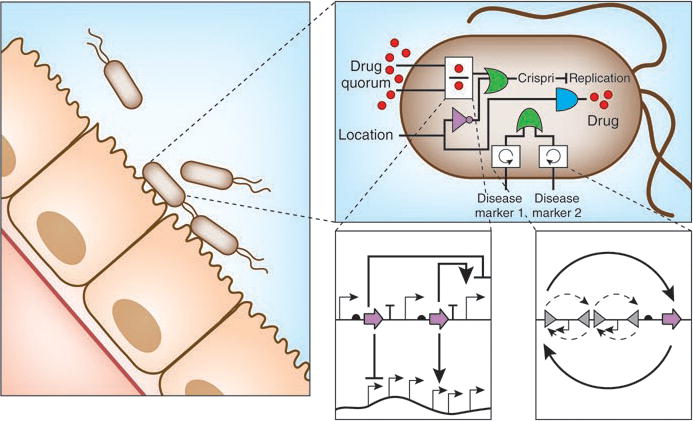
This circuit demonstrates how the different classes of regulators and circuits described in this review could be combined into a single system. The leftmost panel shows genetically modified bacteria that have colonized the interior of a human gastrointestinal tract. The upper right panel focuses on the conceptual circuit that the bacteria use to regulate their growth and deliver drugs to the human patient. An analog circuit103 (left) and irreversible recombinases (right) are highlighted in the insets to emphasize the diverse biochemistries used to build this circuit.
Acknowledgments
CAV and JANB are supported by the National Institute of General Medical Sciences (NIGMS grant P50 GMO98792 and R01 GM095765), Office of Naval Research (ONR) Multidisciplinary University Research Initiative (MURI grant 4500000552), the National Science Foundation Synthetic Biology Engineering Research Center (SynBERC EEC0540879), and Life Technologies (A114510). JANB is supported by a National Science Foundation Graduate Research Fellowship.
References
- 1.Dahl RH, et al. Engineering dynamic pathway regulation using stress-response promoters. Nat Biotechnol. 2013;31:1039–1046. doi: 10.1038/nbt.2689. [DOI] [PubMed] [Google Scholar]
- 2.Moser F, et al. Genetic Circuit performance under conditions relevant for industrial bioreactors. ACS Synth Biol. 2012;1:555–564. doi: 10.1021/sb3000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holtz WJ, Keasling JD. Engineering static and dynamic control of synthetic pathways. Cell. 2010;140:19–23. doi: 10.1016/j.cell.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Anesiadis N, Kobayashi H, Cluett WR, Mahadevan R. Analysis and design of a genetic circuit for dynamic metabolic engineering. ACS Synth Biol. 2013;2:442–452. doi: 10.1021/sb300129j. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, Keasling J. Biosensors and their applications in microbial metabolic engineering. Trends Microbiol. 2011;19:323–329. doi: 10.1016/j.tim.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich JA, Shis DL, Alikhani A, Keasling JD. Transcription factor-based screens and synthetic selections for microbial small-molecule biosynthesis. ACS Synth Biol. 2013;2:47–58. doi: 10.1021/sb300091d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schendzielorz G, et al. Taking control over control: use of product sensing in single cells to remove flux control at key enzymes in biosynthesis pathways. ACS Synth Biol. 2014;3:21–29. doi: 10.1021/sb400059y. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Carothers JM, Keasling JD. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat Biotechnol. 2012;30:354–359. doi: 10.1038/nbt.2149. [DOI] [PubMed] [Google Scholar]
- 9.Yi TM, Huang Y, Simon MI, Doyle J. Robust perfect adaptation in bacterial chemotaxis through integral feedback control. Proc Natl Acad Sci. 2000;97:4649–4653. doi: 10.1073/pnas.97.9.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnanathan K, Anderson SR, Billings SA, Kadirkamanathan V. A data-driven framework for identifying nonlinear dynamic models of genetic parts. ACS Synth Biol. 2012;1:375–384. doi: 10.1021/sb300009t. [DOI] [PubMed] [Google Scholar]
- 11.Carbonell P, Parutto P, Baudier C, Junot C, Faulon JL. Retropath: automated pipeline for embedded metabolic circuits. ACS Synth Biol. 2013 doi: 10.1021/sb4001273. [DOI] [PubMed] [Google Scholar]
- 12.Adams BL, et al. Evolved quorum sensing regulator, LsrR, for altered switching functions. ACS Synth Biol. 2013 doi: 10.1021/sb400068z. [DOI] [PubMed] [Google Scholar]
- 13.Umeyama T, Okada S, Ito T. Synthetic gene circuit-mediated monitoring of endogenous metabolites: identification of GAL11 as a novel multicopy Enhancer of s-adenosylmethionine level in yeast. ACS Synth Biol. 2013;2:425–430. doi: 10.1021/sb300115n. [DOI] [PubMed] [Google Scholar]
- 14.Stapleton JA, et al. Feedback control of protein expression in mammalian cells by tunable synthetic translational inhibition. ACS Synth Biol. 2012;1:83–88. doi: 10.1021/sb200005w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D, Xiao Y, Evans B, Zhang F. Negative feedback regulation of fatty acid production based on a malonyl-CoA sensor–actuator. ACS Synth Biol. 2013 doi: 10.1021/sb400158w. [DOI] [PubMed] [Google Scholar]
- 16.Siedler S, et al. SoxR as a single-cell biosensor for NADPH-consuming enzymes in Escherichia coli. ACS Synth Biol. 2014;3:41–47. doi: 10.1021/sb400110j. [DOI] [PubMed] [Google Scholar]
- 17.Medema MH, Breitling R, Bovenberg R, Takano E. Exploiting plug-and-play synthetic biology for drug discovery and production in microorganisms. Nat Rev Microbiol. 2011;9:131–137. doi: 10.1038/nrmicro2478. [DOI] [PubMed] [Google Scholar]
- 18.Fischbach M, Voigt CA. Prokaryotic gene clusters: A rich toolbox for synthetic biology. Biotechnol J. 2010;5:1277–1296. doi: 10.1002/biot.201000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frasch HJ, Medema MH, Takano E, Breitling R. Design-based re-engineering of biosynthetic gene clusters: plug-and-play in practice. Curr Opin Biotechnol. 2013;24:1144–1150. doi: 10.1016/j.copbio.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Temme K, Zhao D, Voigt CA. Refactoring the nitrogen fixation gene cluster from Klebsiella oxytoca. Proc Natl Acad Sci. 2012;109:7085–7090. doi: 10.1073/pnas.1120788109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao Z, et al. Refactoring the silent spectinabilin gene cluster using a plug-and-play scaffold. ACS Synth Biol. 2013;2:662–669. doi: 10.1021/sb400058n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oßwald C, et al. Modular construction of a functional artificial epothilone polyketide pathway. ACS Synth Biol. 2012 doi: 10.1021/sb300080t. [DOI] [PubMed] [Google Scholar]
- 23.Steidler L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 24.Anderson JC, Clarke EJ, Arkin AP, Voigt CA. Environmentally controlled invasion of cancer cells by engineered bacteria. J Mol Biol. 2006;355:619–627. doi: 10.1016/j.jmb.2005.10.076. [DOI] [PubMed] [Google Scholar]
- 25.Ruder WC, Lu T, Collins JJ. Synthetic biology moving into the clinic. Science. 2011;333:1248–1252. doi: 10.1126/science.1206843. [DOI] [PubMed] [Google Scholar]
- 26.Motta JP, et al. Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci Transl Med. 2012;4:1–12. doi: 10.1126/scitranslmed.3004212. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Kong Q, Curtiss R., III New technologies in developing recombinant attenuated Salmonella vaccine vectors. Microb Pathog. 2013;58:17–28. doi: 10.1016/j.micpath.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huh JH, Kittleson JT, Arkin AP, Anderson JC. Modular design of a synthetic payload delivery device. ACS Synth Biol. 2013;2:418–424. doi: 10.1021/sb300107h. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Bram EE, Weiss R. Genetically programmable pathogen sense and destroy. ACS Synth Biol. 2013;2:715–723. doi: 10.1021/sb4000417. [DOI] [PubMed] [Google Scholar]
- 30.Hwang IY, et al. Reprogramming microbes to be pathogen-seeking killers. ACS Synth Biol. 2013 doi: 10.1021/sb400077j. [DOI] [PubMed] [Google Scholar]
- 31.Prindle A, et al. Genetic circuits in Salmonella typhimurium. ACS Synth Biol. 2012;1:458–464. doi: 10.1021/sb300060e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volzing K, Borrero J, Sadowsky MJ, Kaznessis YN. Antimicrobial peptides targeting gram-negative pathogens, produced and delivered by lactic acid bacteria. ACS Synth Biol. 2013;2:643–650. doi: 10.1021/sb4000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasty J. Engineered microbes for therapeutic applications. ACS Synth Biol. 2012;1:438–439. doi: 10.1021/sb300105b. [DOI] [PubMed] [Google Scholar]
- 34.Danino T, Lo J, Prindle A, Hasty J, Bhatia SN. In vivo gene expression dynamics of tumor-targeted bacteria. ACS Synth Biol. 2012;1:465–470. doi: 10.1021/sb3000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Archer EJ, Robinson AB, Süel GM. Engineered E. coli that detect and respond to gut inflammation through nitric oxide sensing. ACS Synth Biol. 2012;1:451–457. doi: 10.1021/sb3000595. [DOI] [PubMed] [Google Scholar]
- 36.Antunes MS, et al. Programmable ligand detection system in plants through a synthetic signal transduction pathway. PLoS ONE. 2011;6:e16292. doi: 10.1371/journal.pone.0016292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Widmaier DM, et al. Engineering the Salmonella type III secretion system to export spider silk monomers. Mol Syst Biol. 2009;5 doi: 10.1038/msb.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernhardt K, et al. New tools for self-organized pattern formation. BMC Syst Biol. 2007;1:S10. [Google Scholar]
- 39.Xia XX, et al. Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber. Proc Natl Acad Sci. 2010;107:14059–14063. doi: 10.1073/pnas.1003366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Widmaier DM, Voigt CA. Quantification of the physiochemical constraints on the export of spider silk proteins by Salmonella type III secretion. Microb Cell Factories. 2010;9:78. doi: 10.1186/1475-2859-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aquea F, et al. A molecular framework for the inhibition of Arabidopsis root growth in response to boron toxicity. Plant Cell Environ. 2012;35:719–734. doi: 10.1111/j.1365-3040.2011.02446.x. [DOI] [PubMed] [Google Scholar]
- 42.Antunes MS, et al. A synthetic de-greening gene circuit provides a reporting system that is remotely detectable and has a re-set capacity. Plant Biotechnol J. 2006;4:605–622. doi: 10.1111/j.1467-7652.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- 43.Purnick PEM, Weiss R. The second wave of synthetic biology: from modules to systems. Nat Rev Mol Cell Biol. 2009;10:410–422. doi: 10.1038/nrm2698. [DOI] [PubMed] [Google Scholar]
- 44.Khoury GA, Smadbeck J, Kieslich CA, Floudas CA. Protein folding and de novo protein design for biotechnological applications. Trends Biotechnol. 2014;32:99–109. doi: 10.1016/j.tibtech.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis NE, Nagarajan H, Palsson BO. Constraining the metabolic genotype–phenotype relationship using a phylogeny of in silico methods. Nat Rev Microbiol. 2012;10:291–305. doi: 10.1038/nrmicro2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss R. Cellular computation and communications using engineered genetic regulatory networks. 2001 < http://dspace.mit.edu/handle/1721.1/8228>.
- 47.Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403:339–342. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- 48.Salis HM, Mirsky EA, Voigt CA. Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol. 2009;27:946–950. doi: 10.1038/nbt.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mutalik VK, et al. Precise and reliable gene expression via standard transcription and translation initiation elements. Nat Methods. 2013;10:354–360. doi: 10.1038/nmeth.2404. [DOI] [PubMed] [Google Scholar]
- 50.Cambray G, et al. Measurement and modeling of intrinsic transcription terminators. Nucleic Acids Res. 2013;41:5139–5148. doi: 10.1093/nar/gkt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodrigo G, Jaramillo A. AutoBioCAD: full biodesign automation of genetic circuits. ACS Synth Biol. 2013;2:230–236. doi: 10.1021/sb300084h. [DOI] [PubMed] [Google Scholar]
- 52.Voigt CA. Genetic parts to program bacteria. Curr Opin Biotechnol. 2006;17:548–557. doi: 10.1016/j.copbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Yokobayashi Y, Weiss R, Arnold FH. Directed evolution of a genetic circuit. Proc Natl Acad Sci. 2002;99:16587–16591. doi: 10.1073/pnas.252535999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellefson JW, et al. Directed evolution of genetic parts and circuits by compartmentalized partnered replication. Nat Biotechnol. 2014;32:97–101. doi: 10.1038/nbt.2714. [DOI] [PubMed] [Google Scholar]
- 55.Moon TS, Lou C, Tamsir A, Stanton BC, Voigt CA. Genetic programs constructed from layered logic gates in single cells. Nature. 2012;491:249–253. doi: 10.1038/nature11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haseltine EL, Arnold FH. Synthetic gene circuits: design with directed evolution. Annu Rev Biophys Biomol Struct. 2007;36:1–19. doi: 10.1146/annurev.biophys.36.040306.132600. [DOI] [PubMed] [Google Scholar]
- 57.Collins CH, Arnold FH, Leadbetter JR. Directed evolution of Vibrio fischeri LuxR for increased sensitivity to a broad spectrum of acyl-homoserine lactones. Mol Microbiol. 2005;55:712–723. doi: 10.1111/j.1365-2958.2004.04437.x. [DOI] [PubMed] [Google Scholar]
- 58.Sleight SC, Sauro HM. Randomized BioBrick assembly: a novel DNA assembly method for randomizing and optimizing genetic circuits and metabolic pathways. ACS Synth Biol. 2013;2:506–518. doi: 10.1021/sb4000542. [DOI] [PubMed] [Google Scholar]
- 59.Shong J, Collins CH. Engineering the esaR promoter for tunable quorum sensing-dependent gene expression. ACS Synth Biol. 2013;2:568–575. doi: 10.1021/sb4000433. [DOI] [PubMed] [Google Scholar]
- 60.Balagaddé FK, You L, Hansen CL, Arnold FH, Quake SR. Long-term monitoring of bacteria undergoing programmed population control in a microchemostat. Science. 2005;309:137–140. doi: 10.1126/science.1109173. [DOI] [PubMed] [Google Scholar]
- 61.Cardinale S, Arkin AP. Contextualizing context for synthetic biology – identifying causes of failure of synthetic biological systems. Biotechnol J. 2012;7:856–866. doi: 10.1002/biot.201200085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engler C, Gruetzner R, Kandzia R, Marillonnet S. Golden gate shuffling: a one-pot DNA shuffling Method based on type IIs restriction enzymes. PLoS ONE. 2009;4:e5553. doi: 10.1371/journal.pone.0005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gibson DG, et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 64.Hillson NJ, Rosengarten RD, Keasling JD. j5 DNA assembly design automation software. ACS Synth Biol. 2012;1:14–21. doi: 10.1021/sb2000116. [DOI] [PubMed] [Google Scholar]
- 65.Leguia M, Brophy JA, Densmore D, Asante A, Anderson JC. 2ab assembly: a methodology for automatable, high-throughput assembly of standard biological parts. J Biol Eng. 2013;7:2. doi: 10.1186/1754-1611-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Kok S, et al. Rapid and reliable DNA assembly via ligase cycling reaction. ACS Synth Biol. 2014;3:97–106. doi: 10.1021/sb4001992. [DOI] [PubMed] [Google Scholar]
- 67.Paetzold B, Carolis C, Ferrar T, Serrano L, Lluch-Senar M. In situ overlap and sequence synthesis during DNA assembly. ACS Synth Biol. 2013;2:750–755. doi: 10.1021/sb400067v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clancy K, Voigt CA. Programming cells: towards an automated ‘Genetic Compiler’. Curr Opin Biotechnol. 2010;21:572–581. doi: 10.1016/j.copbio.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Friedland AE, et al. Synthetic gene networks that count. Science. 2009;324:1199–1202. doi: 10.1126/science.1172005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stricker J, et al. A fast, robust and tunable synthetic gene oscillator. Nature. 2008;456:516–519. doi: 10.1038/nature07389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stanton BC, et al. Genomic mining of prokaryotic repressors for orthogonal logic gates. Nat Chem Biol. 2014;10:99–105. doi: 10.1038/nchembio.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Endy D. Foundations for engineering biology. Nature. 2005;438:449–453. doi: 10.1038/nature04342. [DOI] [PubMed] [Google Scholar]
- 73.Khalil AS, Collins JJ. Synthetic biology: applications come of age. Nat Rev Genet. 2010;11:367–379. doi: 10.1038/nrg2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liang JC, Bloom RJ, Smolke CD. Engineering biological systems with synthetic RNA molecules. Mol Cell. 2011;43:915–926. doi: 10.1016/j.molcel.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lim WA. Designing customized cell signalling circuits. Nat Rev Mol Cell Biol. 2010;11:393–403. doi: 10.1038/nrm2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weber W, Fussenegger M. Synthetic gene networks in mammalian cells. Curr Opin Biotechnol. 2010;21:690–696. doi: 10.1016/j.copbio.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Liu W, Yuan JS, Stewart CN., Jr Advanced genetic tools for plant biotechnology. Nat Rev Genet. 2013;14:781–793. doi: 10.1038/nrg3583. [DOI] [PubMed] [Google Scholar]
- 78.Bikard D, et al. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013;41:7429–7437. doi: 10.1093/nar/gkt520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qi LS, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu CC, et al. An adaptor from translational to transcriptional control enables predictable assembly of complex regulation. Nat Methods. 2012;9:1088–1094. doi: 10.1038/nmeth.2184. [DOI] [PubMed] [Google Scholar]
- 81.Mutalik VK, Qi L, Guimaraes JC, Lucks JB, Arkin AP. Rationally designed families of orthogonal RNA regulators of translation. Nat Chem Biol. 2012;8:447–454. doi: 10.1038/nchembio.919. [DOI] [PubMed] [Google Scholar]
- 82.Beerli RR, Barbas CF. Engineering polydactyl zinc-finger transcription factors. Nat Biotechnol. 2002;20:135–141. doi: 10.1038/nbt0202-135. [DOI] [PubMed] [Google Scholar]
- 83.Garg A, Lohmueller JJ, Silver PA, Armel TZ. Engineering synthetic TAL effectors with orthogonal target sites. Nucleic Acids Res. 2012;40:7584–7595. doi: 10.1093/nar/gks404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moscou MJ, Bogdanove AJ. A Simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501–1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 85.Takeda Y, Folkmanis A, Echols H. Cro regulatory protein specified by bacteriophage lambda. Structure, DNA-binding, and repression of RNA synthesis. J Biol Chem. 1977;252:6177–6183. [PubMed] [Google Scholar]
- 86.Ptashne M, Hopkins N. The operators controlled by the lambda phage repressor. Proc Natl Acad Sci U S A. 1968;60:1282–1287. doi: 10.1073/pnas.60.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhan J, et al. Develop reusable and combinable designs for transcriptional logic gates. Mol Syst Biol. 2010;6 doi: 10.1038/msb.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elowitz MB, Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000;403:335–338. doi: 10.1038/35002125. [DOI] [PubMed] [Google Scholar]
- 89.Guet CC, Elowitz MB, Hsing W, Leibler S. Combinatorial synthesis of genetic networks. Science. 2002;296:1466–1470. doi: 10.1126/science.1067407. [DOI] [PubMed] [Google Scholar]
- 90.Hasty J, Dolnik M, Rottschäfer V, Collins JJ. Synthetic gene network for entraining and amplifying cellular oscillations. Phys Rev Lett. 2002;88:148101. doi: 10.1103/PhysRevLett.88.148101. [DOI] [PubMed] [Google Scholar]
- 91.Hooshangi S, Thiberge S, Weiss R. Ultrasensitivity and noise propagation in a synthetic transcriptional cascade. Proc Natl Acad Sci U S A. 2005;102:3581–3586. doi: 10.1073/pnas.0408507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaber R, et al. Designable DNA-binding domains enable construction of logic circuits in mammalian cells. Nat Chem Biol. 2014;10:203–208. doi: 10.1038/nchembio.1433. [DOI] [PubMed] [Google Scholar]
- 93.Lohmueller JJ, Armel TZ, Silver PA. A tunable zinc finger-based framework for Boolean logic computation in mammalian cells. Nucleic Acids Res. 2012;40:5180–5187. doi: 10.1093/nar/gks142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peacock RWS, Sullivan KA, Wang CL. Tetracycline-regulated expression implemented through transcriptional activation combined with proximal and distal repression. ACS Synth Biol. 2012;1:156–162. doi: 10.1021/sb200029a. [DOI] [PubMed] [Google Scholar]
- 95.Mercer AC, Gaj T, Sirk SJ, Lamb BM, Barbas CF. Regulation of endogenous human gene expression by ligand-inducible TALE transcription factors. ACS Synth Biol. 2013 doi: 10.1021/sb400114p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Purcell O, Peccoud J, Lu TK. Rule-based design of synthetic transcription factors in eukaryotes. ACS Synth Biol. 2013 doi: 10.1021/sb400134k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lienert F, et al. Two- and three-input TALE-based AND logic computation in embryonic stem cells. Nucleic Acids Res. 2013;41:9967–9975. doi: 10.1093/nar/gkt758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Temme K, Hill R, Segall-Shapiro TH, Moser F, Voigt CA. Modular control of multiple pathways using engineered orthogonal T7 polymerases. Nucleic Acids Res. 2012;40:8773–8781. doi: 10.1093/nar/gks597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Esvelt KM, Carlson JC, Liu DR. A system for the continuous directed evolution of biomolecules. Nature. 2011;472:499–503. doi: 10.1038/nature09929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rhodius VA, et al. Design of orthogonal genetic switches based on a crosstalk map of σs, anti-σs, and promoters. Mol Syst Biol. 2013;9 doi: 10.1038/msb.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang B, Kitney RI, Joly N, Buck M. Engineering modular and orthogonal genetic logic gates for robust digital-like synthetic biology. Nat Commun. 2011;2:508. doi: 10.1038/ncomms1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anderson JC, Voigt CA, Arkin AP. Environmental signal integration by a modular AND gate. Mol Syst Biol. 2007;3 doi: 10.1038/msb4100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Daniel R, Rubens JR, Sarpeshkar R, Lu TK. Synthetic analog computation in living cells. Nature. 2013;497:619–623. doi: 10.1038/nature12148. [DOI] [PubMed] [Google Scholar]
- 104.Buchler NE, Gerland U, Hwa T. On schemes of combinatorial transcription logic. Proc Natl Acad Sci. 2003;100:5136–5141. doi: 10.1073/pnas.0930314100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Calles B, Lorenzo V. de. Expanding the boolean logic of the prokaryotic transcription factor XylR by functionalization of permissive sites with a protease-target sequence. ACS Synth Biol. 2013;2:594–603. doi: 10.1021/sb400050k. [DOI] [PubMed] [Google Scholar]
- 106.Ramalingam KI, Tomshine JR, Maynard JA, Kaznessis YN. Forward engineering of synthetic bio-logical AND gates. Biochem Eng J. 2009;47:38–47. [Google Scholar]
- 107.Lou C, et al. Synthesizing a novel genetic sequential logic circuit: a push-on push-off switch. Mol Syst Biol. 2010;6 doi: 10.1038/msb.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Regot S, et al. Distributed biological computation with multicellular engineered networks. Nature. 2011;469:207–211. doi: 10.1038/nature09679. [DOI] [PubMed] [Google Scholar]
- 109.Ausländer S, Ausländer D, Müller M, Wieland M, Fussenegger M. Programmable single-cell mammalian biocomputers. Nature. 2012;487:123–127. doi: 10.1038/nature11149. [DOI] [PubMed] [Google Scholar]
- 110.Tamsir A, Tabor JJ, Voigt CA. Robust multicellular computing using genetically encoded NOR gates and chemical/‘wires/’. Nature. 2011;469:212–215. doi: 10.1038/nature09565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shis DL, Bennett MR. Library of synthetic transcriptional AND gates built with split T7 RNA polymerase mutants. Proc Natl Acad Sci. 2013;110:5028–5033. doi: 10.1073/pnas.1220157110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Basu S, Mehreja R, Thiberge S, Chen MT, Weiss R. Spatiotemporal control of gene expression with pulse-generating networks. Proc Natl Acad Sci U S A. 2004;101:6355–6360. doi: 10.1073/pnas.0307571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen D, Arkin AP. Sequestration-based bistability enables tuning of the switching boundaries and design of a latch. Mol Syst Biol. 2012;8 doi: 10.1038/msb.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Atkinson MR, Savageau MA, Myers JT, Ninfa AJ. Development of genetic circuitry exhibiting toggle switch or oscillatory behavior in Escherichia coli. Cell. 2003;113:597–607. doi: 10.1016/s0092-8674(03)00346-5. [DOI] [PubMed] [Google Scholar]
- 115.Fung E, et al. A synthetic gene–metabolic oscillator. Nature. 2005;435:118–122. doi: 10.1038/nature03508. [DOI] [PubMed] [Google Scholar]
- 116.Tigges M, Dénervaud N, Greber D, Stelling J, Fussenegger M. A synthetic low-frequency mammalian oscillator. Nucleic Acids Res. 2010;38:2702–2711. doi: 10.1093/nar/gkq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Argos P, et al. The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 1986;5:433–440. doi: 10.1002/j.1460-2075.1986.tb04229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gopaul DN, Van Duyne GD. Structure and mechanism in site-specific recombination. Curr Opin Struct Biol. 1999;9:14–20. doi: 10.1016/s0959-440x(99)80003-7. [DOI] [PubMed] [Google Scholar]
- 119.Ham TS, Lee SK, Keasling JD, Arkin AP. A tightly regulated inducible expression system utilizing the fim inversion recombination switch. Biotechnol Bioeng. 2006;94:1–4. doi: 10.1002/bit.20916. [DOI] [PubMed] [Google Scholar]
- 120.Ham TS, Lee SK, Keasling JD, Arkin AP. Design and construction of a double inversion recombination switch for heritable sequential genetic memory. PLoS ONE. 2008;3:e2815. doi: 10.1371/journal.pone.0002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moon TS, et al. Construction of a genetic multiplexer to toggle between chemosensory pathways in Escherichia coli. J Mol Biol. 2011;406:215–227. doi: 10.1016/j.jmb.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bonnet J, Yin P, Ortiz ME, Subsoontorn P, Endy D. Amplifying genetic logic gates. Science. 2013;340:599–603. doi: 10.1126/science.1232758. [DOI] [PubMed] [Google Scholar]
- 123.Siuti P, Yazbek J, Lu TK. Synthetic circuits integrating logic and memory in living cells. Nat Biotechnol. 2013;31:448–452. doi: 10.1038/nbt.2510. [DOI] [PubMed] [Google Scholar]
- 124.Bonnet J, Subsoontorn P, Endy D. Rewritable digital data storage in live cells via engineered control of recombination directionality. Proc Natl Acad Sci. 2012;109:8884–8889. doi: 10.1073/pnas.1202344109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sorek R, Lawrence CM, Wiedenheft B. CRISPR-mediated adaptive immune systems in bacteria and archaea. Annu Rev Biochem. 2013;82:237–266. doi: 10.1146/annurev-biochem-072911-172315. [DOI] [PubMed] [Google Scholar]
- 126.Sashital DG, Wiedenheft B, Doudna JA. Mechanism of foreign DNA selection in a bacterial adaptive immune system. Mol Cell. 2012;46:606–615. doi: 10.1016/j.molcel.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mali P, et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol. 2013;31:833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Farzadfard F, Perli SD, Lu TK. Tunable and multifunctional eukaryotic transcription factors based on CRISPR/Cas. ACS Synth Biol. 2013;2:604–613. doi: 10.1021/sb400081r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gilbert LA, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maeder ML, et al. CRISPR RNA-guided activation of endogenous human genes. Nat Methods. 2013;10:977–979. doi: 10.1038/nmeth.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Perez-Pinera P, et al. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat Methods. 2013;10:973–976. doi: 10.1038/nmeth.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Esvelt KM, et al. Orthogonal Cas9 proteins for RNA-guided gene regulation and editing. Nat Methods. 2013;10:1116–1121. doi: 10.1038/nmeth.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hsu PD, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Larson MH, et al. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc. 2013;8:2180–2196. doi: 10.1038/nprot.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gossen M, Bonin AL, Bujard H. Control of gene activity in higher eukaryotic cells by prokaryotic regulatory elements. Trends Biochem Sci. 1993;18:471–475. doi: 10.1016/0968-0004(93)90009-c. [DOI] [PubMed] [Google Scholar]
- 136.Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507:62–67. doi: 10.1038/nature13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nishimasu H, et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014;156:935–949. doi: 10.1016/j.cell.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Del Vecchio D, Ninfa AJ, Sontag ED. Modular cell biology: retroactivity and insulation. Mol Syst Biol. 2008;4 doi: 10.1038/msb4100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Deltcheva E, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Simons RW, Kleckner N. Translational control of IS10 transposition. Cell. 1983;34:683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- 141.Kittle JD, Simons RW, Lee J, Kleckner N. Insertion sequence IS10 anti-sense pairing initiates by an interaction between the 5′ end of the target RNA and a loop in the anti-sense RNA. J Mol Biol. 1989;210:561–572. doi: 10.1016/0022-2836(89)90132-0. [DOI] [PubMed] [Google Scholar]
- 142.Ma C, Simons RW. The IS10 antisense RNA blocks ribosome binding at the transposase translation initiation site. EMBO J. 1990;9:1267–1274. doi: 10.1002/j.1460-2075.1990.tb08235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qi L, Lucks JB, Liu CC, Mutalik VK, Arkin AP. Engineering naturally occurring trans-acting non-coding RNAs to sense molecular signals. Nucleic Acids Res. 2012;40:5775–5786. doi: 10.1093/nar/gks168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu CC, Qi L, Yanofsky C, Arkin AP. Regulation of transcription by unnatural amino acids. Nat Biotechnol. 2011;29:164–168. doi: 10.1038/nbt.1741. [DOI] [PubMed] [Google Scholar]
- 145.Callura JM, Dwyer DJ, Isaacs FJ, Cantor CR, Collins JJ. Tracking, tuning, and terminating microbial physiology using synthetic riboregulators. Proc Natl Acad Sci. 2010;107:15898–15903. doi: 10.1073/pnas.1009747107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nielsen AA, Segall-Shapiro TH, Voigt CA. Advances in genetic circuit design: novel biochemistries, deep part mining, and precision gene expression. Curr Opin Chem Biol. 2013;17:878–892. doi: 10.1016/j.cbpa.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 147.Bintu L, et al. Transcriptional regulation by the numbers: models. Curr Opin Genet Dev. 2005;15:116–124. doi: 10.1016/j.gde.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bintu L, et al. Transcriptional regulation by the numbers: applications. Curr Opin Genet Dev. 2005;15:125–135. doi: 10.1016/j.gde.2005.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Voigt CA, Wolf DM, Arkin AP. The Bacillus subtilis sin operon an evolvable network motif. Genetics. 2005;169:1187–1202. doi: 10.1534/genetics.104.031955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Strogatz SH. Nonlinear dynamics and chaos: with application to physics, biology, chemistry, and engineering. Westview press; 2000. [Google Scholar]
- 151.Ang J, Harris E, Hussey BJ, Kil R, McMillen DR. Tuning response curves for synthetic biology. ACS Synth Biol. 2013;2:547–567. doi: 10.1021/sb4000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gottesman S. Proteases and their targets in Escherichia coli. Annu Rev Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- 153.Naryshkin N, Revyakin A, Kim Y, Mekler V, Ebright RH. Structural organization of the RNA polymerase-promoter open complex. Cell. 2000;101:601–611. doi: 10.1016/s0092-8674(00)80872-7. [DOI] [PubMed] [Google Scholar]
- 154.Davis JH, Rubin AJ, Sauer RT. Design, construction and characterization of a set of insulated bacterial promoters. Nucleic Acids Res. 2011;39:1131–1141. doi: 10.1093/nar/gkq810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kittleson JT, Cheung S, Anderson JC. Rapid optimization of gene dosage in E. coli using DIAL strains. J Biol Eng. 2011;5:10. doi: 10.1186/1754-1611-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cox RS, Surette MG, Elowitz MB. Programming gene expression with combinatorial promoters. Mol Syst Biol. 2007;3 doi: 10.1038/msb4100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen S, et al. Automated design of genetic toggle switches with predetermined bistability. ACS Synth Biol. 2012;1:284–290. doi: 10.1021/sb300027y. [DOI] [PubMed] [Google Scholar]
- 158.Koshland DE, Goldbeter A, Stock JB. Amplification and adaptation in regulatory and sensory systems. Science. 1982;217:220–225. doi: 10.1126/science.7089556. [DOI] [PubMed] [Google Scholar]
- 159.Vilar JM, Saiz L. DNA looping in gene regulation: from the assembly of macromolecular complexes to the control of transcriptional noise. Curr Opin Genet Dev. 2005;15:136–144. doi: 10.1016/j.gde.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 160.Johnson S, Lindén M, Phillips R. Sequence dependence of transcription factor-mediated DNA looping. Nucleic Acids Res. 2012;40:7728–7738. doi: 10.1093/nar/gks473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Legewie S, Dienst D, Wilde A, Herzel H, Axmann IM. Small RNAs establish delays and temporal thresholds in gene expression. Biophys J. 2008;95:3232–3238. doi: 10.1529/biophysj.108.133819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Levine E, Zhang Z, Kuhlman T, Hwa T. Quantitative characteristics of gene regulation by small RNA. PLoS Biol. 2007;5:e229. doi: 10.1371/journal.pbio.0050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Buchler NE, Cross FR. Protein sequestration generates a flexible ultrasensitive response in a genetic network. Mol Syst Biol. 2009;5 doi: 10.1038/msb.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lu MS, Mauser JF, Prehoda KE. Ultrasensitive synthetic protein regulatory networks using mixed decoys. ACS Synth Biol. 2012;1:65–72. doi: 10.1021/sb200010w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee TH, Maheshri N. A regulatory role for repeated decoy transcription factor binding sites in target gene expression. Mol Syst Biol. 2012;8 doi: 10.1038/msb.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cookson NA, et al. Queueing up for enzymatic processing: correlated signaling through coupled degradation. Mol Syst Biol. 2011;7 doi: 10.1038/msb.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shen J, Liu Z, Zheng W, Xu F, Chen L. Oscillatory dynamics in a simple gene regulatory network mediated by small RNAs. Phys Stat Mech Its Appl. 2009;388:2995–3000. [Google Scholar]
- 168.Borujeni AE, Channarasappa AS, Salis HM. Translation rate is controlled by coupled trade-offs between site accessibility, selective RNA unfolding and sliding at upstream standby sites. Nucleic Acids Res. 2013;42:2646–2659. doi: 10.1093/nar/gkt1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Geyer PK. The role of insulator elements in defining domains of gene expression. Curr Opin Genet Dev. 1997;7:242–248. doi: 10.1016/s0959-437x(97)80134-7. [DOI] [PubMed] [Google Scholar]
- 170.Lou C, Stanton B, Chen YJ, Munsky B, Voigt CA. Ribozyme-based insulator parts buffer synthetic circuits from genetic context. Nat Biotechnol. 2012;30:1137–1142. doi: 10.1038/nbt.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jeong W, Kang C. Start site selection at lacUV5 promoter affected by the sequence context around the initiation sites. Nucleic Acids Res. 1994;22:4667–4672. doi: 10.1093/nar/22.22.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Walker KA, Osuna R. Factors affecting start site selection at the Escherichia coli fis promoter. J Bacteriol. 2002;184:4783–4791. doi: 10.1128/JB.184.17.4783-4791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kudla G, Murray AW, Tollervey D, Plotkin JB. Coding-Sequence Determinants of Gene Expression in Escherichia coli. Science. 2009;324:255–258. doi: 10.1126/science.1170160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Goodman DB, Church GM, Kosuri S. Causes and effects of N-terminal codon bias in bacterial genes. Science. 2013;342:475–479. doi: 10.1126/science.1241934. [DOI] [PubMed] [Google Scholar]
- 175.Kosuri S, et al. Composability of regulatory sequences controlling transcription and translation in Escherichia coli. Proc Natl Acad Sci. 2013;110:14024–14029. doi: 10.1073/pnas.1301301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Qi L, Haurwitz RE, Shao W, Doudna JA, Arkin AP. RNA processing enables predictable programming of gene expression. Nat Biotechnol. 2012;30:1002–1006. doi: 10.1038/nbt.2355. [DOI] [PubMed] [Google Scholar]
- 177.Chen YJ, et al. Characterization of 582 natural and synthetic terminators and quantification of their design constraints. Nat Methods. 2013;10:659–664. doi: 10.1038/nmeth.2515. [DOI] [PubMed] [Google Scholar]
- 178.Yao AI, et al. Promoter element arising from the fusion of standard BioBrick parts. ACS Synth Biol. 2013;2:111–120. doi: 10.1021/sb300114d. [DOI] [PubMed] [Google Scholar]
- 179.Villalobos A, Ness JE, Gustafsson C, Minshull J, Govindarajan S. Gene Designer: a synthetic biology tool for constructing artificial DNA segments. BMC Bioinformatics. 2006;7:1–8. doi: 10.1186/1471-2105-7-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rhodius VA, Mutalik VK, Gross CA. Predicting the strength of UP-elements and full-length E. coli σE promoters. Nucleic Acids Res. 2012;40:2907–2924. doi: 10.1093/nar/gkr1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brewster RC, Jones DL, Phillips R. Tuning promoter strength through RNA polymerase binding site design in Escherichia coli. PLoS Comput Biol. 2012;8:e1002811. doi: 10.1371/journal.pcbi.1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Weller K, Recknagel RD. Promoter strength prediction based on occurrence frequencies of consensus patterns. J Theor Biol. 1994;171:355–359. doi: 10.1006/jtbi.1994.1239. [DOI] [PubMed] [Google Scholar]
- 183.Seo SW, et al. Predictive design of mRNA translation initiation region to control prokaryotic translation efficiency. Metab Eng. 2013;15:67–74. doi: 10.1016/j.ymben.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 184.De Hoon MJL, Makita Y, Nakai K, Miyano S. Prediction of transcriptional terminators in Bacillus subtilis and related species. PLoS Comput Biol. 2005;1:e25. doi: 10.1371/journal.pcbi.0010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lesnik EA, et al. Prediction of rho-independent transcriptional terminators in Escherichia coli. Nucleic Acids Res. 2001;29:3583–3594. doi: 10.1093/nar/29.17.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rackham O, Chin JW. A network of orthogonal ribosome mRNA pairs. Nat Chem Biol. 2005;1:159–166. doi: 10.1038/nchembio719. [DOI] [PubMed] [Google Scholar]
- 187.Sleight SC, Sauro HM. Visualization of evolutionary stability dynamics and competitive fitness of Escherichia coli engineered with randomized multigene circuits. ACS Synth Biol. 2013;2:519–528. doi: 10.1021/sb400055h. [DOI] [PubMed] [Google Scholar]
- 188.Lovett ST, Hurley RL, Sutera VA, Aubuchon RH, Lebedeva MA. Crossing over between regions of limited homology in Escherichia coli: RecA-dependent and RecA-independent pathways. Genetics. 2002;160:851–859. doi: 10.1093/genetics/160.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sleight SC, Bartley BA, Lieviant JA, Sauro HM. Designing and engineering evolutionary robust genetic circuits. J Biol Eng. 2010;4:12. doi: 10.1186/1754-1611-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Arkin AP, Fletcher DA. Fast, cheap and somewhat in control. Genome Biol. 2006;7:114. doi: 10.1186/gb-2006-7-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dong H, Nilsson L, Kurland CG. Gratuitous overexpression of genes in Escherichia coli leads to growth inhibition and ribosome destruction. J Bacteriol. 1995;177:1497–1504. doi: 10.1128/jb.177.6.1497-1504.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mather WH, Hasty J, Tsimring LS, Williams RJ. Translational cross talk in gene networks. Biophys J. 2013;104:2564–2572. doi: 10.1016/j.bpj.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Grigorova IL, Phleger NJ, Mutalik VK, Gross CA. Insights into transcriptional regulation and σ competition from an equilibrium model of RNA polymerase binding to DNA. Proc Natl Acad Sci. 2006;103:5332–5337. doi: 10.1073/pnas.0600828103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Levine JH, Lin Y, Elowitz MB. Functional roles of pulsing in genetic circuits. Science. 2013;342:1193–1200. doi: 10.1126/science.1239999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jayanthi S, Nilgiriwala KS, Del Vecchio D. Retroactivity controls the temporal dynamics of gene transcription. ACS Synth Biol. 2013;2:431–441. doi: 10.1021/sb300098w. [DOI] [PubMed] [Google Scholar]
- 196.Cardinale S, Joachimiak MP, Arkin AP. Effects of genetic variation on the E. coli host-circuit interface. Cell Rep. 2013;4:231–237. doi: 10.1016/j.celrep.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 197.Canton B, Labno A, Endy D. Refinement and standardization of synthetic biological parts and devices. Nat Biotechnol. 2008;26:787–793. doi: 10.1038/nbt1413. [DOI] [PubMed] [Google Scholar]
- 198.Tagami H, Inada T, Kunimura T, Aiba H. Glucose lowers CRP* levels resulting in repression of the lac operon in cells lacking cAMP. Mol Microbiol. 1995;17:251–258. doi: 10.1111/j.1365-2958.1995.mmi_17020251.x. [DOI] [PubMed] [Google Scholar]
- 199.Purcell O, Grierson CS, di Bernardo M, Savery NJ. Temperature dependence of ssrA-tag mediated protein degradation. J Biol Eng. 2012;6:10. doi: 10.1186/1754-1611-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kelly JR, et al. Measuring the activity of BioBrick promoters using an in vivo reference standard. J Biol Eng. 2009;3:4. doi: 10.1186/1754-1611-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cho BK, Charusanti P, Herrgård MJ, Palsson BØ. Microbial regulatory and metabolic networks. Curr Opin Biotechnol. 2007;18:360–364. doi: 10.1016/j.copbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 202.Yaman F, Bhatia S, Adler A, Densmore D, Beal J. Automated selection of synthetic biology parts for genetic regulatory networks. ACS Synth Biol. 2012;1:332–344. doi: 10.1021/sb300032y. [DOI] [PubMed] [Google Scholar]
- 203.Bhatia S, Densmore D. Pigeon: a design visualizer for synthetic biology. ACS Synth Biol. 2013;2:348–350. doi: 10.1021/sb400024s. [DOI] [PubMed] [Google Scholar]
- 204.Huynh L, Tsoukalas A, Köppe M, Tagkopoulos I. SBROME: a scalable optimization and module matching framework for automated biosystems design. ACS Synth Biol. 2013;2:263–273. doi: 10.1021/sb300095m. [DOI] [PubMed] [Google Scholar]
- 205.Roehner N, Myers CJ. A methodology to annotate systems biology markup language models with the synthetic biology open language. ACS Synth Biol. 2013 doi: 10.1021/sb400066m. [DOI] [PubMed] [Google Scholar]
- 206.Arkin AP. A wise consistency: engineering biology for conformity, reliability, predictability. Curr Opin Chem Biol. 2013;17:893–901. doi: 10.1016/j.cbpa.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 207.Ceroni F, Furini S, Stefan A, Hochkoeppler A, Giordano E. A synthetic post-transcriptional controller to explore the Mmodular design of gene circuits. ACS Synth Biol. 2012;1:163–171. doi: 10.1021/sb200021s. [DOI] [PubMed] [Google Scholar]
- 208.Seo JH, et al. Multiple-omic data analysis of Klebsiella pneumoniae MGH 78578 reveals its transcriptional architecture and regulatory features. BMC Genomics. 2012;13:679. doi: 10.1186/1471-2164-13-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ingolia NT, Brar GA, Rouskin S, McGeachy AM, Weissman JS. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat Protoc. 2012;7:1534–1550. doi: 10.1038/nprot.2012.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Becker AH, Oh E, Weissman JS, Kramer G, Bukau B. Selective ribosome profiling as a tool for studying the interaction of chaperones and targeting factors with nascent polypeptide chains and ribosomes. Nat Protoc. 2013;8:2212–2239. doi: 10.1038/nprot.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shin J, Noireaux V. An E. coli cell-free expression toolbox: application to synthetic gene circuits and artificial cells. ACS Synth Biol. 2012;1:29–41. doi: 10.1021/sb200016s. [DOI] [PubMed] [Google Scholar]
- 212.Siegal-Gaskins D, Noireaux V, Murray RM. Biomolecular resource utilization in elementary cell-free gene circuits. Proceedings of the American Control Conference. 2013 < http://arxiv.org/abs/1307.0178>.
- 213.Karzbrun E, Shin J, Bar-Ziv RH, Noireaux V. Coarse-grained dynamics of protein synthesis in a cell-free system. Phys Rev Lett. 2011;106:048104. doi: 10.1103/PhysRevLett.106.048104. [DOI] [PubMed] [Google Scholar]
- 214.Noireaux V, Bar-Ziv R, Libchaber A. Principles of cell-free genetic circuit assembly. Proc Natl Acad Sci. 2003;100:12672–12677. doi: 10.1073/pnas.2135496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Karig DK, Jung SY, Srijanto B, Collier CP, Simpson ML. Probing cell-free gene expression noise in femtoliter volumes. ACS Synth Biol. 2013;2:497–505. doi: 10.1021/sb400028c. [DOI] [PubMed] [Google Scholar]
- 216.Lentini R, et al. Fluorescent proteins and in vitro genetic organization for cell-free synthetic biology. ACS Synth Biol. 2013;2:482–489. doi: 10.1021/sb400003y. [DOI] [PubMed] [Google Scholar]
- 217.Davidson EA, Meyer AJ, Ellefson JW, Levy M, Ellington AD. An in vitro Autogene. ACS Synth Biol. 2012;1:190–196. doi: 10.1021/sb3000113. [DOI] [PubMed] [Google Scholar]
- 218.Niederholtmeyer H, Xu L, Maerkl SJ. Real-Time mRNA Measurement during an in vitro transcription and translation reaction using binary probes. ACS Synth Biol. 2013;2:411–417. doi: 10.1021/sb300104f. [DOI] [PubMed] [Google Scholar]
- 219.Chizzolini F, Forlin M, Cecchi D, Mansy SS. Gene position more strongly influences cell-free protein expression from operons than T7 transcriptional promoter strength. ACS Synth Biol. 2013 doi: 10.1021/sb4000977. [DOI] [PubMed] [Google Scholar]
- 220.Sun ZZ, Yeung E, Hayes CA, Noireaux V, Murray RM. Linear DNA for rapid prototyping of synthetic biological circuits in an Escherichia coli based TX-TL cell-free system. ACS Synth Biol. 2013 doi: 10.1021/sb400131a. [DOI] [PubMed] [Google Scholar]
- 221.Peralta-Yahya PP, et al. Identification and microbial production of a terpene-based advanced biofuel. Nat Commun. 2011;2:483. doi: 10.1038/ncomms1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jonnalagadda SB, Becker JU, Sel’kov EE, Betz A. Flux regulation in glycogen-induced oscillatory glycolysis in cell-free extracts of Saccharomyces carlsbergensis. Biosystems. 1982;15:49–58. doi: 10.1016/0303-2647(82)90016-8. [DOI] [PubMed] [Google Scholar]
- 223.Loo LWM, et al. cis-Expression QTL analysis of established colorectal cancer risk variants in colon tumors and adjacent normal tissue. PLoS ONE. 2012;7:e30477. doi: 10.1371/journal.pone.0030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Klavins E. Proportional-integral control of stochastic gene regulatory networks. 2010 49th IEEE Conf Decis Control CDC. 2010:2547–2553. [Google Scholar]
- 225.Bernard P, Couturier M. Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes. J Mol Biol. 1992;226:735–745. doi: 10.1016/0022-2836(92)90629-x. [DOI] [PubMed] [Google Scholar]
- 226.Hussein R, Lim HN. Direct comparison of small RNA and transcription factor signaling. Nucleic Acids Res. 2012;40:7269–7279. doi: 10.1093/nar/gks439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hucka M, et al. The systems biology markup language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics. 2003;19:524–531. doi: 10.1093/bioinformatics/btg015. [DOI] [PubMed] [Google Scholar]
- 228.Gottesman S, Roche E, Zhou Y, Sauer RT. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Scott M, Gunderson CW, Mateescu EM, Zhang Z, Hwa T. Interdependence of Cell Growth and Gene Expression: Origins and Consequences. Science. 2010;330:1099–1102. doi: 10.1126/science.1192588. [DOI] [PubMed] [Google Scholar]


