Abstract
Transplantation of islet or beta cells is seen as the cure for type 1 diabetes since it allows physiological regulation of blood glucose levels without requiring any compliance from the patients. In order to circumvent the use of immunosuppressive drugs (and their side effects), semipermeable membranes have been developed to encapsulate and immunoprotect transplanted cells. This review presents the historical developments of immunoisolation and provides an update on the current research in this field. A particular emphasis is laid on the fabrication, characterization and performance of membranes developed for immunoisolation applications.
Keywords: Cell Immunoisolation, Cell Transplantation, Inorganic Membranes, Macrocapsules, Microcapsules, Organic Membranes, Type 1 Diabetes, Review
2. INTRODUCTION
As of today, it is estimated that diabetes affects 346 million people worldwide(1). This chronic disease is characterized by high levels of blood glucose (hyperglycemia) that, if untreated, lead to devastating complications such as heart disease, stroke, loss of vision, retinopathy, kidney failure, nervous system damage and even death(2). Type 1 diabetes (T1D), also known as “juvenile diabetes” or “insulin dependent diabetes mellitus”, represents about 10% of all cases. It is the most severe form of diabetes: the pancreatic beta cells (located in the islets of Langerhans) are progressively destroyed by the patient's immune system (autoimmune attack). These cells are essential since they normally produce the hormone insulin in amounts that regulate the blood glucose concentration. Their destruction reduces and then permanently stops the insulin production which translates in high blood glucose levels. The current treatment consists of several subcutaneous injections of insulin every day (based on a careful monitoring of blood glucose levels via finger pricking). This treatment is obviously inconvenient for patients and the bolus-type administration of insulin is not physiological. This has lead to the development of portable insulin pumps(3) and the hope to develop an artificial pancreas that would combine an implantable insulin pump, a continuous glucose monitoring system and a control algorithm(4, 5). However, before the development of a clinical application, a closed-loop insulin delivery approach must still surmount obstacles related to the delay of insulin action when infused subcutaneously and to the blood glucose estimation made by subcutaneous interstitial measurement(5). More complex algorithms are thus needed to compensate for the time lags impairing the system reactivity.
An alternate approach that would be a real cure for type 1 diabetes patients is to replace their pancreas(6) or alternatively to transplant functional islets of Langerhans or beta cells alone(7-11). This constitutes the best solution in terms of physiological regulation of blood glucose and patient compliance. However, two major problems have hindered the successful development of transplantations up to now: 1) the supply of pancreas/islet cells available for transplantation is very limited; 2) the transplanted organs/cells are subject to the host's immune attack and destruction (resulting in brief viability and efficacy of the graft in the best case scenario). A lot of progress has been made to tackle both of these problems over the last few years but more effort will be needed to bring a safe transplantation approach to the whole community of type 1 diabetic patients.
2.1. Immunosuppressed pancreas/islet transplantation
Approximately 30,000 pancreases have been transplanted worldwide since 1966 with the annual numbers of transplants reaching a steady state since the late 1990s(6). The procedure is usually performed in conjunction with a kidney transplant for patients with type 1 diabetes and chronic renal failure (the patients receive both a pancreas and a kidney from a single deceased organ donor). With this method, the overall 1-year pancreas graft survival rate that achieves insulin independence is 85% and decreases to about 50% 10 years after transplantation(6, 12, 13). However, recipients of these transplants must adhere to a strict lifelong immunosuppressive therapy in order to avoid rejection of the grafts(6, 12). The currently used anti-rejection medications present side effects that are not acceptable for patients with type 1 diabetes only (insulin injections still constitute a better treatment for those). The complications associated with immunosuppressive drugs include increased incidence of infection and malignancy, decreased wound healing, renal dysfunction...(14-16) Only patients who require a kidney transplant are then incentivized in a whole pancreas graft.
An alternative therapy is to transplant isolated islet cells instead of a whole pancreas (here again, graft recipients have to take immunosuppressive drugs for the rest of their lives, with the associated complications)(11, 16-18). Only the endocrine component of the pancreas is transplanted in this case (~2-3% of the pancreas mass), considerably reducing the risks of the surgical procedure(17). Islets are usually injected in the portal vein and transported via the bloodstream into the liver where they take up residence(16). Over 1400 islet transplantation procedures have been performed worldwide since 1974(17). These transplantations lacked success before 2000: only 8% of recipients maintained insulin independence one year after transplantation from 1990 to 1998(19). However, in 2000, Shapiro et al. reported 7 consecutive recipients who were all insulin independent one year after transplantation, which is commonly referred to as the Edmonton Protocol(7). Success rates decreased after 5 years, with only 10% of patients still achieving insulin independence(8). Nevertheless, clinical benefits are observed after islet transplantation even in the absence of insulin independence since the incidence of life-threatening hypoglycemia decreases dramatically(20).
2.2. Cell sources for islet transplantation
A normal human pancreas contains roughly 1 million islets(11); however islets purified from donor pancreases require several steps to be ready for transplantation, and all these steps can be detrimental to the harvested islets(17). Consequently, 2-4 donor pancreases are required to perform a successful islet transplantation procedure(17). The significant mismatch between the number of islets needed for transplantation and the islet availability highlights the urgent need to find additional islet sources. Various cell sources are currently envisaged to overcome this obstacle(17, 18, 21-24): expansion/replication of existing human beta cells(25-28), differentiation of human embryonic stem cells (hESC) to beta cells(29-35), conversion of either pancreatic or nonpancreatic adult stem/progenitor cells to beta cells(36-48) and animal islet cells. Among xenogeneic sources, porcine islets are particularly interesting due to the close homology between porcine and human insulin and the similarity of islets between both species(49).
Despite great promises from these diversified islet sources, several issues must be overcome before large-scale utilization will be made possible. Cells derived from stem cells are not yet fully functional beta cells and animal cells induce a more aggressive immune rejection than human cells. Moreover, the risk of transmittable diseases between animal and human will have to be carefully investigated(50).
2.3. Immunoisolated islet/cell transplantation
In order to circumvent the use of immunosuppressive drugs and their side effects following transplantation of islet or beta cells, the idea of encapsulating the cells in a protective semipermeable membrane has been developed. Such a membrane has to be immunoisolating (i.e. impede contact with immune cells, antibodies, complement...) yet at the same time this membrane must allow rapid transport of glucose, insulin, nutrients (oxygen (O2)...) and waste products. Conceptually immunoisolation membranes are possible given the relatively smaller size of glucose (180 Da; Stokes radius: 0.4 nm)(51) and insulin (monomer/hexamer: 5.8/34.2 kDa; 1.35-2.75 nm)(52) compared to inflammatory cells (size of ~10 μm) and molecules responsible for immune rejection such as immunoglobulin G (IgG: 150 kDa; Stokes radius: 5.9 nm)(53, 54), complement C1q (410 kDa)(55), immunoglobulin M (IgM: 910 kDa)(55). Figure 1 presents the concept of immunoisolation on a molecular weight scale.
Figure 1.
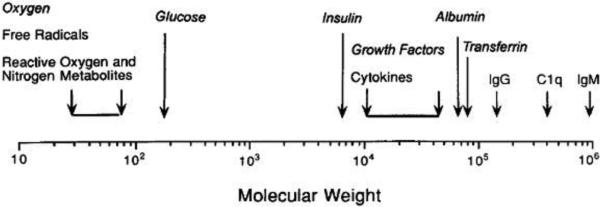
Molecular weight spectrum in immunoisolation: molecules that should pass the immunoisolation barrier are in italics, all other molecules may be deleterious to implanted tissue. Reproduced with permission from (56).
Cell encapsulation is sometimes referred to as cell-based drug delivery: in the case of islet or beta cells, they secrete insulin (a therapeutic protein) in quantities related to external glucose stimulation.
Two distinct approaches have been developed to immunoisolate cells using semipermeable membranes (see Figure 2): macrocapsules (macroencapsulation) confine a large number of transplanted cells in an implantable device (a macrocapsule can be transplanted extravascularly or intravascularly) and microcapsules (microencapsulation) only contain from 1 to 3 islet cells in each device (typically 400-800 μm in diameter)(56, 57) (a very large number of these microbeads need to be transplanted in this case). New microencapsulation techniques with thinner or even nanoscaled coatings have recently been developed, introducing the terms conformal coatings and nanoencapsulation in the community(57). Nanoencapsulated islets could also be used in conjunction with macrocapsules to enhance the immune protection.
Figure 2.
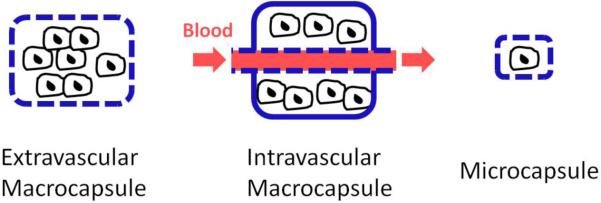
Schematic representation of different cellular encapsulation approaches.
Possible transplantation sites are different for each type of device(58). Extravascular macrocapsules are generally transplanted intraperitoneally or subcutaneously whereas intravascular macrocapsules are connected as a shunt to systemic blood circulation. With macrocapsules, it is possible to encapsulate islet cells at a high tissue-like density or dispersed in a chosen extracellular gel matrix (alginate, chitosan, agarose...)(55). Extravascular microcapsules are usually transplanted in the peritoneal cavity.
All of these immunoisolation approaches present advantages and disadvantages that will be detailed in section 3. Other reviews of interest may be found elsewhere as well(49, 56-65).
Despite many promising encapsulation studies and the development of numerous devices, cell encapsulation has yet to make an impact in the clinical setting. Some of the factors limiting widespread application of encapsulated islets include incomplete isolation of islets from the immune system and inadequate physiological nutrient accessibility for cells within the devices.
In fact, transplanting immunoisolated islet cells is challenging since both the innate and the adaptive immune responses have to be overcome. Membranes presenting pores smaller than 1 μm easily block the passage of immune cells but blockage of antibodies (the smallest being IgG) or cytokines is much more challenging(55). These problems are even more important for xenogeneic transplants. Avgoustiniatos et al. estimated that both IgM and C1q should be completely blocked by a membrane with a maximum pore diameter of 30 nm(55). However, IgG will require smaller pores to be fully blocked and this will significantly hinder the diffusion of glucose and insulin. Thus, a compromise has to be found. It is also interesting to note that a tiny permeability of IgG may not be so detrimental to encapsulated cells: Iwata et al. showed that complement components are rapidly inactivated, and therefore it should be sufficient to hinder IgG diffusion in the first days after transplantation rather than totally block it(66).
As mentioned before, the other issue with encapsulated cells is poor access to oxygen and nutrients caused by the membrane barrier. In a healthy pancreas, islets are perfused by blood and supplied with O2 at arterial levels(55, 56). When encapsulated, islets can easily be located more than 150-200 μm away from the nearest blood vessel, which can induce hypoxic conditions leading to cell necrosis(55, 56, 67, 68). Furthermore, biocompatibility of the device material is extremely important(56): if the foreign body response induces the formation of an avascular layer on the membrane (typically on the order of 100 μm), there is little chance that the cells will be able to survive. On the other hand, biocompatible materials can induce neovascularization (growth and proliferation of new blood vessels near the membrane interface) that will drastically improve diffusion of O2 and nutrients. However, the development of such vasculature takes 2-3 weeks, meaning that grafted cells will experience the most severe nutrient limitations immediately after transplantation since they will only depend on peripheral diffusion from the surrounding tissue(55). Some solutions are currently investigated to tackle this problem: prevascularization of the macrocapsule before adding cells(69), faster vascularization with growth factors(70), incorporation of oxygen carriers or oxygen-generating biomaterials(71-78).
Another interesting idea to improve islet transplant success would be to use controlled size beta cell clusters(79, 80) within the capsules. It has been shown that clusters of cells secrete insulin more efficiently than single cells(80-84), indicating that communication between cells should be preserved in transplantation situations to improve cell function. Moreover, cluster size could be optimized to avoid insufficient O2 or nutrient supply to the cells.
3. IMMUNOISOLATION OF TRANSPLANTED ISLETS: DIFFERENT APPROACHES
3.1. Extravascular macrocapsules
3.1.1. Advantages and disadvantages
Extravascular macrocapsules present several advantages for cell encapsulation: they can be made from a variety of different materials, they are easily retrievable and/or reloadable (clear advantage if an issue arises after implantation), they can be implanted with minimally invasive surgeries and the extracellular matrix can be chosen independently (important to ensure a suitable environment for encapsulated cells). Pancreatic beta cell behavior is known to depend on the surrounding matrix environment(85). Moreover, a very tight pore size distribution is now achievable for inorganic nanoporous membranes(61), which is of utmost importance for immunoisolation properties. Finally, the fact that cells can exist as clusters (as in a healthy pancreas) within the macrocapsules is beneficial regarding the communication between cells and the synchronization of insulin secretion pulses(86).
The main drawback of these macrocapsules is their lack of direct vascular access. This results in increased diffusion times for O2 and glucose. As a consequence, the production of insulin and its release are also delayed. Moreover, if large concentrations of insulin build up inside the chamber, the enclosed islets may be subject to insulin inhibition from their own products(87). Another problem of macroencapsulation is the potential lack of oxygenation for the islets located far away from the membranes, creating risks of central necrosis and cellular death. It is well known that cells have to be close to blood vessels, typically at a distance less than 150-200 μm to allow diffusion of O2 and nutrients and to perform metabolic processes appropriately(55, 56, 67, 68). Thus, membranes have to be thin (to address the possible hypoxic conditions for the inner part of the graft) and at the same time mechanically and chemically robust.
3.1.2. Historical aspects and developments
The first study that used encapsulated biological material for diabetes treatment was reported in 1933 by Bisceglie who placed human insulinoma tissue in membranous bags transplanted into rats(88). However, the concept of immunoisolated transplantation was really developed in the early 1950's by Algire et al.(89-93). These researchers were interested in immune rejection mechanisms and wanted to know if cellular or humoral factors were responsible for the destruction of nonvascularized transplants. In order to answer that question, they designed a diffusion chamber by gluing together two thin membrane disks made of porous cellulose (supported by plastic rings) around the cells (see Figure 3). They used different pore sizes: some allowing free passage of host immune cells (leukocytes and macrophages) and others blocking those entities (pore diameters < 0.45 μm). Their results showed that allogenic tissue transplanted in mice was destroyed more rapidly with large pore membranes that permitted external cellular invasion. The lack of contact with immune cells prevented the direct antigen presentation pathway that leads to immune-mediated destruction.
Figure 3.
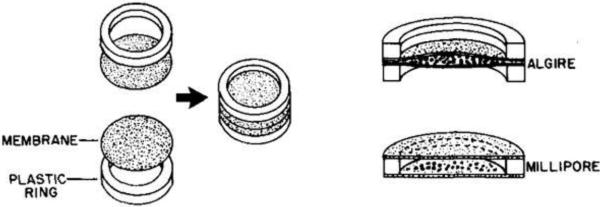
Algire and Millipore diffusion chambers for extravascular macroencapsulation. Reproduced with permission from (64).
Subsequently, many endocrine tissues were transplanted in similar extravascular diffusion chambers. However, only after the isolation of the islets of Langerhans in 1965 by Moskalewski(94) did immunoisolated islet cell research really begin.
The company Millipore produced a commercial extravascular transplantation chamber with 0.45 μm pores by modifying the design from Algire(64) (see Figure 3). Researchers used this chamber to confirm the improved survival of grafts within protective capsules(95, 96). Other types of membranes were developed and tested(64): nitrocellulose ester membranes, cuprophane (cellulose) bags, hydrogel membranes, hollow fibers... In 1991, Lacy et al. developed hollow fibers fabricated from an acrylic copolymer and used them to encapsulate rat islets immobilized in an alginate hydrogel. They transplanted these fibers either subcutaneously or intraperitoneally in diabetic mice(97) and these implants reverted diabetes for up to 60 days.
Transplant failure occurred sooner or later with these early extravascular macrocapsules due to fibroblastic overgrowth inside or/and outside the chamber(64). Inadequate oxygenation of the grafted cells was also advocated as a significant issue with macrocapsules(56).
Major advances have been made since the early developments of extravascular macrocapsules, which have been reviewed in details elsewhere(61, 64). Section 4 will also give a complete update about current designs for immunoprotective membranes for extravascular macroencapsulation of islet/beta cells.
3.1.3. Commercialization of extravascular macrocapsules
Several companies have produced extravascular chambers for islet encapsulation since the 1980s. Baxter Healthcare Corp. (Round Lake, Illinois) designed an encapsulation planar device called TheraCyte (see Figure 4) that is still in use today in several laboratories around the world(98, 99). The TheraCyte system is made of polytetrafluoroethylene (PTFE) and is composed of a cell impermeable membrane (400 nm pore diameter) laminated to another membrane (5 μm pore diameter) that promotes neovascularization (angiogenesis). This double layer approach seeks to reduce diffusion time delays by development of a new vasculature within the large pores while the small pores immunoprotect the encapsulated cells. Angiogenesis can also be promoted by infusion of vascular endothelial growth factor (VEGF) transcutaneously into the device(70). Some success has been achieved in animal models using islets encapsulated in TheraCyte devices(100-102); however, there are no reported studies of clinical success in human subjects and it is improbable that the 400 nm porous structure would lead to a full immunoisolation (IgG and cytokines will be able to cross the membrane of these devices).
Figure 4.
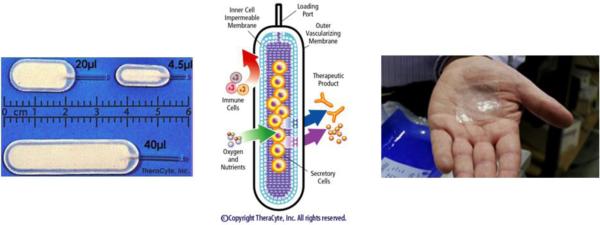
Commercial macroencapsulation devices: TheraCyte (left and middle) and Islet Sheet (right).
Several other companies have also come and gone over the years: Encelle (A.-L. Usala) (pig islets macroencapsulated in a hydrogel matrix wrapped in a polyester net coated with a stealth polymer, device transplanted intramuscularly), BetaGene (C. Newgard) partnered with Gore Hybrid Technologies (cartridge of immortalized cells inserted in a prevascularized flexible tube transplanted subcutaneously) and iMedd (T. Desai and M. Ferrari) (macroencapsulation of islets with nanoporous silicon membranes)(103-107). The failure of these companies was mainly related to difficulties in achieving long-term viability of the encapsulated islets (fibroblastic growth over membranes, poor islet oxygenation and poor diffusion of nutrients) and lack of funding due to unmet objectives.
Two other companies are still in operation today: Cerco Medical and ViaCyte. Cerco Medical (formerly Islet Sheet Medical, Scott R. King, San Francisco) is developing the Islet Sheet, which consists of islets encapsulated in an alginate sheet(108, 109). This very thin (0.3 mm) device is the size of a business card (4 cm × 6 cm) and can sustain approximately 100,000 islets (see Figure 4). About 6 sheets will be necessary per transplanted human patient to achieve insulin independence. The envisioned implantation sites are the peritoneal cavity or a subcutaneous space. The Islet Sheet is intended to be fully retrievable and replaceable, ensuring safety. Cerco Medical is probably the most advanced company working with macroencapsulation today and is currently performing trials on pancreatectomized dogs (their pancreas has been totally removed to mimic type 1 diabetes) with encapsulated canine islets.
ViaCyte (formerly Novocell) (San Diego) is developing a macroencapsulation device called Encaptra (based on the TheraCyte device) that is designed to be transplanted subcutaneously. This retrievable and vascularizing capsule will contain pancreatic progenitor cells that are expected to differentiate into functioning islet cells(29-32, 110). Stem-cell derived pancreatic islets represent a promising alternative to the short supply of human islets available for transplantation. However, Matveyenko et al. are currently doubtful about the clinical application of such engineered cell types(33). They believe the extent of endocrine cell formation and secretory function is insufficient to be clinically relevant.
3.2. Intravascular macrocapsules
3.2.1. Intravascular diffusion chambers: advantages and disadvantages
Intravascular diffusion chambers (see schematic representation in Figure 5) present a clear advantage over extravascular devices: they have a direct access to blood and thus more accurate tracking of blood glucose levels. This reduces delays for insulin secretion and the islets are also very well oxygenated due to the blood proximity. Extracellular matrix can also be chosen independently with intravascular macrocapsules to ensure the most suitable environment for cells.
Figure 5.
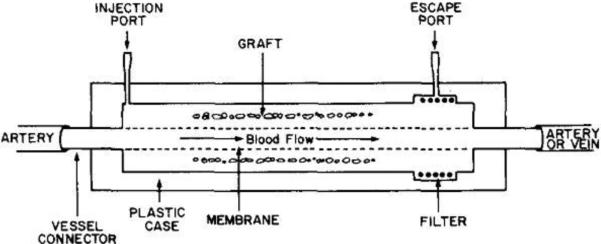
Schematic representation of an intravascular diffusion chamber. Reproduced with permission from (64).
However, these devices also present disadvantages, including a complicated and risky surgical procedure and blood coagulation issues after transplantation(64). Blood flow distortion at the interface between the blood vessel and the device can induce platelet deposition leading to thrombosis. Moreover, the tubing and membranes themselves can cause blood coagulation. Unfortunately, systemic anticoagulation medication is unadvisable for people suffering from type 1 diabetes.
3.2.2. Intravascular diffusion chambers: historical aspects and developments
Development of intravascular macrocapsules began in 1972 when Knazek et al. fabricated an artificial capillary system for continuous perfusion culture systems(111). Three years later, Chick et al. reported the first culture of islets in such a device and referred to it as an “artificial endocrine pancreas”(112). In 1977, Chick managed to reverse diabetes in rats by transplanting this device in the aorta with heparin anticoagulation(113). The membrane was made of Amicon (polyacrylonitrilepolyvinyl chloride copolymer (PAN/PVC)). Tze(114), Sun(115) and Orsetti(116) published similar results with diabetic rats. These studies constituted a proof of concept for intravascular macrocapsules, although coagulation and hemorrhage complications occurred after several days(117-119). Scharp et al. obtained similar results with tubular polycarbonate membranes(64).
Development of intravascular capsules was subsequently slowed or even stopped because of clotting issues. However, a report from Prochorov et al. published in 2008 has renewed interest in this approach(86). They transplanted a nylon macrocapsule (pore diameter: 1-2 μm) into the arteria profunda femoris or into the forearm cubital vein of 19 diabetic human patients, 3 of them with diabetes resulting from pancreonecrosis (non-immune nature). They used islets from fetal rabbits and no immunosuppressive therapy was used, only standard antithrombotic therapy for 5 days after surgery. Positive results were still observed in 14 patients two years after transplantation. Exogenous insulin demand was reduced by 60-65% and hypo- and hyperglycemic comas disappeared completely. C-peptide and immunoreactive insulin levels increased significantly.
T-cell immunity to grafting was absent and neither vascular lumen narrowing nor thrombosis was observed. However, approximately 40% of the islets died in the first weeks because of poor vascularization in the chamber (neoangiogenesis only developed in the macrocapsule after 2 weeks).
3.2.3. Intravascular ultrafiltration chambers
A slight design modification of intravascular diffusion chambers lead to ultrafiltration chambers (see schematic representation in Figure 6). This configuration eliminates any diffusion-based delay in the transport of nutrients and therapeutic products: blood is ultrafiltered by the membrane, crosses the islets and stimulates them with respect to its glucose concentration and finally delivers the secreted insulin via the venous connection. This approach permits the best oxygen and nutrient availability to encapsulated cells. However, these intravascular macrocapsules present the same blood coagulation problems as the diffusion chambers. Moreover, deposition of proteins can also occur on ultrafiltration membranes over time, ultimately leading to clogging and thrombosis(64).
Figure 6.
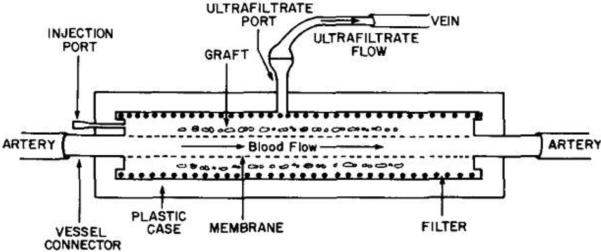
Schematic representation of an intravascular ultrafiltration chamber. Reproduced with permission from (64).
Ultrafiltration chambers have been used in diabetic rats by Reach et al.(120) and Scharp et al.(64). However, the devices only worked for hours before being clotted. Their development has been on hold since then.
3.2.4. Commercialization of intravascular macrocapsules
In 1985, Hayes and Chick founded BioHybrid Technologies that developed a reseedable intravascular chamber with limited success transplanting allogenic islets into pancreatectomized dogs(121). Their device was connected to the vascular system as an arteriovenous shunt. 6 out of 10 dogs remained insulin-independent after 5 months but the glycemic control in response to a meal or an intravenous glucose tolerance test remained abnormal. They also implanted a device with bovine islets in a pancreatectomized dog that remained insulin-independent for 80 days. Developments of this company were halted because of the previously mentioned issues with intravascular approaches.
3.3. Microcapsules
3.3.1. Advantages and disadvantages
Microencapsulation presents several advantages: the microbeads (see Figure 7) are implanted via minimally invasive surgery (simple injections are even possible), and their high surface to volume ratio confers better diffusion characteristics than extravascular macrocapsules (at least in theory). Faster diffusion kinetics are beneficial for cell oxygenation and glucose-stimulated insulin production and release.
Figure 7.
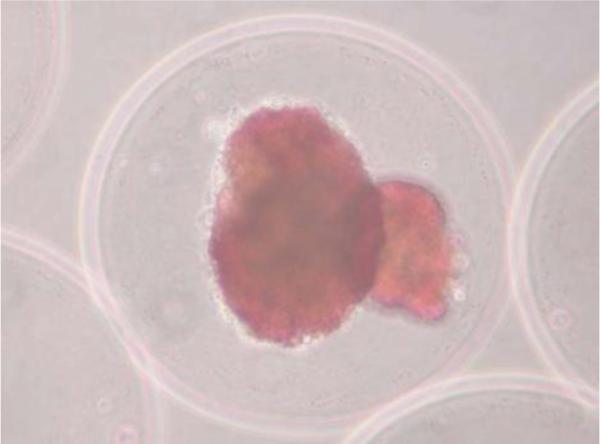
Two human pancreatic islets encapsulated in an alginate-based microcapsule (the red color is obtained after staining with dithizone that binds to zinc ions present in beta cells). Reproduced with permission from (149).
However, microcapsules also present several disadvantages. They are difficult if not impossible to retrieve after implantation (that may be very dangerous in case of a complication). They have indirect access to blood, which causes delays in diffusion of O2, glucose and insulin. The thickness of microcapsules is also a barrier for diffusion, although recent strategies have permitted a reduction of it. Moreover, since the cells are encapsulated while forming the microcapsules, these lack the capacity to choose a different material for the extracellular matrix. Another problem arises from the broad pore size distributions of microcapsules associated with their polymeric nature (with the exception of recently developed self-folding microcontainers presented in section 4.2.4., microcapsules can only be made of polymers). This could be an issue for complete immunoisolation of cells. Indeed, even if only 1% of pores are larger than the cut-off goal, passage of antibodies, complement, and cytokines will be sufficient to initiate immunorejection pathways(56). Finally, microcapsules prevent formation of clusters of encapsulated cells, unlike a real pancreas.
3.3.2. Materials
The most popular materials for microencapsulation are alginates. Alginates (primarily extracted from seaweeds) are natural anionic polysaccharides composed of homopolymeric regions of beta-D-mannuronic acid (“M-blocks”) and alpha-L-guluronic acid (“G-blocks”) interspaced with regions of mixed sequence (“MG-blocks”). They have hydrogel-forming properties with di- or trivalent cations (Ca2+, Ba2+, Fe3+...) used as cross-linking agents(122). Alginates are a good material choice for cell encapsulation due to their good biocompatibility and the fact that the encapsulation procedure can be performed under mild conditions not detrimental to cells(123).
Alginate-based microencapsulation usually consists of extruding a suspension made of a solution of sodium alginate plus islets through a microdroplet generator that incorporates a peristaltic pump and an air flow source (electrostatic droplet generation)(62). The suspension is continuously cut (by air shearing forces) into small spherical droplets. These drop into a positively-charged cation bath (usually CaCl2 or BaCl2) and immediately turn into water-immiscible gel microbeads that contain one or a few islets. The beads are then coated with an aminoacidic cation solution, typically poly-L-lysine (PLL) or poly-L-ornithine (PLO). The amine groups bind to carboxylic alginate radicals, preventing access by unwanted cellular and humoral mediators of the host's immune system(62). Less frequently, non-spherical microcapsules have also been produced by using polymeric replica molds in polydimethylsiloxane (PDMS)(124) or polypropylene (PP)(125).
3.3.3. Historical aspects and developments
The term microencapsulation was first mentioned by Chang in 1964 to describe aqueous solutions of protein within polymer microcapsules of 1-100 μm in diameter(126). However, the first microencapsulation of pancreatic islets was performed by Lim and Sun in 1980(127). They showed that insulin was released from spherical microcapsules made of alginate-polylysine-polyethyleneimine. They also managed to revert diabetes in rats for 3 weeks using intraperitoneal implants. The graft failed after that period due to poor material biocompatibility. Biocompatibility was improved in 1984 by O'shea and Sun who used intraperitoneal implants of islets in alginate-polylysine-alginate microcapsules(128). Diabetes was reverted in rats for up to 1 year. Subsequent chemical purification of alginates further improved the biocompatibility of microcapsules(129-131). Polyethylene glycol (PEG) hydrogels have also been used as a coating on microcapsules to improve their biocompatibility(132). In 1997, Wang et al. evaluated over a thousand combinations of water-soluble polyanions and polycations to find the best polymer for encapsulation of living cells(133). Their most promising combination consisted of sodium alginate, cellulose sulfate, poly(methylene-co-guanidine) hydrochloride, calcium chloride and sodium chloride. This formulation allowed independent control of capsule size, wall thickness, mechanical strength and permeability. Reversal of diabetes was maintained for up to 6 months in mice with intraperitoneal implants.
Besides alginates, other materials have also been studied for microencapsulation, including sol-gel silica (SiO2)(134, 135), polyacrylates(136), agarose(137, 138), chitosan(139)...
In order to improve nutrient availability for microencapsulated cells, several groups tried to decrease the thickness of the capsules. With this perspective, they developed techniques to directly deposit very thin (1.5 to 50 μm)(57) conformal coatings of protective biomaterial (usually alginate) on the surface of islets. The transplant volume in this case is determined only by the size of objects being coated and the coating thickness, reducing void volume and diffusion delays. In theory, immunoisolation could also be achieved by applying even thinner coatings down to submicron or nanoscale thickness. The terms nanoencapsulation and molecular camouflage have been introduced to refer to this subclass of microcapsules(57). Polyethylene glycol (PEG) chains are usually anchored to the cell or islet surface to create a barrier preventing molecular recognition between cell surface receptors and soluble ligands(140, 141). Attachment of PEG is generally performed by covalently coupling PEG to amines of cell surface proteins or carbohydrates, or by direct insertion of PEG-lipid conjugates into the cell membrane(141). Despite promising results with PEG protected islets transplanted in rats, it is unclear how long PEG coatings will remain stable enough to provide protection for a graft(57). Besides PEGylation, it is also possible to construct nanothin films of controlled permeability and surface chemistry directly on the surface of cells via layer-by-layer (LbL) polymer self-assembly(57, 142, 143). Polyelectrolyte multilayer (PEM) films are created that way by using polycations and polyanions: poly(L-lysine)(PLL)/alginate, chitosan/hyaluronic acid, PLL/hyaluronic acid, poly(diallyldimethylammonium chloride)/poly(styrene sulfonate)(PSS)... However, the possible toxicity of polycations and the immunoisolation properties of these multilayers need to be further investigated.
Microcapsules have already been evaluated in clinical tests on humans. Soon-Shiong reported the first case of transplantation into a human in 1994(144). This team used human cadaveric islets microencapsulated in purified alginate with a high guluronic acid content. Insulin independence was demonstrated 9 months after the procedure, yet the patient was already on low-dose immunosuppression due to a kidney transplant.
More recently, in 2006, Calafiore led a clinical trial on non-immunosuppressed patients using alginate microcapsules (containing human islets) that were double-coated with poly-L-ornithine and sodium alginate(145). They first reported results from two patients showing amelioration of their mean daily blood glucose levels and reduction of daily exogenous insulin (although exogenous insulin independence was unsuccessful)(146). In 2011, they reported new results from a total of four non-immunosuppressed patients including the previous two patients(147). Patients were followed through 3 years after transplantation of the microcapsules and no sign of islet rejection was seen (absence of islet cell antibodies and anti-MHC class I-II antibodies). Amelioration of blood glucose levels was achieved for all patients as well as reduced need for exogenous insulin. However, these improvements dissipated over time and patients had reverted to their original exogenous insulin therapy regimen at the end of the trial.
Developments and current research in microencapsulation have also been extensively reviewed elsewhere(57, 62, 148-153). Rabanel specifically reviewed fabrication techniques for microcapsules(123). Among the different reviews, de Vos et al. provided recommendations for characterization of microcapsules for cellular encapsulation(151). They stressed the importance of standardizing characterization procedures to resolve current lab-to-lab variations and lack of reproducibility in organic microcapsules. They have identified five criteria that should be detailed in any research related to microencapsulation: polymer characterization (high-resolution NMR), permeability, surface properties (FT-IR, XPS, TOF-SIMS, Microscopies), biocompatibility and storage conditions.
3.3.4. Commercialization of microcapsules
The company Living Cell Technologies (LCT) (Auckland, New Zealand) has been using alginate microencapsulation over the last few years. LCT is investigating the use of encapsulated porcine islets following a study on a human recipient published in 2007(154). LCT is the first company to enter clinical trials using therapeutic porcine cell implants. They have completed a successful Phase I/IIa clinical trial in Russia and currently have Phase IIb clinical trials underway in New Zealand and Argentina. LCT and Otsuka Pharmaceutical Factory created a new company in 2011, Diatranz Otsuka Limited (DOL), to accelerate development and commercialization of their porcine cell product. LCT is poised to be the first company to launch a product on the market within the next few years.
MicroIslet (San Diego) also developed a strategy to microencapsulate porcine islets to treat type 1 diabetes in humans, but the company is no longer operating.
Microencapsulation is also currently envisaged by the company ALTuCELL (Dix Hills, New York) that plans to transplant microencapsulated porcine-derived Sertoli cells into humans to revert type 1 diabetes (Dr. Calafiore is also involved in this company)(155).
4. CURRENT IMMUNOPROTECTIVE MEMBRANE DESIGNS
This chapter will discuss the current membrane designs for cellular immunoprotection. It is divided in 2 parts: organic (polymeric) membranes and inorganic ones. Another recent review discusses broader medical and biological applications of nanoporous membranes(156).
4.1. Organic membranes
The first and most common materials used to fabricate immunoprotective membranes are polymers. Their main drawback is a relatively broad pore size distribution (variations as large as 30% for polymeric membranes formed by solvent-casting)(157). The use of ion-track etching to form polycarbonate filter membranes (e.g.: Isopore from Millipore) has permitted much tighter pore size distributions (~10%) but these membranes have low porosities (maximum 20%), limited pore sizes and randomly distributed pores across their surface(158). These limited properties have excluded commercial track etch filters from cell encapsulation applications.
The most studied polymeric materials for cell immunoisolation are not synthetic but natural polymers, the alginates (a hydrogel consisting of anionic polysaccharides extracted from seaweeds). They currently lead the field of microencapsulation and are also gaining a lot of attention in macroencapsulation as “islet sheets”, layers of islets sandwiched between thin layers of alginate (described in section 4.1.1.).
Besides alginates, other polymeric materials have also been studied for cell encapsulation. They can mainly be separated in two classes: hydrogels and thermoplastic polymers(63, 159). Hydrogels are water swollen 3D networks of hydrophilic homopolymers or copolymers(160). Their structural integrity relies on cross-links formed between polymer chains (chemical bonds and physical interactions). Due to their viscoelasticity and high H2O content, they resemble natural biological tissues and often induce minimal inflammatory response. Their permeability is adjustable, which is promising for cell immunoisolation, but since they rely on crosslinks, these materials will always present a broad pore size distribution (which is broader than inorganic membranes). This can be an issue for complete immunoprotection of encapsulated cells. Indeed, even if only 1% of pores are larger than the size cut-off goal, the pores will allow sufficient passage of antibodies, complement, and cytokines to initiate immunorejection(56). Besides alginates, hydrogels that have been studied for cell encapsulation include: polyethylene glycol (PEG)(161, 162), agarose (macrobeads)(163, 164), polyvinyl alcohol (PVA) (islet sheet)(165, 166), polyvinyl alcohol and polyacrylic acid copolymers (PVA/PAA) (membrane)(167).
The other category of encapsulating polymers is thermoplastics. They consist of long, linear and water insoluble chains that can be processed into multiple configurations by heat melting followed by cooling. Their chemical and mechanical stability properties are superior to those of hydrogels, which explains why thermoplastics have mainly been used for macrocapsules. However, hydrogels still remain the most popular choice for cell encapsulation due to their very good biocompatibility.
Thermoplastic materials that are currently used for cell immunoprotection are polyacrylonitrile and polyvinyl chloride copolymers (PAN/PVC or Amicon; hollow fibers)(97, 113, 168, 169), polyurethane (PU) and polyurethane and polyvinyl pyrrolidone copolymers (PU/PVP) (macrocapsules)(170, 171), PU (membranes)(172), polysulfone (hollow fibers)(173, 174), AN69 renal dialysis membranes (69% acrylonitrile and 31% sodium methallyl sulphonate) modified by electrical discharges(175), polytetrafluoroethylene (PTFE) membranes(98, 99, 176, 177), dual porosity nylon membrane(178)...
Among organic materials that have been tested for cell immunoisolation, alginates are certainly the most promising, both as microcapsules (see section 3.3.) and as the “islet sheets” (macrocapsules) described below.
Another interesting approach that is still in its infancy is the fabrication of SU-8 (an epoxy) microcapsules with very precise nanopores. The use of photolithographic techniques permits to obtain a very narrow pore size distribution which is normally never achieved with polymeric materials. Section 4.1.2. gives more detail about this technique.
4.1.1. Alginate islet sheets
While alginates have led the microencapsulation field, they have also been used for macroencapsulation by Dufrane et al. who recently developed an alginate device(179, 180). Briefly, their device consists of a collagen matrix on which islets are seeded to produce a cell monolayer, allowing faster diffusion kinetics as compared to clusters of islets. This monolayer is then covered by a gelled layer of alginate 3% wt./vol. rich in mannuronic acid (the other side of the collagen matrix is also covered by an alginate layer). This produces an islet sheet (“monolayer cellular device”, MCD) that is ready to be transplanted (see Figure 8).
Figure 8.
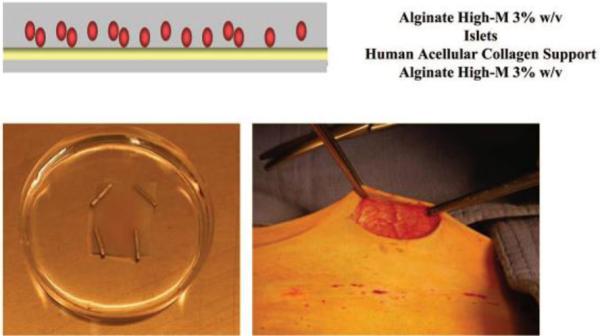
Alginate and collagen macrocapsule containing a monolayer of pig islets, schematic representation (top) and picture (bottom, left) ; Implantation of the device into abdominal subcutaneous tissue of non-human primate (bottom, right). Reproduced with permission from (180).
Dufrane et al. have already reported very promising results with this type of devices in 2010(180). They transplanted (without any immunosuppression) encapsulated pig islets in streptozotocin-induced diabetic monkeys: 4 primates received alginate microcapsules transplanted under the kidney capsule and 5 primates received 3 to 5 alginate MCDs transplanted into abdominal subcutaneous tissue (same batch of alginate for micro and macrocapsules). Only two animals within the microcapsule group showed complete control of diabetes and for a very limited time (2 weeks). The animals that received the macrocapsules performed significantly better and their diabetes was corrected for up to 28 weeks. The MCDs were removed when diabetes reappeared for all 5 animals and 2 of these primates then received a new transplantation of islet sheets. Diabetes was then controlled again for up to 20 weeks.
The micro and macrocapsules were histologically examined after explantation and showed no sign of graft fibrosis or alginate degradation. However, CD3 (lymphocytes) and CD68 (monocytes-macrophages) cells were detected with both types of capsules, but in lower amounts than for free pig islets transplantation (control group). No complement (C3d/C9) deposition was observed on the macrocapsules. Humoral immunoresponse was also evaluated by measuring the level of anti-pig antibodies (IgM and IgG) in the sera of the primates. This level was strongly increased for all graft recipients but it decreased after 2 months for the MCDs group (the second graft with MCDs produced a similar response).
In vitro diffusion studies were also carried out with FITC-lectins (36, 75 and 150 kDa) before and after implantation (the capsules were incubated with lectins for 48h at 4°C and then frozen in order to count the fluorescent lectins inside them). While the 150 kDa lectin (same size as IgG) was able to diffuse into the microcapsules, it did not cross the MCDs barrier, even after the 20 week transplantation. The smaller molecules were able to diffuse into all capsules at any time. The MCDs thus present very interesting semipermeable properties, although a more rigorous testing of the diffusion properties will have to be carried out at 37°C.
This study presents very encouraging results since it shows that alginate subcutaneous devices containing porcine islets can control diabetes up to 6 months in primates without immunosuppression. The authors associate the failure of the grafts after 6 months with the limited lifespan of adult pig islets and expect better islet sources to overcome the half year limitation.
The MCDs are also currently being tested in a phase I clinical trial by Dufrane in Belgium (ClinicalTrials.gov Identifier: NCT00790257). The aim of this trial is to test the safety and efficacy of encapsulated human islets in an MCD for allogeneic islet transplantation in type 1 diabetic patients. Encapsulated human islets are transplanted in the subcutaneous tissue of patients (1 device of 1-3 cm2 per patient; total of 15 patients). The first phase of the trial will deal with patients already with a transplanted organ and under immunosuppression, whereas the second phase will be carried out in patients without immunosuppression. The estimated completion date of this clinical trial is December 2013.
4.1.2. SU-8 nanoporous membranes
With the development of nanofabrication technologies such as electron-beam lithography and nanoimprinting, nanoporous membranes have been fabricated in the commonly used photosensitive polymer SU-8 (epoxy-based) by Gimi et al.(181-184). Biocompatibility of SU-8 has already been studied and found satisfactory(185). The fabrication of nanoporous SU-8 membranes occurs in two steps. A silicon (Si) mold is first created by conventional electron beam lithography(183, 184). In order to reduce the dimensions of the mold features down to the final target of a 20 nm wide grating and 200 nm pitch, controlled and cyclic oxidation and etching steps are applied. Then, the mold is used to superficially imprint nanoslots in a SU-8 membrane. A metal is then deposited at an oblique angle to the membrane to protect the superficial imprint features. The nanoslots are finally etched chemically and anisotropically through the entire cross section of the membrane producing a semipermeable membrane that can be used for cell immunoisolation. The final pores present a width of 20 nm that is precisely controlled over the whole membrane surface and a length of about 1 μm. The reusable mold permits to achieve reproducibility and allows possible high-throughput fabrication.
The nanoslotted membranes are then integrated into the surfaces of SU-8 cuboid microcapsules fabricated by lithography. The microcapsules provide support to the thin (350-450 nm) nanoporous membranes and consist in a base with a female structure that hosts the insulin-secreting cells and a lid with a male structure (see Figure 9).
Figure 9.
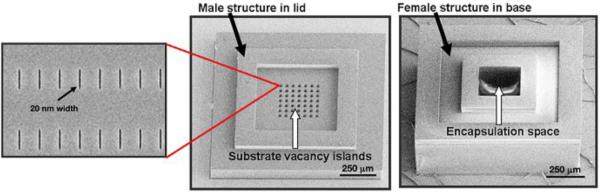
SU-8 nanoporous microcapsule micrographs showing the lid, the base and a close-up of the nanoslots in the lid. Reproduced with permission from (184).
One difference of this approach as compared to other photolithographic techniques is that it aims to produce microcapsules, rather than macrocapsules like those presented in section 4.2.1. on Si nanoporous membranes. These SU-8 microcapsules are designed to house a single pancreatic islet, or an equivalent cluster of insulin-secreting cells, with an encapsulation space of 200 × 200 × 200 μm3. They thus present the advantages of microencapsulation but have better diffusion characteristics due to their very thin membranes (as compared to the thicker alginate microcapsules). Moreover, the pore width is very precise and uniform, comparing favorably with polymer conformal coatings.
A key feature of these nanoporous containers is their optical and MRI transparency, allowing multimodal imaging of encapsulated islets post-transplantation (two-photon confocal microscopy and MRI)(181). In vitro experiments showed that islet function is unimpaired after 48 h of encapsulation(181). Other experiments studied the diffusion of fluorescent probe molecules across the nanoporous membranes: IgG-FITC(182), lectin-FITC (140 kDa) and FM 4-64 (608 Da)(183). While FM 4-64 diffused without any problem, some diffusion of the larger molecules (that would ideally be blocked) was also observed. This may be attributed either to the slit shape of the nanopores, possibly enabling flexible proteins to cross the barrier, or to a gap between the lid and the base of the microcapsules (no experiment has been carried out yet to determine the degree of sealing of the assembly). In order to investigate cell oxygenation within these nanoporous containers, 9L rat glioma cells were engineered to bioluminesce under hypoxic conditions(182), which could potentially be used for future in vivo experiments. The preliminary results indicate that the nanoporous capsules may provide restricted oxygenation of the encapsulated cells.
It is also important to mention the robustness of these microcapsules(181), which do not fracture or rupture when manipulated during manufacture and encapsulation. This is another advantage over alginate-based microcapsules that are not so mechanically stable.
Although this research is still in its infancy, nanoporous SU-8 containers may prove useful to encapsulate islet cells in the future. Concerns about the lack of biocompatibility of this polymer may be solved by applying coatings of biofriendly molecules or bioinert materials, such as gold(181).
4.2. Inorganic membranes
Advances in inorganic materials research in the electronics, sensors and photovoltaics industries have enabled the development of inorganic nanoporous membranes with well controlled pore sizes and geometries. The produced nanoporous membranes have inspired researchers active in drug delivery and cell encapsulation. For instance, silicon microfabricated membranes were already proposed for biomedical applications in 1995(186, 187).
Three inorganic materials currently present very promising properties for the production of immunoisolating membranes(61): silicon (Si), aluminum/aluminum oxide (Al/Al2O3) and titanium/titanium oxide (Ti/TiO2). The extravascular macrocapsules that use those membranes present several advantages over their polymeric counterparts: a tighter pore size distribution and faster diffusion kinetics due to decreased membrane thickness(105). For Si and Al/Al2O3 membranes, these advantages have to be balanced with decreased levels of biocompatibility. The three materials will be presented in the following sections. The fabrication techniques discussed here are not the only ones available, yet they present a major advantage over other techniques: they lead to materials with straight nanopores, ensuring the fastest diffusion possible for nutrients and therapeutic products. Indeed, powder sintering and sol-gel methods also produce nanoporous SiO2, Al2O3 and TiO2 - but the pores are always tortuous in these cases(156). Commercial porous Al2O3 filter membranes, Anopore from Whatman, could also be interesting to encapsulate cells. They present uniform pore sizes with high pore densities (>1010/cm2), but available pore size is quite limited.
Finally, a last section will present a different and very innovative approach to produce nanoporous microcontainers that assemble by self-folding. One interesting feature of this technique is that numerous materials can be used to form these microcapsules.
Since the science behind these inorganic nanoporous membranes is rather new, these materials have not been tested as much as the polymeric membranes for cell encapsulation. However, they have the potential to catch up with the currently popular alginate microcapsules and sheets by offering better stability and tighter control of porosity.
4.2.1. Silicon nanoporous membranes
Inspired by the fabrication techniques used for the production of silicon computer chips, silicon nanoporous membranes were initially developed and used to encapsulate pancreatic islets by Desai and Ferrari(103). The development of this silicon membrane technology has been extensively reviewed elsewhere(53, 105, 158, 188). The process scheme is presented in Figure 10.
Figure 10.
Process scheme for the fabrication of Si nanoporous membranes. Reproduced with permission from (158).
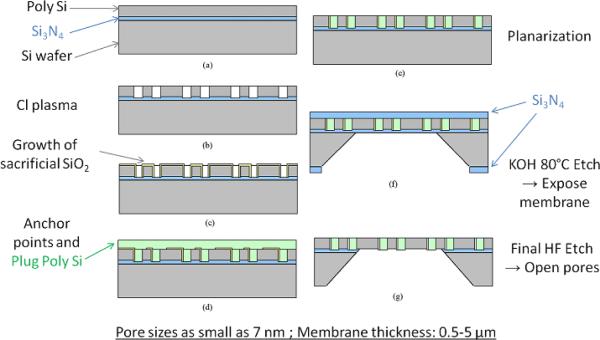
Fabrication of Si nanoporous membranes starts with a Si wafer. A support ridge structure is first etched by photo-lithography to provide mechanical support to the final structure (not shown in Figure 10). A very thin silicon nitride (Si3N4) layer is then deposited on top of the wafer, serving as an etch-stop for future processes. A structural base layer of polysilicon is deposited on top of the Si3N4 (Figure 10a), and its thickness will determine the overall thickness of the final nanoporous membrane (0.5 μm to 5 μm). Holes are then etched through poly Si by a chlorine plasma (Figure 10b), and a sacrificial silicon oxide (SiO2) layer is thermally grown over the Si base layer (Figure 10c). The sacrificial oxide thickness determines the pore size in the final membrane, and pores ranging from 7 nm to 100 nm have been obtained with tight pore size distributions (<5% pore width variation)(105, 158). The next step consists in depositing plug poly Si in the holes of the base layer (Figure 10d). The surface is then planarized, leaving a smooth exposed surface of sacrificial oxide (Figure 10e). Subsequently, a Si3N4 protective layer is deposited uniformly across the wafer. Windows are etched through this layer on the bottom side of the wafer to expose the bulk Si in specified areas. The wafer is then placed in a KOH bath at 80°C where bulk Si is dissolved up to the Si3N4 etch stop layer (Figure 10f). Finally, the protective and etch-stop Si3N4 layers and the sacrificial SiO2 layer are removed by etching in hydrofluoric acid (HF) (g). The finished product is a Si nanoporous membrane of controlled thickness presenting slit nanopores of controlled channel widths. These pores are organized in parallel arrays along the membrane major dimension. The length of the pores is fixed at 45 μm and there are 10,000 pores/mm2 (105). Hence, the total pore area increases linearly with the pore size.
Figure 11 shows a picture of one Si nanoporous membrane as well as SEM micrographs illustrating the pore structure.
Figure 11.

Picture of a Si nanoporous membrane showing the porous area surrounded by the support ridge (left), SEM micrographs: top view (middle) and cross-section showing the nanopores across the whole thickness of the membrane (right). Reproduced with permission from (191).
The very tight pore size distributions (<5%)(158) of these Si nanoporous membranes make them advantageous over their polymer counterparts for which pore size distributions of 30% are common(157).
Desai et al. used 18, 66 and 78 nm porous Si membranes to encapsulate rat islets. They performed a glucose stimulated insulin secretion test and showed that encapsulated islets presented a similar release profile as compared to unencapsulated islets(103, 189). Moreover, 18 nm pores significantly hindered the passage of IgG as compared to the larger pores, although with incomplete blockage(189). They also incubated islet-filled capsules in a serum complement/antibody solution during 2 weeks. At day 14, the insulin secretion following stimulation by glucose was approximately 5 times higher for the encapsulated islets (18 and 78 nm pores) as compared to free islets, proving the potential of this immunoisolation technology.
In vivo tests were also performed with encapsulated rat and mouse insulinoma cells implanted intraperitoneally in mice(190). After 8 days, biocapsules were removed for cell viability and functionality tests. The insulinoma cells encapsulated by 18 nm membranes exhibited higher insulin secretion upon glucose stimulation than the cells in 66 nm porous capsules, highlighting the correlation between smaller pores and immunoprotection. Membranes with pores below 18 nm have also been tested for diffusion of glucose, albumin (67 kDa) and IgG(53, 191). The diffusion of glucose was constrained with pores below 13 nm (non-Fickian diffusion) and albumin was unable to pass through 7 nm pores. Diffusion of IgG was greatly hindered, especially for 7 nm pores. However, total immunoisolation was not achieved, despite early estimations that pore sizes between 30 and 50 nm should be able to exclude IgG(192). This can be explained by the flexible characteristics of the Y-shaped IgG protein that can adopt different conformational changes(53), easing its diffusion through the slit pores (a few nm wide but 45 μm long). Unfolding of the 3D protein structure may also occur, easing the passage of IgG as well. Therefore, slow diffusion of IgG may be expected even for pore sizes below 20 nm. A strategy that could be applied to block IgG is to decrease the length of the pores. However, a very tiny leak of IgG may not be so detrimental to the cells. Iwata and others showed that complement components are rapidly inactivated, and therefore it should be good enough to hinder IgG diffusion in the first days after implantation rather than completely block it(66, 193, 194).
Thus, a Si membrane with pores just below 20 nm should be able to immunoprotect cells while allowing sufficient diffusion of glucose and insulin (due to their very low thickness of a few micrometers).
Silicon membranes can also be modified in terms of surface chemistry. Strategies have been developed to decrease unwanted adsorption by coating Si with polyethylene glycol (PEG)(195, 196). The PEG chains can be covalently attached to Si and permit reduced adsorption of albumin, IgG and fibrinogen by 76, 82 and 64%, respectively, as compared to untreated Si.
The assembly of these Si nanoporous membranes into actual biocapsules has been performed differently over the years. The interested reader can find information on that matter elsewhere(53, 105-107, 189, 197).
4.2.2. Alumina nanoporous membranes
It is well known that surfaces of aluminum (Al) present a high affinity for oxygen, resulting in the formation of an aluminum oxide layer covering the metal. However, this natural oxide layer is uncontrolled, and this has led researchers to develop anodization techniques to grow controlled porous or non-porous layers of aluminum oxide (Al2O3) on Al substrates(198). An interesting feature of these porous layers is that they present ordered straight circular pores in a hexagonal arrangement. Provided the layers are separated from the underlying Al, they could be used as membranes for cell immunoisolation.
Following Itoh et al. in 1996(199, 200), Gong et al. reported in 2003 a sequential etching technique to produce an Al2O3 nanoporous membrane embedded in a cylindrical Al tube(201) (see Figure 12). They showed that such devices could control release of dextran conjugates of varying molecular weight by adapting the pore size, via the anodization voltage. The same fabrication process has also been used to produce flat membranes of Al2O3 (202).
Figure 12.
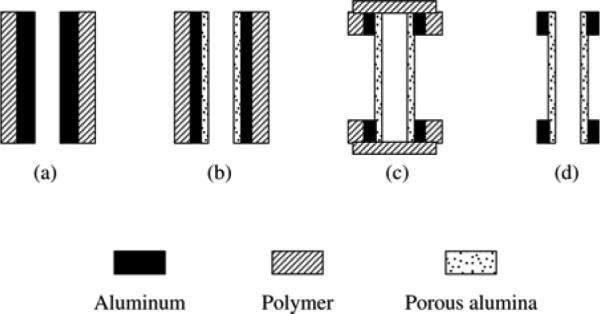
Process scheme for the fabrication of Al2O3 nanoporous membranes embedded in an Al cylinder (side view). Reproduced with permission from (201).
Briefly, the external surface of an Al tube is coated with a thin layer of protective polymer, e.g. nail polish (Figure 12a). Then, an anodization step is performed from the inner side of the tube in 0.25 M oxalic acid, producing a layer of nanoporous Al2O3. Voltage selection determines the pore size (diameter). This layer is then etched in a mixture of H2CrO4 and H3PO4, leaving a uniform concave array of nucleation sites that are critical to obtaining narrow pore size distributions during the subsequent anodization step. A second anodization step is then performed at the same voltage, producing the final Al2O3 nanoporous membrane with circular pores (Figure 12b). The duration of this anodization determines the membrane thickness. In order to expose the nanoporous membrane, window-areas are created with acetone in the protective outer polymer film. Both ends of the Al tube are then protected with Parafilm and the tube is dipped in a 10 wt. % HCl and 0.1M CuCl2 solution that selectively etches unprotected Al areas, thereby revealing a transparent Al2O3 membrane (Figure 12c). Since the nanoporous membranes are incorporated in the Al tube, they are strong enough for easy handling and use. The final step is to etch the barrier layer present at the outer surface of the Al2O3 membranes in a mixture of H2CrO4 and H3PO4. The Parafilm and polymer protection layers are then removed (Figure 12d), resulting in an Al cylinder with Al2O3 nanoporous membrane windows (see Figure 13).
Figure 13.
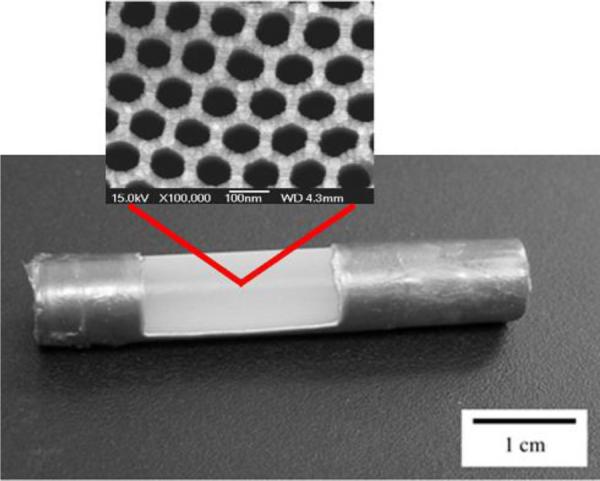
Cylindrical Al macrocapsule with Al2O3 nanoporous membrane windows, close-up: SEM micrograph of the top of the membrane. Reproduced with permission from (201, 205).
Superior control over the size and shape of the nanoporous Al2O3 windows within a flat Al frame can be achieved by using a photoresist polymer as the initial protective coating that can then be removed at selected locations by photolithography(203).
The general technique presented here produces nanoporous Al2O3 membranes in a variety of configurations that may be used for immunoisolated cell encapsulation(204). Anodization technology is really simple and inexpensive, especially if no photolithography step is involved. Diameters of circular vertical nanopores reported in the literature are comprised between 25 and 80 nm and the thicknesses of these membranes range from 55 to 100 μm(201-204). Pore size distributions of Al2O3 membranes(204) are worse than those of microfabricated Si membranes but they are still better than those of their polymeric counterparts. The thicknesses of Al2O3 membranes are larger than those of Si. Thicker membranes obviously impede diffusion of oxygen, glucose and insulin but simultaneously render the whole device more robust and resistant. Moreover, the high density of pores (~1010/cm2)(204) achievable for alumina membranes could compensate for their increased thickness from a diffusional point of view.
La Flamme et al. compared diffusion coefficients of glucose and IgG for an Al2O3 75 nm membrane, for a Si 49 nm membrane, and for a poly(vinyl alcohol) 10-30 nm hydrogel(204). Transport of glucose was comparable for all 3 membranes, but diffusion of IgG was significantly reduced for Al2O3 vs. Si and the hydrogel, even though these two presented a smaller pore size. The circular shape of Al2O3 nanopores seems to be really advantageous over rectangular slit pores of Si or the hydrogel pores regarding blockage of the flexible IgG. In vitro encapsulation studies have also been performed by La Flamme with MIN6 insulinoma cells(204). They showed insulin secretion upon glucose stimulation although the secreted insulin amounts were lower with the 75 nm Al2O3 membranes than with unencapsulated cells due to the physical barrier of the membrane.
The main concern for the use of Al2O3 nanoporous membranes is the extent of biocompatibility for this material. However, in vitro tests have shown that these membranes are nontoxic to fibroblast cells and do not induce significant complement activation(205). It has also been shown that incorporation of a polyethylene glycol (PEG) coating(202, 206) reduces the interactions of serum albumin with the material(205). This reduction in protein adsorption will certainly help to prevent any clogging of the pores. Finally, in vivo tests of up to 4 weeks with empty Al/Al2O3 capsules have demonstrated that implantation of these capsules into the abdominal cavity of rats induces a transient inflammatory response (probably due to the surgery and not to the capsule), and that PEG coatings were useful in minimizing the host response(205). The membranes were fully intact and free from fibrous growth at the end of the 4 weeks.
Despite encouraging preliminary results, Al2O3 nanoporous membranes have not yet been tested in vivo with encapsulated cells.
4.2.3. Titania nanotubular membranes
As for aluminum, surfaces of titanium (Ti) present a high affinity for oxygen, resulting in the formation of an oxide layer covering the metal. Since this natural oxide layer is not controlled, anodization techniques have also been developed for this material. Less studied than Al anodization, Ti surface modification encountered a major breakthrough in 1999 when Zwilling et al. anodized a Ti sample in a solution of H2CrO4 and HF and observed the formation of a porous layer of TiO2 nanotubes in a hexagonal arrangement(207). In 2001, Gong et al. obtained uniform arrays of well-aligned TiO2 nanotubes after anodization of a Ti foil in an aqueous solution containing 0.5 to 3.5 wt. % HF(208).
Numerous studies of the formation and characterization of TiO2 nanotubes have been published afterwards(209-220). The influence of anodization experimental parameters (electrolyte composition, temperature, voltage, current, anodization time...) on the resulting nanotubular TiO2 arrays has been thoroughly investigated in these studies. Layers of vertically-oriented nanotubes with diameters from 15 to 150 nm (mainly controlled by anodization voltage) and lengths from a few nm to 1000 μm (mainly controlled by anodization time) have been produced. Synthesis of TiO2 nanotubes by anodization, properties of these tubes and their applications have been reviewed extensively elsewhere(221-224).
Arrays of TiO2 nanotubes present several excellent characteristics for biofiltration applications such as cell-based drug delivery and immunoisolation: they have circular nanopores that are ideal for blocking flexible proteins like IgG and a very narrow pore size distribution coupled to a very high pore density(217), their thickness can be varied over a broad range, and their biocompatibility is excellent(225).
A variety of procedures are given in the literature to produce a uniform TiO2 nanotubular layer. A simple recipe that is widely used currently is to anodize a Ti foil in an ethylene glycol solution containing 0.3 wt. % of NH4F and 2 vol. % of H2O (220). A nanotube length of about 200 μm (pore diameter 125 nm, standard deviation 10 nm) is obtained after anodization of the Ti foil at 60V for 72 h. SEM micrographs of such a TiO2 nanotubular layer are presented in Figure 14.
Figure 14.
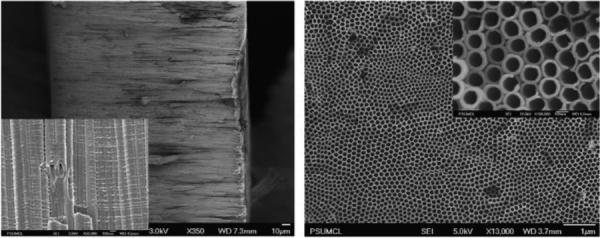
SEM micrographs of a TiO2 nanotubular membrane: cross-section of free-standing membrane with inset showing high magnification (left); nanotubular array top surface with inset showing high magnification (right). Reproduced with permission from (220).
A common practice to improve the close-packed hexagonal arrangement of the nanotubes is to carry out 2 subsequent anodizations on the same Ti foil(217). A first anodization produces a layer of TiO2 nanotubes that is not perfectly ordered, which is then removed. This leaves a dimpled pattern on the Ti surface. A second anodization conducted with this textured surface then produces a layer of TiO2 nanotubes with improved ordering.
Despite promising properties for immunoisolation, TiO2 nanotubes have not yet been applied to cell encapsulation. This is mainly due to the fact that this avenue of research is still in its infancy for Ti. Techniques to produce a free-standing membrane of TiO2 nanotubes are not as mature as they are for nanoporous Al2O3. TiO2 nanotubes have to be detached as an array from the Ti foil and their bottom ends (barrier layer) need to be opened.
Different techniques have been reported so far(219, 220, 226-234) but these approaches remain difficult to realize in practice, especially for large area membranes.
Another very interesting approach to produce membranes was developed in 2010 by Albu et al. (see Figure 15)(235). They evaporated a 5 μm film of Al on a 6 μm Ti foil. After depositing a positive photoresist on Ti (Figure 15a), they defined a grid structure via photolithography (Figure 15b). They subsequently performed anodization in an ethylene glycol electrolyte containing NH4F and DI H2O, producing TiO2 nanotubes connected to Al2O3 nanopores (no TiO2 barrier layer in this case) (Figure 15c). The Al substrate and the Al2O3 porous area were then selectively etched in an acidic solution of 19(H3PO4):1(HNO3):1(CH3COOH):2(H2O) parts by volume (Figure 15d). The remaining Ti frame allows for a high mechanical flexibility. The nanopores present a diameter of 30±10 nm. These membranes have been tested for diffusion of methylene blue and nanospheres of 20 and 200 nm, showing total blockage of the latter while 20 nm spheres diffused slower than methylene blue.
Figure 15.
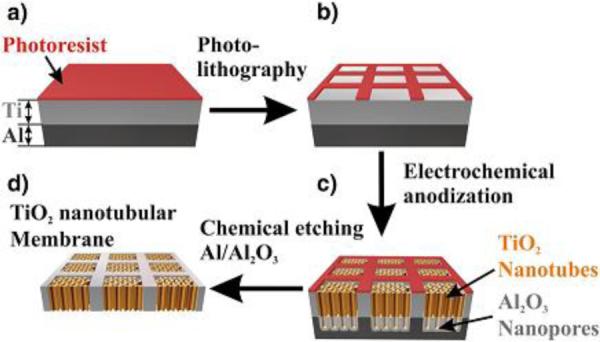
Process scheme for the fabrication of flexible TiO2 nanotubular membranes. Reproduced with permission from (235).
Other studies on TiO2 nanotubular arrays for membrane applications have investigated diffusion of methylene blue(226), phenol red(219), phenol red, glucose, bovine serum albumin (BSA) and IgG(220). This last study used a 200 μm thick membrane with 125±10 nm pores and showed that the diffusion coefficient of IgG was about 100 times smaller than that of BSA and 1000 times smaller than that of glucose, which are encouraging results for islet encapsulation applications.
TiO2 nanotubular layers seem to be an excellent choice for incorporation into macrocapsules for cell immunoisolation. However, the research in this field is still in its infancy and a lot of work will need to be carried out to assess the potential of this material for biofiltration applications.
4.2.4. Self-folding microencapsulation devices
An innovative microencapsulation approach that was developed by Gracias et al. utilizes patterned self-folding devices to host and immunoprotect cells(236-238).
Current microfabrication and lithography techniques can produce very precise pores at the nanoscale on 2D surfaces, and resulting cell-containing capsules typically incorporate 1 or 2 of these membranes. This can result in hypoxic conditions for cells residing far away from these porous surfaces. In order to tackle this problem, Gracias et al. developed a technique derived from the ancient Japanese art of paper folding, origami. Using lithography techniques, they produce a patterned 2D precursor structure with hinges. The next step involves self-folding of the 2D structure along the hinges to produce a precise 3D hollow structure. Different polyhedra have been produced this way, and the main advantage resides in that nearly all faces of these objects can be porous. Precisely patterned hollow polyhedra with overall dimensions from 100 nm to 1 cm have been fabricated with a variety of materials, including metals, ceramics and polymers(236-242). Straight or curved pores as narrow as 15 nm have been produced on the faces of these objects using electron beam lithography(240). These very small containers present all the advantages of microencapsulation but have better diffusion characteristics due to their very thin walls (as compared to thicker alginate microcapsules). Moreover, their pores are very uniform and present a narrow size distribution due to lithography. These microcontainers thus compare favorably with polymer conformal coatings. Very similar in size to SU-8 nanoporous microcapsules from Gimi et al., self-folded capsules present the advantage of being porous on all of their faces, improving oxygenation of the encapsulated cells and enhancing diffusion of nutrients and therapeutic products. The variety of materials that are compatible with this approach is another advantage of this technique.
The self-folding phenomenon is usually driven by minimization of surface energy(238, 239, 243) or the release of thin film stress(244). The first technique is currently the most popular, and it is the only one described here. Briefly, a low melting point material is deposited between panels to generate folding hinges and at panel edges to generate locking hinges. After 2D microfabrication, the templates are released from the substrate and heated to liquefy the solid solder hinges. Upon heating, the hinges fold and lead to the final 3D structure, where liquefied locking hinges fuse and subsequently harden upon cooling. The use of locking hinges produces well-sealed, mechanically robust hollow polyhedra that can be manipulated without breaking. Figure 16A presents video snapshots of the self-folding phenomenon that is typically carried out in a high-boiling-point solvent (e.g. N-methylpyrrolidone)(238, 239). Figure 16B shows a gold (Au)-coated hollow cube with patterned pores on 5 faces (the bottom face is left open for subsequent cell loading) and Figure 16C shows a close-up of different pores. It is also possible to induce self-folding by exposing Ni/Sn objects to a CF4/O2 plasma (the angular orientation between panels can be controlled by altering the flow rate of O2 gas)(240). However, gaps are present in between panels with this approach that is thus not directly applicable to cell immunoprotection.
Figure 16.
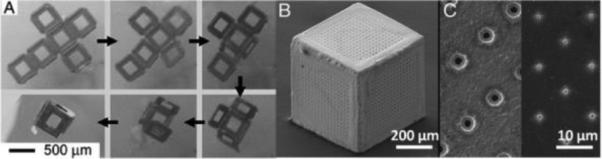
Video snapshots showing the self-assembly of a lithographically fabricated template into a 3D hollow container (A) ; SEM micrograph of a 3D porous container (B) ; SEM micrograph of pores on the faces of containers of different pore sizes (C). Reproduced with permission from (236, 246).
As already mentioned, different materials have been successfully used to fabricate these patterned 3D microcapsules. The faces of the cubes can easily be made with copper (Cu) or nickel (Ni)(238, 239), using pure tin (Sn) or tin/lead (Sn/Pb) as the solder hinge material. Before any contact with biological molecules, gold (Au) is electrodeposited onto all surfaces of these microcontainers to improve their biocompatibility(245, 246). The Au electrodeposition time can also be varied to control the final pore size of the membranes with considerable precision down to the nanoscale(246).
In order to encapsulate cells, 3D cubes with one missing face (microwells) are produced by self-assembly(245). Cells can then be loaded by tumbling the microwells in a concentrated cell solution (~104 cells/ml). Loaded microwells are then oriented with their open face upwards using a glass pipette. An adhesive tape (or polyurethane adhesive spin-coated on a glass slide) is then brought into contact with the open face of multiple microwells. The polymer cures typically within 30 minutes in cell media, thereby sealing the microwells. Arrays of microcontainers can then be formed on rigid or flexible (curved) geometries. It is also possible to create arrays of microcontainers by first positioning the microwells in an SU-8 holder patterned with recessed slots. The cells can also be loaded by simple settling after positioning the microwells in the holder.
Insulinoma cells (beta-TC-6) have been encapsulated for up to 1 month into such 500 μm-sized cube arrays with 1, 3 and 5 porous faces (6-7 μm pores)(245). These in vitro tests showed increased and faster glucose-stimulated insulin production for cubes with higher number of porous faces after 7 days of encapsulation. Moreover, the steady-state release of insulin following glucose stimulation (after 240 minutes) was compared between the 1, 3 and 5 porous-faced microwell arrays at different time points following encapsulation. The insulin steady-state release was similar after 1 day for the 3 categories but decreased over time for 1 and 3 porous faces. The 1 porous face group did not produce insulin any more after 28 days whereas the 5 porous faces group maintained its insulin concentration levels. Some microwells were also peeled from the substrates at different time points to study cell viability using a live/dead cytotoxicity assay. Higher numbers of live cells were consistently observed within the 5 porous faces cubes as compared to the 1 porous face cubes. These experiments clearly demonstrate the advantage of 3D porous microcontainers (cubes with 5 porous faces) as compared to 2D porous microcapsules (cubes with 1 porous face) for cell encapsulation. Cells encapsulated in the latter suffer from inadequate oxygenation and die after some weeks. It is interesting to note that these experimental results are in agreement with cell viability simulations solely based on O2 diffusion(245).
Diffusion of insulin and IgG has also been studied with 500 μm-sized cubes presenting different precise pore sizes (from 78 nm to 2 μm) on 5 faces(246). It has been shown that 78 nm pores permitted diffusion of insulin over one week while blocking IgG diffusion, which is encouraging for cell immunoisolation applications. Insulinoma cells (beta-TC-6) have also been encapsulated within 78 nm porous microcontainers. After 2 days in the capsules, the cells produced insulin within 5 minutes in response to a glucose challenge and a continuous insulin production was measured for up to 2 hours.
These results show a proof-of-concept for this self-folding encapsulation technology, although this procedure is still in its infancy. Numerous in vitro and in vivo tests will have to be carried out to assess the stability and biocompatibility of these microcontainers over time. The hinges will have to be carefully studied for potential toxicity or leaking after several weeks in media.
The self-folding microcapsules have also recently been produced from all-polymer materials(242). The polyhedral objects, made of SU-8 faces and biodegradable polycaprolactone (PCL) hinges, spontaneously assemble upon heating at 58°C. This approach produces transparent microcontainers, enabling in situ imaging of encapsulated content using bright-field or fluorescence microscopy. Here again, all faces of the polyhedra can be porous which represents an improvement over Gimi's approach discussed previously. However, since PCL degrades over time in the body, this solder material is not suitable for islet/beta cell encapsulation (however, it is interesting for drug delivery applications). Indeed, the current SU-8/PCL containers release their content both through pores patterned on the faces of the capsules and by hinge degradation.
5. CONCLUSIONS
While encapsulation of islet or beta cells by semipermeable membranes has a history of more than 50 years, no definitive cure for type 1 diabetes has been seen so far. This situation is explained by the complexity of the compromise that must be reached: membrane pores have to be small enough to block the molecules and cells responsible for immune rejection but the very same pores need to be as large as possible to facilitate transport of O2, glucose and insulin. While prevention of an immune attack on encapsulated cells seems to be nearly achieved today, the adequate supply of nutrients, especially O2, is still a problem. This explains why capsule designs that minimize the distance between cells and the surrounding environment have gained a lot of interest in the past few years. For instance, alginate microcapsules and alginate islet sheets are the most advanced realizations in terms of clinical application. However, the broad pore size distribution of polymeric materials (such as alginates) may be a problem for immunoisolation over long periods. On the other hand, nanoporous inorganic membranes (Si, TiO2, Al2O3...) possess well defined pore sizes that are expected to perform better in immunoprotection applications. The research in this field is still in its infancy but could lead to interesting results in the near future.
Besides membrane biocompatibility and design, several other approaches could help to produce a long-lasting implantable capsule with islet or beta cells: prevascularization of macrocapsules prior to cell transplantation, use of growth factors and oxygen generating materials, or arrangement of beta cells in controlled size clusters.
Apart from the materials science point-of-view, a cure for type 1 diabetes will require additional reliable sources of islet or beta cells since islet availability from donor pancreases is insignificant as compared to demand. Xenogeneic cells and cells derived from stem cells seem to be promising substitutes for human islets, but several remaining issues with these cell sources have yet to be overcome.
A lot of research and development is still required before encapsulated islets or beta cells will be able to cure type 1 diabetes, but such a treatment would represent a tremendous improvement over the multiple injections of insulin that patients currently have to undergo every day.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Daniel Bernards for valuable review of this manuscript. This work was supported by the Juvenile Diabetes Research Foundation. J.S. is also grateful for a postdoctoral grant (2010-2011) from the King Baudouin Foundation (Belgium) and the Belgian American Educational Foundation.
REFERENCES
- 1.Scully T. Diabetes in numbers. Nature. 2012;485(7398):S2–S3. doi: 10.1038/485s2a. [DOI] [PubMed] [Google Scholar]
- 2.Van Belle TL, Coppieters KT, von Herrath MG. Type 1 Diabetes: Etiology, Immunology, and Therapeutic Strategies. Physiological Reviews. 2011;91(1):79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 3.Didangelos T, Iliadis F. Insulin pump therapy in adults. Diabetes Research and Clinical Practice. 2011;93(1):S109–S113. doi: 10.1016/S0168-8227(11)70025-0. [DOI] [PubMed] [Google Scholar]
- 4.Renard E. Implantable closed-loop glucose-sensing and insulin delivery: the future for insulin pump therapy. Current Opinion in Pharmacology. 2002;2(6):708–716. doi: 10.1016/s1471-4892(02)00216-3. [DOI] [PubMed] [Google Scholar]
- 5.Renard E, Farret A, Place J, Wojtusciszyn A, Bringer J. Towards an artificial pancreas at home. Diabetes and Metabolism. 2011;37(4):S94–S98. doi: 10.1016/S1262-3636(11)70973-9. [DOI] [PubMed] [Google Scholar]
- 6.Vogel T, Friend P. Pancreas transplantation. Surgery (Oxford) 2011;29(7):348–352. [Google Scholar]
- 7.Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. New England Journal of Medicine. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 8.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JRT, Shapiro AMJ. Five-Year Follow-Up After Clinical Islet Transplantation. Diabetes. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 9.Kessler L. La therapie cellulaire du diabete de type 1. Situation actuelle et perspective. Medecine Nucleaire. 2010;34(10):589–596. [Google Scholar]
- 10.Shapiro AMJ, Shaw JAM. Islet Transplantation and Beta Cell Replacement Therapy. Informa Healthcare; New York: 2007. [Google Scholar]
- 11.Robertson RP. Islet Transplantation as a Treatment for Diabetes - A Work in Progress. New England Journal of Medicine. 2004;350(7):694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 12.Andreoni KA, Brayman KL, Guidinger MK, Sommers CM, Sung RS. Kidney and Pancreas Transplantation in the United States, 1996-2005. American Journal of Transplantation. 2007;7(9):1359–1375. doi: 10.1111/j.1600-6143.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- 13.Stratta RJ. Immunosuppression in pancreas transplantation: progress, problems and perspective. Transplant Immunology. 1998;6(2):69–77. doi: 10.1016/s0966-3274(98)80020-8. [DOI] [PubMed] [Google Scholar]
- 14.Gummert JF, Ikonen T, Morris RE. Newer Immunosuppressive Drugs. Journal of the American Society of Nephrology. 1999;10(6):1366–1380. doi: 10.1681/ASN.V1061366. [DOI] [PubMed] [Google Scholar]
- 15.Penn I. Post-Transplant Malignancy: The Role Of Immunosuppression. Drug Safety. 2000;23(2):101–113. doi: 10.2165/00002018-200023020-00002. [DOI] [PubMed] [Google Scholar]
- 16.Naftanel MA, Harlan DM. Pancreatic Islet Transplantation. PLoS Medicine. 2004;1(3):198–201. doi: 10.1371/journal.pmed.0010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson PRV, Jones KE. Pancreatic islet transplantation. Seminars in Pediatric Surgery. 2012;21(3):272–280. doi: 10.1053/j.sempedsurg.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Stock PG, Bluestone JA. Beta-Cell Replacement for Type I Diabetes. Annual Review of Medicine. 2004;55(1):133–156. doi: 10.1146/annurev.med.55.091902.103539. [DOI] [PubMed] [Google Scholar]
- 19.Bretzel RG. Current status and perspectives in clinical islet transplantation. Journal of Hepato-Biliary-Pancreatic Surgery. 2000;7(4):370–373. doi: 10.1007/s005340070031. [DOI] [PubMed] [Google Scholar]
- 20.Leitao CB, Tharavanij T, Cure P, Pileggi A, Baidal DA, Ricordi C, Alejandro R. Restoration of Hypoglycemia Awareness After Islet Transplantation. Diabetes Care. 2008;31(11):2113–2115. doi: 10.2337/dc08-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonner-Weir S, Weir GC. New sources of pancreatic B-cells. Nature Biotechnology. 2005;23(7):857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 22.Sahu S, Tosh D, Hardikar AA. New sources of B-cells for treating diabetes. Journal of Endocrinology. 2009;202(1):13–16. doi: 10.1677/JOE-09-0097. [DOI] [PubMed] [Google Scholar]
- 23.Miszta-Lane H, Mirbolooki M, James Shapiro AM, Lakey JRT. Stem cell sources for clinical islet transplantation in type 1 diabetes: Embryonic and adult stem cells. Medical Hypotheses. 2006;67(4):909–913. doi: 10.1016/j.mehy.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Serup P, Madsen OD, Mandrup-Poulsen T. Islet and stem cell transplantation for treating diabetes. BMJ. 2001;322(7277):29–32. doi: 10.1136/bmj.322.7277.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Muller B, Vallejo M, Thomas MK, Habener JF. Multipotential Nestin-Positive Stem Cells Isolated From Adult Pancreatic Islets Differentiate Ex Vivo Into Pancreatic Endocrine, Exocrine, and Hepatic Phenotypes. Diabetes. 2001;50(3):521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 26.Linning KD, Tai M-H, Madhukar BV, Chang CC, Reed DNJ, Ferber S, Trosko JE, Olson LK. Redox-Mediated Enrichment of Self-Renewing Adult Human Pancreatic Cells That Possess Endocrine Differentiation Potential. Pancreas. 2004;29(3):e64–e76. doi: 10.1097/00006676-200410000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Epithelial-to-Mesenchymal Transition Generates Proliferative Human Islet Precursor Cells. Science. 2004;306(5705):2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 28.Lechner A, Nolan AL, Blacken RA, Habener JF. Redifferentiation of insulin-secreting cells after in vitro expansion of adult human pancreatic islet tissue. Biochemical and Biophysical Research Communications. 2005;327(2):581–588. doi: 10.1016/j.bbrc.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 29.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nature Biotechnology. 2006;24(11):1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 30.Baetge EE. Production of B-cells from human embryonic stem cells. Diabetes, Obesity and Metabolism. 2008;10(4):186–194. doi: 10.1111/j.1463-1326.2008.00956.x. [DOI] [PubMed] [Google Scholar]
- 31.Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, Richardson M, Carpenter MK, D'Amour KA, Kroon E, Moorman M, Baetge EE, Bang AG. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nature Biotechnology. 2011;29(8):750–756. doi: 10.1038/nbt.1931. [DOI] [PubMed] [Google Scholar]
- 32.Schulz TC, Young HY, Agulnick AD, Babin MJ, Baetge EE, Bang AG, Bhoumik A, Cepa I, Cesario RM, Haakmeester C, Kadoya K, Kelly JR, Kerr J, Martinson LA, McLean AB, Moorman MA, Payne JK, Richardson M, Ross KG, Sherrer ES, Song X, Wilson AZ, Brandon EP, Green CE, Kroon EJ, Kelly OG, D'Amour KA, Robins AJ. A Scalable System for Production of Functional Pancreatic Progenitors from Human Embryonic Stem Cells. PLoS ONE. 2012;7(5):1–17. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matveyenko AV, Georgia S, Bhushan A, Butler PC. Inconsistent formation and nonfunction of insulin-positive cells from pancreatic endoderm derived from human embryonic stem cells in athymic nude rats. American Journal of Physiology - Endocrinology And Metabolism. 2010;299(5):E713–E720. doi: 10.1152/ajpendo.00279.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nature Chemical Biology. 2009;5(4):258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 35.Van Hoof D, Mendelsohn AD, Seerke R, Desai TA, German MS. Differentiation of human embryonic stem cells into pancreatic endoderm in patterned size-controlled clusters. Stem Cell Research. 2011;6(3):276–285. doi: 10.1016/j.scr.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Bonner-Weir S, Toschi E, Inada A, Reitz P, Fonseca SY, Aye T, Sharma A. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatric Diabetes. 2004;5(s2):16–22. doi: 10.1111/j.1399-543X.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 37.Baeyens L, De Breuck S, Lardon J, Mfopou J, Rooman I, Bouwens L. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia. 2005;48(1):49–57. doi: 10.1007/s00125-004-1606-1. [DOI] [PubMed] [Google Scholar]
- 38.Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song K-H, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proceedings of the National Academy of Sciences. 2000;97(14):7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao R, Ustinov J, Pulkkinen M-A, Lundin K, Korsgren O, Otonkoski T. Characterization of Endocrine Progenitor Cells and Critical Factors for Their Differentiation in Human Adult Pancreatic Cell Culture. Diabetes. 2003;52(8):2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- 40.Suarez-Pinzon WL, Lakey JRT, Brand SJ, Rabinovitch A. Combination Therapy with Epidermal Growth Factor and Gastrin Induces Neogenesis of Human Islet B-Cells from Pancreatic Duct Cells and an Increase in Functional B-Cell Mass. Journal of Clinical Endocrinology & Metabolism. 2005;90(6):3401–3409. doi: 10.1210/jc.2004-0761. [DOI] [PubMed] [Google Scholar]
- 41.Fujita Y, Cheung AT, Kieffer TJ. Harnessing the gut to treat diabetes. Pediatric Diabetes. 2004;5(s2):57–69. doi: 10.1111/j.1399-543X.2004.00080.x. [DOI] [PubMed] [Google Scholar]
- 42.Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, Benvenisti-Zarum L, Meivar-Levy I, Ferber S. Functional, Persistent, and Extended Liver to Pancreas Transdifferentiation. Journal of Biological Chemistry. 2003;278(34):31950–31957. doi: 10.1074/jbc.M303127200. [DOI] [PubMed] [Google Scholar]
- 43.Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, Zern MA, Fleischer N, Efrat S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proceedings of the National Academy of Sciences. 2003;100(12):7253–7258. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, Peck AB. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proceedings of the National Academy of Sciences. 2002;99(12):8078–8083. doi: 10.1073/pnas.122210699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao L-Z, Tang D-Q, Horb ME, Li S-W, Yang L-J. High Glucose Is Necessary for Complete Maturation of Pdx1-VP16 - Expressing Hepatic Cells into Functional Insulin-Producing Cells. Diabetes. 2004;53(12):3168–3178. doi: 10.2337/diabetes.53.12.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aviv V, Meivar-Levy I, Rachmut IH, Rubinek T, Mor E, Ferber S. Exendin-4 Promotes Liver Cell Proliferation and Enhances the PDX-1-induced Liver to Pancreas Transdifferentiation Process. Journal of Biological Chemistry. 2009;284(48):33509–33520. doi: 10.1074/jbc.M109.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagaya M, Katsuta H, Kaneto H, Bonner-Weir S, Weir GC. Adult mouse intrahepatic biliary epithelial cells induced in vitro to become insulin-producing cells. Journal of Endocrinology. 2009;201(1):37–47. doi: 10.1677/JOE-08-0482. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to B-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Sullivan ES, Vegas A, Anderson DG, Weir GC. Islets Transplanted in Immunoisolation Devices: A Review of the Progress and the Challenges that Remain. Endocrine Reviews. 2011;32(6):827–844. doi: 10.1210/er.2010-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abrahante JE, Martins K, Papas KK, Hering BJ, Schuurman H-J, Murtaugh MP. Microbiological safety of porcine islets: comparison with source pig. Xenotransplantation. 2011;18(2):88–93. doi: 10.1111/j.1399-3089.2011.00632.x. [DOI] [PubMed] [Google Scholar]
- 51.Pappenheimer JR, Renkin EM, Borrero LM. Filtration, Diffusion and Molecular Sieving Through Peripheral Capillary Membranes: A Contribution to the Pore Theory of Capillary Permeability. American Journal of Physiology. 1951;167(1):13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- 52.Oliva A, Fariña J, Llabrés M. Development of two high-performance liquid chromatographic methods for the analysis and characterization of insulin and its degradation products in pharmaceutical preparations. Journal of Chromatography B: Biomedical Sciences and Applications. 2000;749(1):25–34. doi: 10.1016/s0378-4347(00)00374-1. [DOI] [PubMed] [Google Scholar]
- 53.Leoni L, Desai TA. Micromachined biocapsules for cell-based sensing and delivery. Advanced Drug Delivery Reviews. 2004;56(2):211–229. doi: 10.1016/j.addr.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Kang J, Erdodi G, Kennedy JP. Third-generation amphiphilic conetworks. III. Permeabilities and mechanical properties. J. Polym. Sci. A Polym. Chem. 2007;45(18):4276–4283. [Google Scholar]
- 55.Avgoustiniatos ES, Wu H, Colton CK. In: Principles of Tissue Engineering. Lanza RP, Langer R, Vacanti J, editors. Academic Press; San Diego: 2000. pp. 331–350. [Google Scholar]
- 56.Colton CK. Implantable biohybrid artificial organs. Cell Transplantation. 1995;4(4):415–436. doi: 10.1177/096368979500400413. [DOI] [PubMed] [Google Scholar]
- 57.Wilson JT, Chaikof EL. Challenges and emerging technologies in the immunoisolation of cells and tissues. Advanced Drug Delivery Reviews. 2008;60(2):124–145. doi: 10.1016/j.addr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Vos P, Marchetti P. Encapsulation of pancreatic islets for transplantation in diabetes: the untouchable islets. Trends in Molecular Medicine. 2002;8(8):363–366. doi: 10.1016/s1471-4914(02)02381-x. [DOI] [PubMed] [Google Scholar]
- 59.Lysaght MJ, Frydel B, Gentile F, Emerich D, Winn S. Recent progress in immunoisolated cell therapy. J. Cell. Biochem. 1994;56(2):196–203. doi: 10.1002/jcb.240560214. [DOI] [PubMed] [Google Scholar]
- 60.Lanza RP, Kuhtreiber WM, Chick WL. Encapsulation Technologies. Tissue Engineering. 1995;1(2):181–196. doi: 10.1089/ten.1995.1.181. [DOI] [PubMed] [Google Scholar]
- 61.Mendelsohn A, Desai T. In: Therapeutic Applications of Cell Microencapsulation. Pedraz JL, Orive G, editors. Vol. 670. Springer; New York: 2010. pp. 104–125. [Google Scholar]
- 62.Basta G, Calafiore R. Immunoisolation of Pancreatic Islet Grafts with No Recipient's Immunosuppression: Actual and Future Perspectives. Current Diabetes Reports. 2011;11(5):384–391. doi: 10.1007/s11892-011-0219-6. [DOI] [PubMed] [Google Scholar]
- 63.Nafea EH, L.A. P-W, Marson A, Martens PJ. Immunoisolating semi-permeable membranes for cell encapsulation: Focus on hydrogels. Journal of Controlled Release. 2011;154(2):110–122. doi: 10.1016/j.jconrel.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 64.Scharp DW, Mason NS, Sparks RE. Islet immuno-isolation: The use of hybrid artificial organs to prevent islet tissue rejection. World Journal of Surgery. 1984;8(2):221–229. doi: 10.1007/BF01655139. [DOI] [PubMed] [Google Scholar]
- 65.Beck J, Angus R, Madsen B, Britt D, Vernon B, Nguyen KT. Islet Encapsulation: Strategies to Enhance Islet Cell Functions. Tissue Engineering. 2007;13(3):589–599. doi: 10.1089/ten.2006.0183. [DOI] [PubMed] [Google Scholar]
- 66.Iwata H, Morikawa N, Fujii T, Takagi T, Samejima T, Ikada Y. Does immunoisolation need to prevent the passage of antibodies and complement? Tranplantation Proceedings. 1995;27(6):3224–3226. [PubMed] [Google Scholar]
- 67.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. British Journal of Cancer. 1955;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Avgoustiniatos ES, Colton CK. Effect of External Oxygen Mass Transfer Resistances on Viability of Immunoisolated Tissuea. Annals of the New York Academy of Sciences. 1997;831(1):145–166. doi: 10.1111/j.1749-6632.1997.tb52192.x. [DOI] [PubMed] [Google Scholar]
- 69.Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L. Reversal of Diabetes by Pancreatic Islet Transplantation into a Subcutaneous, Neovascularized Device. Transplantation. 2006;81(9):1318–1324. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 70.Trivedi N, Steil GM, Colton CK, Bonner-Weir S, Weir GC. Improved vascularization of planar membrane diffusion devices following continuous infusion of vascular endothelial growth factor. Cell Transplantation. 2000;9(1):115–124. doi: 10.1177/096368970000900114. [DOI] [PubMed] [Google Scholar]
- 71.Adlercreutz P, Mattiasson B. Oxygen Supply to Immobilized Cells: Oxygen Supply by Hemoglobin or Emulsions of Perfluorochemicals. European Journal of Applied Microbiology and Biotechnology. 1982;16(4):165–170. [Google Scholar]
- 72.Fraker CA, Alejandro R, Ricordi C. Use of Oxygenated Perfluorocarbon Toward Making Every Pancreas Count. Transplantation. 2002;74(12):1811–1812. doi: 10.1097/00007890-200212270-00032. [DOI] [PubMed] [Google Scholar]
- 73.Wu H, Avgoustiniatos ES, Swette L, Bonner-Weir S, Weir GC, Colton CK. In Situ Electrochemical Oxygen Generation with an Immunoisolation Device. Annals of the New York Academy of Sciences. 1999;875(1):105–125. doi: 10.1111/j.1749-6632.1999.tb08497.x. [DOI] [PubMed] [Google Scholar]
- 74.Bloch K, Papismedov E, Yavriyants K, Vorobeychik M, Beer S, Vardi P. Photosynthetic Oxygen Generator for Bioartificial Pancreas. Tissue Engineering. 2006;12(2):337–344. doi: 10.1089/ten.2006.12.337. [DOI] [PubMed] [Google Scholar]
- 75.Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ. Oxygen producing biomaterials for tissue regeneration. Biomaterials. 2007;28(31):4628–4634. doi: 10.1016/j.biomaterials.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 76.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30(5):757–762. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 77.Ludwig B, Zimerman B, Steffen A, Yavriants K, Azarov D, Reichel A, Vardi P, German T, Shabtay N, Rotem A, Evron Y, Neufeld T, Mimon S, Ludwig S, Brendel M,D, Bornstein S,R, Barkai U. A Novel Device for Islet Transplantation Providing Immune Protection and Oxygen Supply. Horm Metab Res. 2010;42(13):918–922. doi: 10.1055/s-0030-1267916. [DOI] [PubMed] [Google Scholar]
- 78.Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proceedings of the National Academy of Sciences. 2012;109(11):4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mendelsohn AD, Bernards DA, Lowe RD, Desai TA. Patterning of Mono- and Multilayered Pancreatic Beta-Cell Clusters. Langmuir. 2010;26(12):9943–9949. doi: 10.1021/la1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bernard AB, Lin C-C, Anseth KS. A Microwell Cell Culture Platform for the Aggregation of Pancreatic B-Cells. Tissue Engineering Part C: Methods. 2012;18(8):583–592. doi: 10.1089/ten.tec.2011.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meda P, Bosco D, Chanson M, Giordano E, Vallar L, Wollheim C, Orci L. Rapid and reversible secretion changes during uncoupling of rat insulin-producing cells. J Clin Invest. 1990;86(3):759–768. doi: 10.1172/JCI114772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jaques F, Jousset H.l.n., Tomas A, Prost A-L, Wollheim CB, Irminger J-C, Demaurex N, Halban PA. Dual Effect of Cell-Cell Contact Disruption on Cytosolic Calcium and Insulin Secretion. Endocrinology. 2008;149(5):2494–2505. doi: 10.1210/en.2007-0974. [DOI] [PubMed] [Google Scholar]
- 83.Brereton HC, Carvell MJ, Asare-Anane H, Roberts G, Christie MR, Persaud SJ, Jones PM. Homotypic cell contact enhances insulin but not glucagon secretion. Biochemical and Biophysical Research Communications. 2006;344(3):995–1000. doi: 10.1016/j.bbrc.2006.03.214. [DOI] [PubMed] [Google Scholar]
- 84.Mendelsohn AD, Nyitray C, Sena M, Desai TA. Size-controlled Insulin Secreting Cell Clusters. Acta Biomaterialia. 2012;8(12):4278–4284. doi: 10.1016/j.actbio.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang R, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. Journal of Endocrinology. 1999;163(2):181–190. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- 86.Prochorov A, Tretjak S, Goranov V, Glinnik A, Goltsev M. Treatment of insulin dependent diabetes mellitus with intravascular transplantation of pancreatic islet cells without immunosuppressive therapy. Advances in Medical Sciences. 2008;53(2):240–244. doi: 10.2478/v10039-008-0045-5. [DOI] [PubMed] [Google Scholar]
- 87.Bergen JF, Mason NS, Scharp DW, Sparks RE. Insulin Feedback Inhibition as a Factor in the Reduction of Insulin Release from Islet Transplantation Diffusion Chambers. Artificial Organs. 1981;5(A):67–67. [Google Scholar]
- 88.Bisceglie V. Uber die antineoplastische Immunitat. Zeitschrift für Krebsforschung. 1933;40(1):122–140. [Google Scholar]
- 89.Algire GH, Legallais FY. Recent Developments in the Transparent-Chamber Technique as Adapted to the Mouse. Journal of the National Cancer Institute. 1949;10(2):225–253. [PubMed] [Google Scholar]
- 90.Algire GH, Weaver JM, Prehn RT. Growth of Cells In Vivo in Diffusion Chambers. I. Survival of Homografts in Immunized Mice. Journal of the National Cancer Institute. 1954;15(3):493–507. [PubMed] [Google Scholar]
- 91.Prehn RT, Weaver JM, Algire GH. The Diffusion-Chamber Technique Applied to a Study of the Nature of Homograft Resistance. Journal of the National Cancer Institute. 1954;15(3):509–517. [PubMed] [Google Scholar]
- 92.Weaver JM, Algire GH, Prehn RT. The Growth of Cells in Vivo in Diffusion Chambers. II. The Role of Cells in the Destruction of Homografts in Mice. Journal of the National Cancer Institute. 1955;15(6):1737–1767. [PubMed] [Google Scholar]
- 93.Algire GH. Diffusion-Chamber Techniques for Studies of Cellular Immunity. Annals of the New York Academy of Sciences. 1957;69(4):663–667. doi: 10.1111/j.1749-6632.1957.tb49705.x. [DOI] [PubMed] [Google Scholar]
- 94.Moskalewski S. Isolation and culture of the islets of Langerhans of the guinea pig. General and Comparative Endocrinology. 1965;5(3):342–353. doi: 10.1016/0016-6480(65)90059-6. [DOI] [PubMed] [Google Scholar]
- 95.Strautz R. Studies of hereditary-obese mice (obob) after implantation of pancreatic islets in Millipore filter capsules. Diabetologia. 1970;6(3):306–312. doi: 10.1007/BF01212243. [DOI] [PubMed] [Google Scholar]
- 96.Gates R, Smith R, Hunt MI, Lazarus N. Return to Normal of Blood-Glucose, Plasma-Insulin, and Weight Gain in New Zealand Obese Mice after Implantation of Islets of Langerhans. The Lancet. 1972;300(7777):567–570. doi: 10.1016/s0140-6736(72)91960-5. [DOI] [PubMed] [Google Scholar]
- 97.Lacy PE, Hegre OD, Gerasimidi-Vazeou A, Gentile FT, Dionne KE. Maintenance of normoglycemia in diabetic mice by subcutaneous xenografts of encapsulated islets. Science. 1991;254(5039):1782–1784. doi: 10.1126/science.1763328. [DOI] [PubMed] [Google Scholar]
- 98.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. Journal of Biomedical Materials Research. 1995;29(12):1517–1524. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 99.Loudovaris T, Jacobs S, Young S, Maryanov D, Brauker J, Johnson RC. Correction of diabetic nod mice with insulinomas implanted within Baxter immunoisolation devices. Journal of Molecular Medicine. 1999;77(1):219–222. doi: 10.1007/s001090050340. [DOI] [PubMed] [Google Scholar]
- 100.Binette TM, Seeberger KL, Lyon JG, Rajotte RV, Korbutt GS. Porcine Endogenous Retroviral Nucleic Acid in Peripheral Tissues Is Associated with Migration of Porcine Cells Post Islet Transplant. American Journal of Transplantation. 2004;4(7):1051–1060. doi: 10.1111/j.1600-6143.2004.00460.x. [DOI] [PubMed] [Google Scholar]
- 101.Sorenby AK, Kumagai-Braesch M, Sharma A, Hultenby KR, Wernerson AM, Tibell AB. Preimplantation of an Immunoprotective Device Can Lower the Curative Dose of Islets to That of Free Islet Transplantation-Studies in a Rodent Model. Transplantation. 2008;86(2):364–366. doi: 10.1097/TP.0b013e31817efc78. [DOI] [PubMed] [Google Scholar]
- 102.Sweet IR, Yanay O, Waldron L, Gilbert M, Fuller JM, Tupling T, Lernmark A, Osborne WRA. Treatment of diabetic rats with encapsulated islets. Journal of Cellular and Molecular Medicine. 2008;12(6b):2644–2650. doi: 10.1111/j.1582-4934.2008.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Desai TA, Chu WH, Tu JK, Beattie GM, Hayek A, Ferrari M. Microfabricated immunoisolating biocapsules. Biotechnol. Bioeng. 1998;57(1):118–120. doi: 10.1002/(sici)1097-0290(19980105)57:1<118::aid-bit14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 104.Desai TA, Hansford DJ, Kulinsky L, Nashat AH, Rasi G, Tu J, Wang Y, Zhang M, Ferrari M. Nanopore Technology for Biomedical Applications. Biomedical Microdevices. 1999;2(1):11–40. [Google Scholar]
- 105.Desai TA, West T, Cohen M, Boiarski T, Rampersaud A. Nanoporous microsystems for islet cell replacement. Advanced Drug Delivery Reviews. 2004;56(11):1661–1673. doi: 10.1016/j.addr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 106.Smith C, Kirk R, West T, Bratzel M, Cohen M, Martin F, Boiarski A, Rampersaud AA. Diffusion Characteristics of Microfabricated Silicon Nanopore Membranes as Immunoisolation Membranes for Use in Cellular Therapeutics. Diabetes Technology & Therapeutics. 2005;7(1):151–162. doi: 10.1089/dia.2005.7.151. [DOI] [PubMed] [Google Scholar]
- 107.Martin F, Walczak R, Boiarski A, Cohen M, West T, Cosentino C, Ferrari M. Tailoring width of microfabricated nanochannels to solute size can be used to control diffusion kinetics. Journal of Controlled Release. 2005;102(1):123–133. doi: 10.1016/j.jconrel.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 108.King SR, Dorian R, Storrs RW. Requirements for Encapsulation Technology and the Challenges for Transplantation of Islets of Langerhans. Graft. 2001;4(7):491–499. [Google Scholar]
- 109.Storrs R, Dorian R, King SR, Lakey J, Rilo H. Preclinical Development of the Islet Sheet. Annals of the New York Academy of Sciences. 2001;944(1):252–266. doi: 10.1111/j.1749-6632.2001.tb03837.x. [DOI] [PubMed] [Google Scholar]
- 110.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature Biotechnology. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 111.Knazek RA, Gullino PM, Kohler PO, Dedrick RL. Cell Culture on Artificial Capillaries: An Approach to Tissue Growth in vitro. Science. 1972;178(4056):65–67. doi: 10.1126/science.178.4056.65. [DOI] [PubMed] [Google Scholar]
- 112.Chick W, Like A, Lauris V. Beta cell culture on synthetic capillaries: an artificial endocrine pancreas. Science. 1975;187(4179):847–849. doi: 10.1126/science.187.4179.847. [DOI] [PubMed] [Google Scholar]
- 113.Chick W, Perna J, Lauris V, Low D, Galletti P, Panol G, Whittemore A, Like A, Colton C, Lysaght M. Artificial pancreas using living beta cells: effects on glucose homeostasis in diabetic rats. Science. 1977;197(4305):780–782. doi: 10.1126/science.407649. [DOI] [PubMed] [Google Scholar]
- 114.Tze WJ, Wong FC, Chen LM, O'Young S. Implantable artificial endocrine pancreas unit used to restore normoglycaemia in the diabetic rat. Nature. 1976;264(5585):466–467. doi: 10.1038/264466a0. [DOI] [PubMed] [Google Scholar]
- 115.Sun AM, Parisius W, Healy GM, Vacek I, Macmorine HG. The Use, in Diabetic Rats and Monkeys, of Artificial Capillary Units Containing Cultured Islets of Langerhans (Artificial Endocrine Pancreas). Diabetes. 1977;26(12):1136–1139. doi: 10.2337/diab.26.12.1136. [DOI] [PubMed] [Google Scholar]
- 116.Orsetti A, Guy C, Zouai N, Deffay R. Implantation du distributeur bio-artificiel d'insuline chez le chien utilisant des ilots de Langerhans d'especes animales differentes. Comptes-Rendus des Seances de la Societe de Biologie (Paris) 1978;172(1):144–150. [PubMed] [Google Scholar]
- 117.Tze W, Wong F, Chen L. Implantable artificial capillary unit for pancreatic islet allograft and xenograft. Diabetologia. 1979;16(4):247–252. doi: 10.1007/BF01221951. [DOI] [PubMed] [Google Scholar]
- 118.Tze W, Tai J, Wong F, Davis H. Studies with implantable artificial capillary units containing rat islets on diabetic dogs. Diabetologia. 1980;19(6):541–545. doi: 10.1007/BF00253182. [DOI] [PubMed] [Google Scholar]
- 119.Sun A, Parisius W, Macmorine H, Sefton M, Stone R. An Artificial Endocrine Pancreas Containing Cultured Islets of Langerhans. Artificial Organs. 1980;4(4):275–278. [Google Scholar]
- 120.Reach G, Poussier P, Sausse A, Assas R, Itoh M, Gerich JE. Functional evaluation of a bioartificial pancreas using isolated islets perfused with blood ultrafiltrate. Diabetes. 1981;30(4):296–301. doi: 10.2337/diab.30.4.296. [DOI] [PubMed] [Google Scholar]
- 121.Sullivan S, Maki T, Borland K, Mahoney M, Solomon B, Muller T, Monaco A, Chick W. Biohybrid artificial pancreas: long-term implantation studies in diabetic, pancreatectomized dogs. Science. 1991;252(5006):718–721. doi: 10.1126/science.2024124. [DOI] [PubMed] [Google Scholar]
- 122.Goh CH, Heng PWS, Chan LW. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydrate Polymers. 2012;88(1):1–12. [Google Scholar]
- 123.Rabanel J-M, Banquy X, Zouaoui H, Mokhtar M, Hildgen P. Progress technology in microencapsulation methods for cell therapy. Biotechnol Progress. 2009;25(4):946–963. doi: 10.1002/btpr.226. [DOI] [PubMed] [Google Scholar]
- 124.McGuigan AP, Bruzewicz DA, Glavan A, Butte M, Whitesides GM. Cell Encapsulation in Sub-mm Sized Gel Modules Using Replica Molding. PLoS ONE. 2008;3(5):e2258. doi: 10.1371/journal.pone.0002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dang TT, Xu Q, Bratlie KM, O'Sullivan ES, Chen XY, Langer R, Anderson DG. Microfabrication of homogenous, asymmetric cell-laden hydrogel capsules. Biomaterials. 2009;30(36):6896–6902. doi: 10.1016/j.biomaterials.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chang TMS. Semipermeable Microcapsules. Science. 1964;146(3643):524–525. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- 127.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 128.O'Shea GM, Goosen MFA, Sun AM. Prolonged survival of transplanted islets of Langerhans encapsulated in a biocompatible membrane. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1984;804(1):133–136. doi: 10.1016/0167-4889(84)90107-1. [DOI] [PubMed] [Google Scholar]
- 129.Zimmermann U, Klock G, Federlin K, Hannig K, Kowalski M, Bretzel RG, Horcher A, Entenmann H, Sieber U, Zekorn T. Production of mitogen-contamination free alginates with variable ratios of mannuronic acid to guluronic acid by free flow electrophoresis. ELECTROPHORESIS. 1992;13(1):269–274. doi: 10.1002/elps.1150130156. [DOI] [PubMed] [Google Scholar]
- 130.Klock G, Frank H, Houben R, Zekorn T, Horcher A, Siebers U, Wohrle M, Federlin K, Zimmermann U. Production of purified alginates suitable for use in immunoisolated transplantation. Applied Microbiology and Biotechnology. 1994;40(5):638–643. doi: 10.1007/BF00173321. [DOI] [PubMed] [Google Scholar]
- 131.de Vos P, Haan BJD, Wolters GHJ, Strubbe JH, Schilfgaarde RV. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia. 1997;40(3):262–270. doi: 10.1007/s001250050673. [DOI] [PubMed] [Google Scholar]
- 132.Sawhney AS, Pathak CP, Hubbell JA. Interfacial photopolymerization of poly(ethylene glycol)-based hydrogels upon alginate-poly(l-lysine) microcapsules for enhanced biocompatibility. Biomaterials. 1993;14(13):1008–1016. doi: 10.1016/0142-9612(93)90194-7. [DOI] [PubMed] [Google Scholar]
- 133.Wang T, Lacik I, Brissova M, Anilkumar AV, Prokop A, Hunkeler D, Green R, Shahrokhi K, Powers AC. An encapsulation system for the immunoisolation of pancreatic islets. Nature Biotechnology. 1997;15(4):358–362. doi: 10.1038/nbt0497-358. [DOI] [PubMed] [Google Scholar]
- 134.Peterson KP, Peterson CM, Pope EJA. Silica Sol-Gel Encapsulation of Pancreatic Islets. Proceedings of the Society for Experimental Biology & Medicine. 1998;218(4):365–369. doi: 10.3181/00379727-218-44305. [DOI] [PubMed] [Google Scholar]
- 135.Boninsegna S, Bosetti P, Carturan G, Dellagiacoma G, Dal Monte R, Rossi M. Encapsulation of individual pancreatic islets by sol-gel SiO2: A novel procedure for perspective cellular grafts. Journal of Biotechnology. 2003;100(3):277–286. doi: 10.1016/s0168-1656(02)00248-1. [DOI] [PubMed] [Google Scholar]
- 136.Sefton M, Stevenson W. Microencapsulation of live animal cells using polyacrylates. Advances in Polymer Science. 1993;107(1):143–197. [Google Scholar]
- 137.Iwata H, Amemiya H, Matsuda T, Takano H, Akutsu T. Microencapsulation of Langerhans Islets in Agarose Microbeads and Their Application for a Bioartificial Pancreas. Journal of Bioactive and Compatible Polymers. 1988;3(4):356–369. [Google Scholar]
- 138.Iwata H, Takagi T, Amemiya H, Shimizu H, Yamashita K, Kobayashi K, Akutsu T. Agarose for a bioartificial pancreas. Journal of Biomedical Materials Research. 1992;26(7):967–977. doi: 10.1002/jbm.820260711. [DOI] [PubMed] [Google Scholar]
- 139.Yang K-C, Qi Z, Wu C-C, Shirouza Y, Lin F-H, Yanai G, Sumi S. The cytoprotection of chitosan based hydrogels in xenogeneic islet transplantation: An in vivo study in streptozotocin-induced diabetic mouse. Biochemical and Biophysical Research Communications. 2010;393(4):818–823. doi: 10.1016/j.bbrc.2010.02.089. [DOI] [PubMed] [Google Scholar]
- 140.Panza JL, Wagner R, W., Rilo HLR, Harsha Rao R, Beckman EJ, Russell AJ. Treatment of rat pancreatic islets with reactive PEG. Biomaterials. 2000;21(11):1155–1164. doi: 10.1016/s0142-9612(99)00283-5. [DOI] [PubMed] [Google Scholar]
- 141.Kellam B, De Bank PA, Shakesheff KM. Chemical modification of mammalian cell surfaces. Chemical Society Reviews. 2003;32(6):327–337. doi: 10.1039/b211643j. [DOI] [PubMed] [Google Scholar]
- 142.Decher G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science. 1997;277(5330):1232–1237. [Google Scholar]
- 143.Wilson JT, Cui W, Kozlovskaya V, Kharlampieva E, Pan D, Qu Z, Krishnamurthy VR, Mets J, Kumar V, Wen J, Song Y, Tsukruk VV, Chaikof EL. Cell Surface Engineering with Polyelectrolyte Multilayer Thin Films. Journal of the American Chemical Society. 2011;133(18):7054–7064. doi: 10.1021/ja110926s. [DOI] [PubMed] [Google Scholar]
- 144.Soon-Shiong P, Heintz RE, Merideth N, Yao QX, Yao Z, Zheng T, Murphy M, Moloney MK, Schmehl M, Harris M, Mendez R, Mendez R, Sandford PA. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. The Lancet. 1994;343(8903):950–951. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 145.Thanos CG, Calafiore R, Basta G, Bintz BE, Bell WJ, Hudak J, Vasconcellos A, Schneider P, Skinner SJM, Geaney M, Tan P, Elliot RB, Tatnell M, Escobar L, Qian H, Mathiowitz E, Emerich DF. Formulating the alginate-polyornithine biocapsule for prolonged stability: Evaluation of composition and manufacturing technique. J. Biomed. Mater. Res. 2007;83A(1):216–224. doi: 10.1002/jbm.a.31472. [DOI] [PubMed] [Google Scholar]
- 146.Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, Racanicchi L, Mancuso F, Brunetti P. Microencapsulated Pancreatic Islet Allografts Into Nonimmunosuppressed Patients With Type 1 Diabetes. Diabetes Care. 2006;29(1):137–138. doi: 10.2337/diacare.29.1.137. [DOI] [PubMed] [Google Scholar]
- 147.Basta G, Montanucci P, Luca G, Boselli C, Noya G, Barbaro B, Qi M, Kinzer KP, Oberholzer J, Calafiore R. Long-Term Metabolic and Immunological Follow-Up of Nonimmunosuppressed Patients With Type 1 Diabetes Treated With Microencapsulated Islet Allografts. Diabetes Care. 2011;34(11):2406–2409. doi: 10.2337/dc11-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Orive G, Maria Hernandez R, Rodriguez Gascon A, Calafiore R, Swi Chang TM, Vos P.d., Hortelano G, Hunkeler D, Lacik I, Luis Pedraz J. History, challenges and perspectives of cell microencapsulation. Trends in Biotechnology. 2004;22(2):87–92. doi: 10.1016/j.tibtech.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 149.de Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27(32):5603–5617. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 150.Calafiore R, Basta G. In: Methods in Molecular Medicine. Hauser H, Fussenegger M, editors. Vol. 140. Humana Press; Totowa: 2007. pp. 197–236. [DOI] [PubMed] [Google Scholar]
- 151.de Vos P, Bucko M, Gemeiner P, Navrátil M, Svitel J, Faas M, Strand BL, Skjak-Braek G, Morch YA, Vikartovská A, Lacík I, Kolláriková G, Orive G, Poncelet D, Pedraz JL, Ansorge-Schumacher MB. Multiscale requirements for bioencapsulation in medicine and biotechnology. Biomaterials. 2009;30(13):2559–2570. doi: 10.1016/j.biomaterials.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 152.Pedraz JL, Orive G. Therapeutic Applications of Cell Microencapsulation. Springer; New York: 2010. [Google Scholar]
- 153.Calafiore R, Basta G. In: Stem Cell Therapy for Diabetes. Efrat S, editor. Vol. 3. Humana Press; New York: 2010. pp. 241–262. [Google Scholar]
- 154.Elliott RB, Escobar L, Tan PLJ, Muzina M, Zwain S, Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5yr after xenotransplantation. Xenotransplantation. 2007;14(2):157–161. doi: 10.1111/j.1399-3089.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 155.Fallarino F, Luca G, Calvitti M, Mancuso F, Nastruzzi C, Fioretti MC, Grohmann U, Becchetti E, Burgevin A, Kratzer R, Endert P.v., Boon L, Puccetti P, Calafiore R. Therapy of experimental type 1 diabetes by isolated Sertoli cell xenografts alone. Journal of Experimental Medicine. 2009;206(11):2511–2526. doi: 10.1084/jem.20090134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Adiga SP, Jin C, Curtiss LA, Monteiro-Riviere NA, Narayan RJ. Nanoporous membranes for medical and biological applications. WIREs: Nanmed Nanobiotech. 2009;1(5):568–581. doi: 10.1002/wnan.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dunleavy M. Polymeric membranes. A review of applications. Medical Device Technology. 1996;7(4):18–21. [PubMed] [Google Scholar]
- 158.Desai TA, Hansford DJ, Leoni L, Essenpreis M, Ferrari M. Nanoporous anti-fouling silicon membranes for biosensor applications. Biosensors and Bioelectronics. 2000;15(9-10):453–462. doi: 10.1016/s0956-5663(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 159.Li RH. Materials for immunoisolated cell transplantation. Advanced Drug Delivery Reviews. 1998;33(1-2):87–109. doi: 10.1016/s0169-409x(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 160.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical Foundations and Structural Design of Hydrogels in Medicine and Biology. Annual Review of Biomedical Engineering. 2000;2(1):9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 161.Weber LM, He J, Bradley B, Haskins K, Anseth KS. PEG-based hydrogels as an in vitro encapsulation platform for testing controlled B-cell microenvironments. Acta Biomaterialia. 2006;2(1):1–8. doi: 10.1016/j.actbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 162.Weber LM, Cheung CY, Anseth KS. Multifunctional Pancreatic Islet Encapsulation Barriers Achieved Via Multilayer PEG Hydrogels. Cell Transplantation. 2008;16(10):1049–1057. [PubMed] [Google Scholar]
- 163.Magarinos AM, Jain K, Blount ED, Reagan L, Smith BH, McEwen BS. Peritoneal implantation of macroencapsulated porcine pancreatic islets in diabetic rats ameliorates severe hyperglycemia and prevents retraction and simplification of hippocampal dendrites. Brain Research. 2001;902(2):282–287. doi: 10.1016/s0006-8993(01)02400-3. [DOI] [PubMed] [Google Scholar]
- 164.Gazda LS, Vinerean HV, Laramore MA, Diehl CH, Hall RD, Rubin AL, Smith BH. Encapsulation of Porcine Islets Permits Extended Culture Time and Insulin Independence in Spontaneously Diabetic BB Rats. Cell Transplantation. 2007;16(6):609–620. doi: 10.3727/000000007783465028. [DOI] [PubMed] [Google Scholar]
- 165.Qi M, Gu Y, Sakata N, Kim D, Shirouzu Y, Yamamoto C, Hiura A, Sumi S, Inoue K. PVA hydrogel sheet macroencapsulation for the bioartificial pancreas. Biomaterials. 2004;25(27):5885–5892. doi: 10.1016/j.biomaterials.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 166.Qi Z, Shen Y, Yanai G, Yang K, Shirouzu Y, Hiura A, Sumi S. The in vivo performance of polyvinyl alcohol macro-encapsulated islets. Biomaterials. 2010;31(14):4026–4031. doi: 10.1016/j.biomaterials.2010.01.088. [DOI] [PubMed] [Google Scholar]
- 167.Lelli L, Barbani N, Bonaretti A, Giusti P, Marchetti P, Giannarelli R, Navalesi R, Viacava P. Preparation and characterization of permselective, biocompatible membranes for the macroencapsulation of pancreatic islets. Journal of Materials Science: Materials in Medicine. 1994;5(12):887–890. [Google Scholar]
- 168.Scharp D, Swanson C, Olack B, Latta P, Hegre O, Doherty E, Gentile F, Flavin K, Ansara M, Lacy P. Protection of encapsulated human islets implanted without immunosuppression in patients with Type I or Type II diabetes and in nondiabetic control subjects. Diabetes. 1994;43(9):1167–1170. doi: 10.2337/diab.43.9.1167. [DOI] [PubMed] [Google Scholar]
- 169.Bridge MJ, Broadhead KW, Hlady V, Tresco PA. Ethanol treatment alters the ultrastructure and permeability of PAN-PVC hollow fiber cell encapsulation membranes. Journal of Membrane Science. 2002;195(1):51–64. [Google Scholar]
- 170.George S, Nair PD, Risbud MV, Bhonde RR. Nonporous Polyurethane Membranes as Islet Immunoisolation Matrices - Biocompatibility Studies. Journal of Biomaterials Applications. 2002;16(4):327–340. doi: 10.1106/088532802024249. [DOI] [PubMed] [Google Scholar]
- 171.Kadam SS, Sudhakar M, Nair PD, Bhonde RR. Reversal of experimental diabetes in mice by transplantation of neo-islets generated from human amnion-derived mesenchymal stromal cells using immuno-isolatory macrocapsules. Cytotherapy. 2010;12(8):982–991. doi: 10.3109/14653249.2010.509546. [DOI] [PubMed] [Google Scholar]
- 172.Zondervan GJ, Hoppen HJ, Pennings AJ, Fritschy W, Wolters G, van Schilfgaard R. Design of a polyurethane membrane for the encapsulation of islets of Langerhans. Biomaterials. 1992;13(3):136–144. doi: 10.1016/0142-9612(92)90061-r. [DOI] [PubMed] [Google Scholar]
- 173.Cheung SSC, Tai J, Tze WJ. Effect of molecular weight exclusion of polysulfone fibers on macroencapsulated pig islet xenograft function in diabetic mice. Transplantation Proceedings. 1997;29(4):2144–2145. doi: 10.1016/s0041-1345(97)00266-2. [DOI] [PubMed] [Google Scholar]
- 174.Silva AI, Mateus M. Development of a polysulfone hollow fiber vascular bio-artificial pancreas device for in vitro studies. Journal of Biotechnology. 2009;139(3):236–249. doi: 10.1016/j.jbiotec.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 175.Kessler L, Legeay G, Jesser C, Damge C, Pinget M. Influence of corona surface treatment on the properties of an artificial membrane used for Langerhans islets encapsulation: permeability and biocompatibility studies. Biomaterials. 1995;16(3):185–191. doi: 10.1016/0142-9612(95)92116-n. [DOI] [PubMed] [Google Scholar]
- 176.Suzuki K, Bonner-Weir S, Hollister J, Weir GC. A method for estimating number and mass of islets transplanted within a membrane device. Cell Transplantation. 1996;5(6):613–625. doi: 10.1177/096368979600500604. [DOI] [PubMed] [Google Scholar]
- 177.Suzuki K, Bonner-Weir S, Hollister-Lock J, Colton CK, Weir GC. Number and Volume of Islets Transplanted in Immunobarrier Devices. Cell Transplantation. 1998;7(1):47–52. doi: 10.1177/096368979800700107. [DOI] [PubMed] [Google Scholar]
- 178.Krishnan L, Clayton LR, Boland ED, Reed RM, Hoying JB, Williams SK. Cellular Immunoisolation for Islet Transplantation by a Novel Dual Porosity Electrospun Membrane. Transplantation Proceedings. 2011;43(9):3256–3261. doi: 10.1016/j.transproceed.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dufrane D, Gianello P, Melvik JE. Alginate coated, collagen matrix cellular device, preparative methods, and uses thereof. 2007 Jun 13; Patent WO/2007/144389.
- 180.Dufrane D, Goebbels R-M, Gianello P. Alginate Macroencapsulation of Pig Islets Allows Correction of Streptozotocin-Induced Diabetes in Primates up to 6 Months Without Immunosuppression. Transplantation. 2010;90(10):1054–1062. doi: 10.1097/TP.0b013e3181f6e267. [DOI] [PubMed] [Google Scholar]
- 181.Gimi B, Kwon J, Kuznetsov A, Vachha B, Magin RL, Philipson LH, Lee J-B. A Nanoporous, Transparent Microcontainer for Encapsulated Islet Therapy. Journal of Diabetes Science and Technology. 2009;3(2):297–303. doi: 10.1901/jaba.2009.3-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gimi B, Kwon J, Liu L, Su Y, Nemani K, Trivedi K, Cui Y, Vachha B, Mason R, Hu W, Lee J-B. Cell encapsulation and oxygenation in nanoporous microcontainers. Biomedical Microdevices. 2009;11(6):1205–1212. doi: 10.1007/s10544-009-9338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kwon J, Trivedi K, Krishnamurthy NV, Hu W, Lee J-B, Gimi B. SU-8-based immunoisolative microcontainer with nanoslots defined by nanoimprint lithography. Journal of Vacuum Science and Technology B. 2009;27(6):2795–2800. doi: 10.1116/1.3258146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gimi B. Making a Case: Nanofabrication Techniques in Encapsulated Cell Therapy for People with Diabetes. Journal of Diabetes Science and Technology. 2010;4(4):1016–1021. doi: 10.1177/193229681000400435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Voskerician G, Shive MS, Shawgo RS, Recum H.v., Anderson JM, Cima MJ, Langer R. Biocompatibility and biofouling of MEMS drug delivery devices. Biomaterials. 2003;24(11):1959–1967. doi: 10.1016/s0142-9612(02)00565-3. [DOI] [PubMed] [Google Scholar]
- 186.van Rijn CJM, Elwenspoek MC. Microfiltration membrane sieve with silicon micromachining for industrial and biomedical applications. Micro Electro Mechanical Systems, 1995, MEMS '95, Proceedings. 1995:83–87. IEEE DO - 10.1109/MEMSYS.1995.472549. [Google Scholar]
- 187.Ferrari M, Chu IW, Desai IT, Hansford D, Mazzoni G, Huen T, Zhang M. Silicon Nanotechnology for Biofiltration and Immunoisolated Cell Xenografts. MRS Proceedings. 1995;414(1):101–106. [Google Scholar]
- 188.Desai TA, Hansford DJ, Ferrari M. Micromachined interfaces: new approaches in cell immunoisolation and biomolecular separation. Biomolecular Engineering. 2000;17(1):23–36. doi: 10.1016/s1389-0344(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 189.Desai TA, Hansford D, Ferrari M. Characterization of micromachined silicon membranes for immunoisolation and bioseparation applications. Journal of Membrane Science. 1999;159(1-2):221–231. [Google Scholar]
- 190.Desai T, Chu W, Rasi G, Sinibaldi-Vallebona P, Guarino E, Ferrari M. Microfabricated Biocapsules Provide Short-Term Immunoisolation of Insulinoma Xenografts. Biomedical Microdevices. 1999;1(2):131–138. doi: 10.1023/A:1009948524686. [DOI] [PubMed] [Google Scholar]
- 191.Leoni L, Boiarski A, Desai TA. Characterization of Nanoporous Membranes for Immunoisolation: Diffusion Properties and Tissue Effects. Biomedical Microdevices. 2002;4(2):131–139. [Google Scholar]
- 192.Colton CK, Avgoustiniatos ES. Bioengineering in Development of the Hybrid Artificial Pancreas. J. Biomech. Eng. 1991;113(2):152–170. doi: 10.1115/1.2891229. [DOI] [PubMed] [Google Scholar]
- 193.Iwata H, Kobayashi K, Takagi T, Oka T, Yang H, Amemiya H, Tsuji T, Ito F. Feasibility of agarose microbeads with xenogeneic islets as a bioartificial pancreas. Journal of Biomedical Materials Research. 1994;28(9):1003–1011. doi: 10.1002/jbm.820280905. [DOI] [PubMed] [Google Scholar]
- 194.Lanza RP, Hayes JL, Chick WL. Encapsulated cell technology. Nature Biotechnology. 1996;14(9):1107–1111. doi: 10.1038/nbt0996-1107. [DOI] [PubMed] [Google Scholar]
- 195.Zhang M, Desai T, Ferrari M. Proteins and cells on PEG immobilized silicon surfaces. Biomaterials. 1998;19(10):953–960. doi: 10.1016/s0142-9612(98)00026-x. [DOI] [PubMed] [Google Scholar]
- 196.Sharma S, Johnson RW, Desai TA. Ultrathin poly(ethylene glycol) films for silicon-based microdevices. Applied Surface Science. 2003;206(1-4):218–229. doi: 10.1021/la034753l. [DOI] [PubMed] [Google Scholar]
- 197.Leoni L, Desai TA. Nanoporous biocapsules for the encapsulation of insulinoma cells: biotransport and biocompatibility considerations. Biomedical Engineering, IEEE Transactions. 2001;48(11):1335–1341. doi: 10.1109/10.959329. [DOI] [PubMed] [Google Scholar]
- 198.Diggle JW, Downie TC, Goulding CW. Anodic oxide films on aluminum. Chemical Reviews. 1969;69(3):365–405. [Google Scholar]
- 199.Itoh N, Kato K, Tsuji T, Hongo M. Preparation of a tubular anodic aluminum oxide membrane. Journal of Membrane Science. 1996;117(1-2):189–196. [Google Scholar]
- 200.Itoh N, Tomura N, Tsuji T, Hongo M. Strengthened porous alumina membrane tube prepared by means of internal anodic oxidation. Microporous and Mesoporous Materials. 1998;20(4-6):333–337. [Google Scholar]
- 201.Gong D, Yadavalli V, Paulose M, Pishko M, Grimes CA. Controlled Molecular Release Using Nanoporous Alumina Capsules. Biomedical Microdevices. 2003;5(1):75–80. [Google Scholar]
- 202.Popat KC, Mor G, Grimes CA, Desai TA. Surface Modification of Nanoporous Alumina Surfaces with Poly(ethylene glycol). Langmuir. 2004;20(19):8035–8041. doi: 10.1021/la049075x. [DOI] [PubMed] [Google Scholar]
- 203.Swan EEL, Popat KC, Grimes CA, Desai TA. Fabrication and evaluation of nanoporous alumina membranes for osteoblast culture. J. Biomed. Mater. Res. 2005;72A(3):288–295. doi: 10.1002/jbm.a.30223. [DOI] [PubMed] [Google Scholar]
- 204.La Flamme KE, Mor G, Gong D, La Tempa T, Fusaro VA, Grimes CA, Desai TA. Nanoporous Alumina Capsules for Cellular Macroencapsulation: Transport and Biocompatibility. Diabetes Technology & Therapeutics. 2005;7(5):684–694. doi: 10.1089/dia.2005.7.684. [DOI] [PubMed] [Google Scholar]
- 205.La Flamme KE, Popat KC, Leoni L, Markiewicz E, La Tempa TJ, Roman BB, Grimes CA, Desai TA. Biocompatibility of nanoporous alumina membranes for immunoisolation. Biomaterials. 2007;28(16):2638–2645. doi: 10.1016/j.biomaterials.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Popat KC, Mor G, Grimes C, Desai TA. Poly (ethylene glycol) grafted nanoporous alumina membranes. Journal of Membrane Science. 2004;243(1-2):97–106. [Google Scholar]
- 207.Zwilling V, Darque-Ceretti E, Boutry-Forveille A, David D, Perrin MY, Aucouturier M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf. Interface Anal. 1999;27(7):629–637. [Google Scholar]
- 208.Gong D, Grimes CA, Varghese OK, Hu W, Singh RS, Chen Z, Dickey EC. Titanium oxide nanotube arrays prepared by anodic oxidation. Journal of Materials Research. 2001;16(12):3331–3334. [Google Scholar]
- 209.Beranek R, Hildebrand H, Schmuki P. Self-Organized Porous Titanium Oxide Prepared in H2SO4/HF Electrolytes. Electrochem. Solid-State Lett. 2003;6(3):B12–B14. [Google Scholar]
- 210.Macak JM, Sirotna K, Schmuki P. Self-organized porous titanium oxide prepared in Na2SO4/NaF electrolytes. Electrochimica Acta. 2005;50(18):3679–3684. [Google Scholar]
- 211.Macak JM, Tsuchiya H, Schmuki P. High-Aspect-Ratio TiO2 Nanotubes by Anodization of Titanium. Angew. Chem. Int. Ed. 2005;44(14):2100–2102. doi: 10.1002/anie.200462459. [DOI] [PubMed] [Google Scholar]
- 212.Macak JM, Tsuchiya H, Taveira L, Aldabergerova S, Schmuki P. Smooth Anodic TiO2 Nanotubes. Angew. Chem. Int. Ed. 2005;44(45):7463–7465. doi: 10.1002/anie.200502781. [DOI] [PubMed] [Google Scholar]
- 213.Cai Q, Paulose M, Varghese OK, Grimes CA. The effect of electrolyte composition on the fabrication of self-organized titanium oxide nanotube arrays by anodic oxidation. Journal of Materials Research. 2005;20(1):230–236. [Google Scholar]
- 214.Ruan C, Paulose M, Varghese OK, Mor GK, Grimes CA. Fabrication of Highly Ordered TiO2 Nanotube Arrays Using an Organic Electrolyte. The Journal of Physical Chemistry B. 2005;109(33):15754–15759. doi: 10.1021/jp052736u. [DOI] [PubMed] [Google Scholar]
- 215.Macak JM, Schmuki P. Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochimica Acta. 2006;52(3):1258–1264. [Google Scholar]
- 216.Paulose M, Shankar K, Yoriya S, Prakasam HE, Varghese OK, Mor GK, Latempa TA, Fitzgerald A, Grimes CA. Anodic Growth of Highly Ordered TiO2 Nanotube Arrays to 134 um in Length. The Journal of Physical Chemistry B. 2006;110(33):16179–16184. doi: 10.1021/jp064020k. [DOI] [PubMed] [Google Scholar]
- 217.Macak JM, Albu SP, Schmuki P. Towards ideal hexagonal self-ordering of TiO2 nanotubes. Phys. Status Solidi RRL. 2007;1(5):181–183. [Google Scholar]
- 218.Prakasam HE, Shankar K, Paulose M, Varghese OK, Grimes CA. A New Benchmark for TiO2 Nanotube Array Growth by Anodization. The Journal of Physical Chemistry C. 2007;111(20):7235–7241. [Google Scholar]
- 219.Paulose M, Prakasam HE, Varghese OK, Peng L, Popat KC, Mor GK, Desai TA, Grimes CA. TiO2 Nanotube Arrays of 1000 um Length by Anodization of Titanium Foil: Phenol Red Diffusion. The Journal of Physical Chemistry C. 2007;111(41):14992–14997. [Google Scholar]
- 220.Paulose M, Peng L, Popat KC, Varghese OK, LaTempa TJ, Bao N, Desai TA, Grimes CA. Fabrication of mechanically robust, large area, polycrystalline nanotubular/porous TiO2 membranes. Journal of Membrane Science. 2008;319(1-2):199–205. [Google Scholar]
- 221.Grimes CA, Mor GK. TiO2 Nanotube Arrays - Synthesis, Properties, and Applications. Springer; Dordrecht: 2009. [Google Scholar]
- 222.Ghicov A, Schmuki P. Self-ordering electrochemistry: a review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chemical Communications. 2009;20(1):2791–2808. doi: 10.1039/b822726h. [DOI] [PubMed] [Google Scholar]
- 223.Rani S, Roy SC, Paulose M, Varghese OK, Mor GK, Kim S, Yoriya S, LaTempa TJ, Grimes CA. Synthesis and applications of electrochemically self-assembled titania nanotube arrays. Physical Chemistry Chemical Physics. 2010;12(12):2780–2800. doi: 10.1039/b924125f. [DOI] [PubMed] [Google Scholar]
- 224.Roy P, Berger S, Schmuki P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011;50(13):2904–2939. doi: 10.1002/anie.201001374. [DOI] [PubMed] [Google Scholar]
- 225.Ainslie KM, Tao SL, Popat KC, Daniels H, Hardev V, Grimes CA, Desai TA. In vitro inflammatory response of nanostructured titania, silicon oxide, and polycaprolactone. J. Biomed. Mater. Res. 2009;91A(3):647–655. doi: 10.1002/jbm.a.32262. [DOI] [PubMed] [Google Scholar]
- 226.Albu SP, Ghicov A, Macak JM, Hahn R, Schmuki P. Self-Organized, Free-Standing TiO2 Nanotube Membrane for Flow-through Photocatalytic Applications. Nano Letters. 2007;7(5):1286–1289. doi: 10.1021/nl070264k. [DOI] [PubMed] [Google Scholar]
- 227.Albu SP, Ghicov A, Schmuki P. Lift Off Strategies for Self-Organized TiO2 Nanotube Layers. ECS Trans. 2009;16(52):195–202. [Google Scholar]
- 228.Wang J, Lin Z. Freestanding TiO2 Nanotube Arrays with Ultrahigh Aspect Ratio via Electrochemical Anodization. Chemistry of Materials. 2008;20(4):1257–1261. [Google Scholar]
- 229.Kant K, Losic D. A simple approach for synthesis of TiO2 nanotubes with through-hole morphology. Phys. Status Solidi RRL. 2009;3(5):139–141. [Google Scholar]
- 230.Li S, Zhang G. One-step realization of open-ended TiO2 nanotube arrays by transition of the anodizing voltage. Journal of the Ceramic Society of Japan. 2010;118(4):291–294. [Google Scholar]
- 231.Wang D, Liu L. Continuous Fabrication of Free-Standing TiO2 Nanotube Array Membranes with Controllable Morphology for Depositing Interdigitated Heterojunctions. Chemistry of Materials. 2010;22(24):6656–6664. [Google Scholar]
- 232.Wang D, Liu L, Zhang F, Tao K, Pippel E, Domen K. Spontaneous Phase and Morphology Transformations of Anodized Titania Nanotubes Induced by Water at Room Temperature. Nano Letters. 2011;11(9):3649–3655. doi: 10.1021/nl2015262. [DOI] [PubMed] [Google Scholar]
- 233.Lin J, Chen J, Chen X. Facile fabrication of free-standing TiO2 nanotube membranes with both ends open via self-detaching anodization. Electrochemistry Communications. 2010;12(8):1062–1065. [Google Scholar]
- 234.Li L-L, Chen Y-J, Wu H-P, Wang NS, Diau EW-G. Detachment and transfer of ordered TiO2 nanotube arrays for front-illuminated dye-sensitized solar cells. Energy & Environmental Science. 2011;4(9):3420–3425. [Google Scholar]
- 235.Albu SP, Ghicov A, Berger S, Jha H, Schmuki P. TiO2 nanotube layers: Flexible and electrically active flow-through membranes. Electrochemistry Communications. 2010;12(10):1352–1355. [Google Scholar]
- 236.Randall CL, Leong TG, Bassik N, Gracias DH. 3D lithographically fabricated nanoliter containers for drug delivery. Advanced Drug Delivery Reviews. 2007;59(15):1547–1561. doi: 10.1016/j.addr.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 237.Randall CL, Gultepe E, Gracias DH. Self-folding devices and materials for biomedical applications. Trends in Biotechnology. 2012;30(3):138–146. doi: 10.1016/j.tibtech.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gimi B, Leong T, Gu Z, Yang M, Artemov D, Bhujwalla Z, Gracias D. Self-Assembled Three Dimensional Radio Frequency (RF) Shielded Containers for Cell Encapsulation. Biomedical Microdevices. 2005;7(4):341–345. doi: 10.1007/s10544-005-6076-9. [DOI] [PubMed] [Google Scholar]
- 239.Leong TG, Lester PA, Koh TL, Call EK, Gracias DH. Surface Tension-Driven Self-Folding Polyhedra. Langmuir. 2007;23(17):8747–8751. doi: 10.1021/la700913m. [DOI] [PubMed] [Google Scholar]
- 240.Cho J-H, Gracias DH. Self-Assembly of Lithographically Patterned Nanoparticles. Nano Letters. 2009;9(12):4049–4052. doi: 10.1021/nl9022176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Leong TG, Zarafshar AM, Gracias DH. Three-Dimensional Fabrication at Small Size Scales. Small. 2010;6(7):792–806. doi: 10.1002/smll.200901704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Azam A, Laflin K, Jamal M, Fernandes R, Gracias D. Self-folding micropatterned polymeric containers. Biomedical Microdevices. 2011;13(1):51–58. doi: 10.1007/s10544-010-9470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gracias DH, Kavthekar V, Love JC, Paul KE, Whitesides GM. Fabrication of Micrometer-Scale, Patterned Polyhedra by Self-Assembly. Adv. Mater. 2002;14(3):235–238. [Google Scholar]
- 244.Leong TG, Benson BR, Call EK, Gracias DH. Thin Film Stress Driven Self-Folding of Microstructured Containers. Small. 2008;4(10):1605–1609. doi: 10.1002/smll.200800280. [DOI] [PubMed] [Google Scholar]
- 245.Randall CL, Kalinin YV, Jamal M, Manohar T, Gracias DH. Three-dimensional microwell arrays for cell culture. Lab on a Chip. 2011;11(1):127–131. doi: 10.1039/c0lc00368a. [DOI] [PubMed] [Google Scholar]
- 246.Randall CL, Kalinin YV, Jamal M, Shah A, Gracias DH. Self-folding immunoprotective cell encapsulation devices. Nanomedicine: Nanotechnology, Biology and Medicine. 2011;7(6):686–689. doi: 10.1016/j.nano.2011.08.020. [DOI] [PubMed] [Google Scholar]