Abstract
Loss of E-cadherin (CDH1), Smad4 and p53 have all been shown to play an integral role in gastric, intestinal and breast cancer formation. Compound conditional knockout mice for Smad4, p53, and E-cadherin were generated to define and compare the roles of these genes in gastric, intestinal and breast cancer development by crossing with Pdx-1-Cre, Villin-Cre and MMTV-Cre transgenic mice. Interestingly, gastric adenocarcinoma was significantly more frequent in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice than in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mice, demonstrating that Cdh1 heterozygosity accelerates the development and progression of gastric adenocarcinoma, in combination with loss of Smad4 and p53. Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice developed gastric adenocarcinomas without E-cadherin expression. However, intestinal and mammary adenocarcinomas with the same genetic background retained E-cadherin expression and were phenotypically similar to mice with both wild-type Cdh1 alleles. Lung metastases were identified in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice, but not in the other genotypes. Nuclear β-catenin accumulation was identified at the invasive tumor front of gastric adenocarcinomas arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice. This phenotype was less prominent in mice with intact E-cadherin or Smad4, indicating that the inhibition of β-catenin signaling by E-cadherin or Smad4 down-regulates signaling pathways involved in metastases in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice. Knockdown of β-catenin significantly inhibited migratory activity of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ cell lines. Thus, loss of E-cadherin and Smad4 cooperate with p53 loss to promote the development and metastatic progression of gastric adenocarcinomas, with similarities to human gastric adenocarcinoma.
Implications
This study demonstrates that inhibition of β-catenin is a converging node for the anti-metastatic signaling pathways driven by E-cadherin and Smad4 in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice, providing novel insights into mechanisms for gastric cancer metastasis.
Keywords: E-cadherin, Smad4, p53, β-catenin, metastasis
Introduction
Gastric cancer is the second most common cause of cancer death worldwide [1]. p53, Smad4, and E-cadherin are frequently inactivated in human gastric cancer. TP53 mutations are reported in 0%–21% of diffuse-and 36%–43% of intestinal-type gastric cancers, respectively [2]. Smad4, a co-Smad involved in both branches of the TGF-β/BMP signaling system [3], is inactivated in 40% of human gastric cancers by loss of heterozygosity (LOH), promoter hypermethylation, and somatic mutation [4]. The CDH1 gene, coding for E-cadherin, is frequently inactivated in familial and sporadic diffuse-type gastric cancers [5]. Germline CDH1 mutations are associated with an 80% lifetime risk of diffuse-type gastric cancer, and somatic inactivating E-cadherin mutations have been reported in 33-50% of sporadic diffuse-type gastric cancers [5]. Promoter hypermethylation of CDH1, identified in up to 80% of patients with diffuse-type gastric cancers, is the most common second hit in the inactivation of wild type CDH1 allele, but mechanisms leading to the inactivation of the wild type CDH1 allele remain largely unknown [6, 7]. A better understanding of the stepwise inactivation of E-cadherin would provide an opportunity for therapeutic intervention.
Valuable biological insights into these gastric tumor suppressors have been obtained through studies of genetically engineered mouse models. Knockout of Smad4 in the germline of mice results in embryonic lethality [8], whereas mice heterozygous for mutant Smad4 in the germline develop in situ gastric carcinomas after 18 month of age [9]. It still remains to be elucidated how the loss of Smad4 in gastric epithelium, alone and in combination with other tumor suppressors, promotes the progression of gastric cancers. While it was recently reported that Atp4b-driven p53 and E-cadherin knockout mice develop diffuse-type gastric cancers [10], the impact of E-cadherin heterozygosity on the development and progression of gastric cancer has not been previously evaluated. Based on human mutation profiles and prior studies, we assessed the role of one allelic loss of E-cadherin, alone or in combination with the loss of Smad4 and p53, on the development, progression, and metastasis of diffuse-type gastric adenocarcinomas in mice. Additionally, we compared this gastric cancer model with intestinal and mammary cancer models of the same genetic background.
Materials and Methods
Mice
Mouse studies were conducted with the approval of the Animal Care and Use Committees of National Cancer Center of Korea and the National Cancer Institute, Bethesda, MD. Pdx-1-Cre mice (B6.FVB-Tg(Ipf1-cre)1Tuv), originally generated by Dr. Lowy [11], were provided through the Mouse Models of Human Cancers Consortium (MMHCC) repository at the NCI Frederick Cancer Research Center. B6.Cg-Tg(Vil-Cre)20Sy, FVB/N-Tg(MMTV-Cre)7Mul, and FVB.129-Trp53tm1Brn [12] mice were also provided by the MMHCC. B6.129-Cdh1tm2Kem/J mice, which have loxP sites flanking exons 6-10, were purchased from The Jackson Laboratory [13]. Conditional Smad4 knockout mice (Smad4F/F) on the Black Swiss, B6 and 129 backgrounds were previously described [14].
Compound conditional knockouts of Smad4, p53, and E-cadherin were bred with Pdx-1-Cre mice, Villin-Cre mice, and MMTV-Cre mice, to perform targeted deletion for these genes in gastric, intestinal, and mammary epithelium, respectively. Pdx-1-Cre mice express Cre in mucosal epithelial cells of the gastric antrum and duodenum as well as the pancreatic islet cells [15]. Villin-Cre mice expressed Cre in progenitor cells of the intestinal epithelial mucosa [16] and of the antrum [17]. MMTV-Cre mice expressed Cre in mammary epithelial cells and striated ductal cells of the salivary gland [18]. The strain background for crosses was controlled in order to avoid confounding variables in comparing tumor-free survival across the genotypes. Offspring mice were genotyped using polymerase chain reaction (PCR) assays for tail DNA. Mice positive for Pdx-1-Cre or Villin-Cre genes were monitored until they became moribund or showed signs of distress, at which time necropsies were performed (Fig S1). Scheduled sacrifice was also conducted for sentinel mutant mice without signs of distress, in order to evaluate the presence or absence of asymptomatic microscopic disease in stomach. Carcinoma-free intervals were compared by log-rank test. To compare gastric adenocarcinoma-free survivals more accurately, stratified log-rank test was additionally performed after the timing of necropsy was dichotomized (≤6 vs >6 months). When no carcinomatous lesions were identified in a given organ, it was censored for the development of carcinoma on the day of necropsy [19]. MMTV-Cre positive female mice were euthanized when mammary tumors reached 2 cm in diameter. Liver, spleen, and lung were harvested at necropsy to assess for metastases.
Immunohistochemistry
Immunohistochemistry analyses were performed on 5-μm, formalin-fixed, paraffin-embedded slides from tumors arising in the mutant mice. The following antibodies were used: rabbit polyclonal anti-E-cadherin antibody (1:200; Cell Signaling, #3195, Danvers, MA), rabbit polyclonal anti-Ki-67 antibody (1:200; Abcam, ab15580, Cambridge, UK), mouse monoclonal anti-β-catenin antibody (1:200; BD Transduction Laboratories, 610154, San Diego, CA), rabbit monoclonal anti-vimentin antibody (1:100; Cell Signaling, #5741), and rabbit monoclonal anti-MMP7 antibody (1:100; Cell Signaling, #3801). The invasive front of the tumor was defined as the microscopic interface between normal tissue of the host mouse and the tumor mass invading the submucosa or deeper regions of the stomach [20]. Differences in the percentage of positive immunostaining between the various genotypes were evaluated using the Student t-test.
β-catenin and the migration assays
For the measurement of β-catenin mRNA expression and activity, we performed quantitative real-time PCR and dual luciferase reporter assay (Promega, Madison, WI). Primary cultured cells (1.5 × 105 cells/well) were treated in quadruplicate for 16 hours in 12 wells with RPMI 1640 media containing 0.5% FBS with 100 ng/mL of BMP2 (R&D Systems, 355-Bm, Minneapolis, MN), 400 ng/mL of noggin (R&D Systems, 6057-NG, 5 ng/ml of TGF-β1 (gift from Dr. Lalage Wakefield, NCI), or Wnt3a conditioned media produced by L Wnt-3A cells (gift from Dr. Tae-Aug Kim, NCI). For migration assays, primary cultured cells (2.5 × 104 cells/well) were plated on 24-well inserts with an 8 μm pore size (353097, BD) in serum-free RPMI 1640 media. Media containing 10% FBS were added to the 24-well lower insert chambers (354578, BD). After 24 hours, cells that migrated to the lower insert chamber were counted at 3 high power fields (200×). Student t-tests were used for statistical analyses.
Results
Gastric tumors formed in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice recapitulate human diffuse-type gastric adenocarcinomas
Twenty one of 25 Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (84%) developed spontaneous tumors in the glandular stomach (Fig 1A and S2). Of 4 Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice without gastric adenocarcinomas, 2 mice were killed by scheduled sacrifice at 6 months, and the other two mice died of duodenal cancer obstruction at 7.4 and 8.2 months, respectively. The most common cause of death in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice was duodenal obstruction, followed by gastric outlet obstruction. Histologically, tumors arising in the glandular stomach of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ resemble human diffuse-type gastric adenocarcinomas based on the histopathological findings, and were invasive into the muscle layers and regional lymph nodes (Fig 1B).
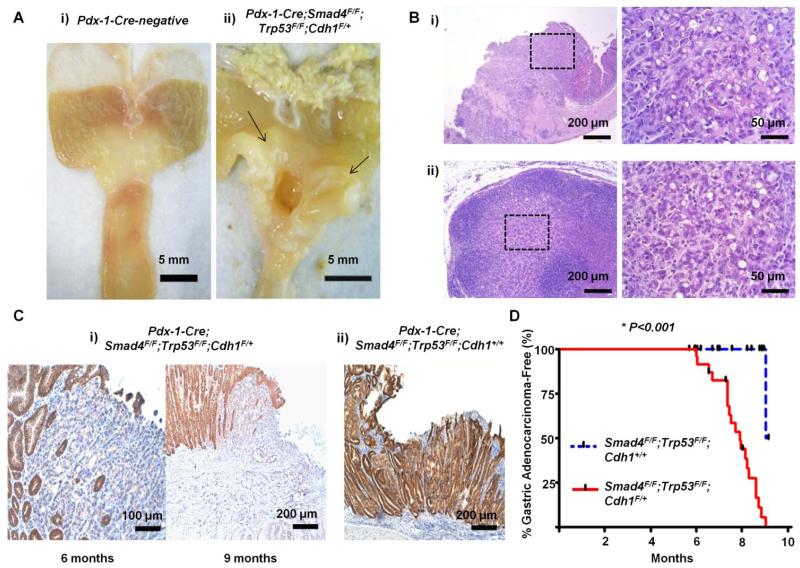
Fig 1.
(A) Representative gross feature of a gastric cancer (arrows), an ulceroinfiltrative mass in the antral mucosa, arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (Aii). Gastric lumen of a Pdx-1-Cre-negative mouse of the same age (Ai). (B) Representative histologic findings of the gastric cancer (Bi) and lymph node metastasis (Bii). Boxed regions of left panels are shown at higher magnification in the right panels. (C) Representative E-cadherin immunohistochemical staining of gastric adenocarcinomas arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice at 6 and 9 months of age (Ci). Gastric adenocaricnoma arising in a Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mouse (Cii). (D) Gastric adenocarcinoma-free survivals of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ (n=25) compared with Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mice (n=28).
Duodenal adenocarcinomas and forestomach squamous cell carcinomas were also identified in 36% and 24% of these mice, respectively (Table S1). In addition, 2 mice were noted to have adenocarcinomas in the pancreas (8%), which were interpreted as invasion of primary duodenal or gastric adenocarcinomas. This pattern of tumor distribution is consistent with the known tissue-specificity of the Pdx-1 promoter [15].
Since gastric adenocarcinoma was the most common type of carcinoma in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice, we focused our analysis on adenocarcinomas arising in the glandular stomach. DNA microarray analyses and immunohistochemistry of 4 gastric cancers from Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice compared to normal stomach epithelium revealed that these tumors were positive for mucin 6 and TFF2, and negative for mucin 5a, pepsinogen C, somatostatin, and gastric intrinsic factors, suggesting the deep antral gland origin of these tumors [21] (Fig S3).
E-cadherin loss is required for the development of diffuse-type gastric adenocarcinoma
Scheduled sacrifices performed at 4 and 5 months revealed no gastric premalignant lesions, such as atrophic gastritis, metaplasia, or dysplasia, in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice. In 2 of 4 Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice screened at 6 months of age, intramucosal adenocarcinomas with signet ring cell feature were observed (Fig 1C). Interestingly, E-cadherin immunostaining was lost in adenocarcinoma cells by 6 months of age, suggesting that E-cadherin loss is an early event required for the diffuse-type gastric carcinogenesis in mice.
To further evaluate the role of E-cadherin in constraining diffuse-type gastric carcinogenesis, a cohort (n=25) of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice was compared with a cohort (n=28) of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mice. The median gastric adenocarcinoma-free survival of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ was 8.0 months (95% Cl, 7.5-8.4), while only one gastric adenocarcinoma was identified by a scheduled sacrifice at 9 month of age, in the Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mouse cohort [Log-rank P<0.001 and stratified log-rank P<0.001] (Fig 1D). Whereas E-cadherin expression was retained in a gastric adenocarcinoma arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ (Fig 1C), all of the gastric adenocarcinomas arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice were negative for E-cadherin immunostaining, and all of the gastric adenocarcinomas analyzed from this group were also found to express low levels of Cdh1 mRNA compared with normal tissue (Fig 2A and 2B).
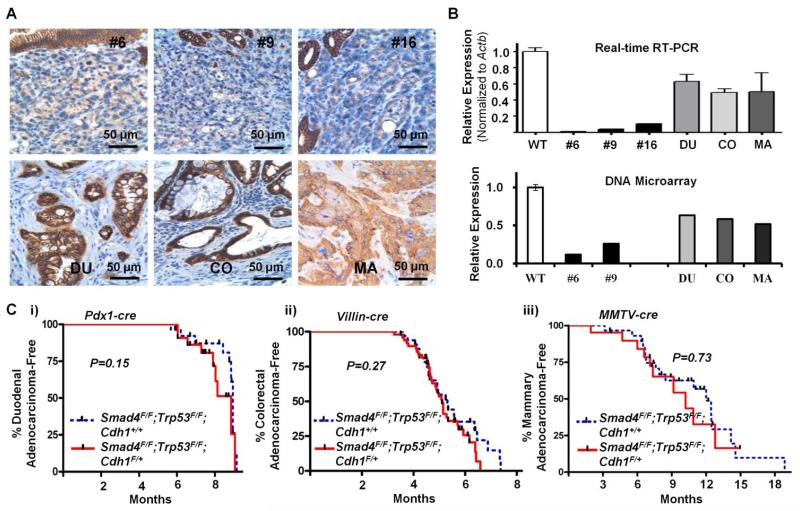
Fig 2.
(A) E-cadherin immunostaining of gastric adenocarcinomas in upper panels(#6, #9, and #16) arising in three representative Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice, duodenal adenocarcinoma (DU) from Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice, colorectal adenocarcinoma (CO) from Villin-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice, and mammary adenocarcinoma (MA) from MMTV-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice. (B) RNA expression levels from Real-time RT-PCR and DNA microarray analysis (in linear scales) for Cdh1 of tumors relative to normal stomach of Cre-negative mice (WT). Error bars represent SDs. (Ci) Duodenal adenocarcinoma-free survival of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice compared with Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mice. (Cii) Colorectal adenocarcinoma-free survival of Villin-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice compared with Villin-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mice. (Ciii) Mammary adenocarcinoma-free survival of MMTV-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice compared with MMTV-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mice.
LOH at the Cdh1 locus was identified in 2 of 17 Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ gastric adenocarcinomas tested (11.8 %) (Fig 3A). Next, we investigated epigenetic changes leading to the loss of E-cadherin, but found no evidence for Cdh1 promoter hypermethylation (Fig 3B and 3C) or deacetylation (Fig 3D). MicroRNA microarray analyses revealed no difference in expression level of microRNAs targeting Cdh1, such as miR-9, between Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ gastric adenocarcinomas and normal tissue (data not shown).
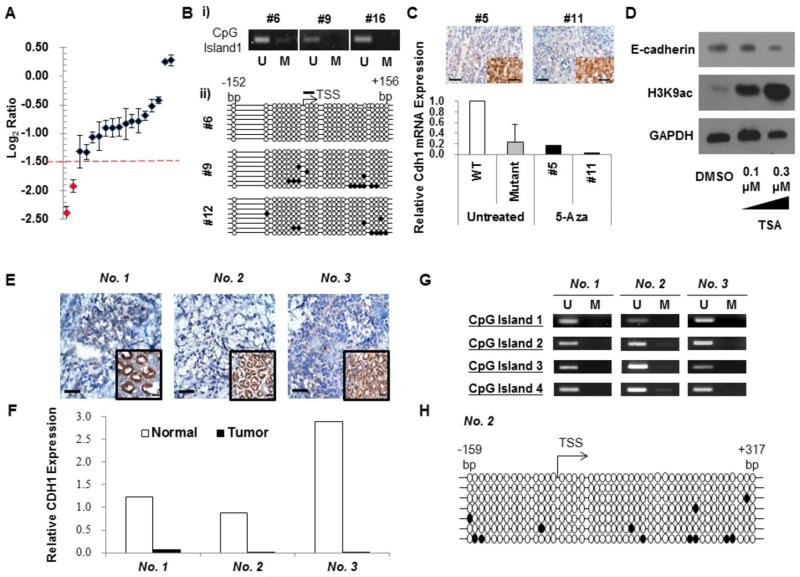
Fig 3.
(A) Genomic DNA real-time PCR for the Cdh1 gene in adenocarcinomas arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice to evaluate LOH. Log2 ratio of each tumor to Cre-negative gastric mucosa is depicted. LOH was defined as the average log2 ratio of three probes (for exons 6, 8, and 10) of tumor to normal DNA < −1.5 (broken red line). (Bi) Methylation-specific PCR of the gastric carcinomas shown in Fig 2A. (Bii) Bisulfite genomic sequencing analyses of the Cdh1 promoter in these gastric adenocarcinomas. Open and closed circles represent unmethylated (U) and methylated (M) CpG sites, respectively (TSS, transcription start site). A black bar indicates a CpG site targeted by (Bi). (C) E-cadherin immunostaining (top panels; Inset, E-cadherin-positive adjacent normal gastric mucosa) and real-time RT-PCR for gastric adenocarcinomas from two Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (#5 and #11) treated with 5-aza-deoxycitidine (5-Aza). Gastric mucosa of a Cre-negative mouse (WT) and a gastric adenocarcinoma from untreated Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mouse (Mutant) were used as references. E-cadherin was not significantly up-regulated after 5-Aza treatment. (D) Western blot analysis for E-cadherin of the tissue explants of a gastric adenocarcinoma from a Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mouse after 24-h exposure to 0.1 μM and 0.3 μM of trichostatin A (TSA). Acetyl-histone H3 lysine 9 (H3K9ac) was immunoblotted as a positive control. (E) E-cadherin immunostaining of an early-onset human gastric cancer. Adjacent normal tissue of the same patient was positive (inset). (F) The CDH1 real-time RT-PCR expression level of each human gastric cancer relative to adjacent normal tissue. (G) Methylation-specific PCR analysis of human gastric cancers for CpG sites of CDH1 promoter (U, unmethylated; M, methylated CpG sites). (H) Bisulfite genomic sequencing analysis of the Cdh1 promoter in the gastric cancer with a germline missense CDH1 mutation.
In contrast to the differences in the incidences of gastric adenocarcinomas between the genotypes, duodenal adenocarcinoma-free survival was similar between Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ and Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mice [9.0 vs 9.1 months; Log-rank P=0.15] (Fig 2C). E-cadherin was retained in duodenal adenocarcinomas arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice, and, therefore, was relatively overexpressed compared with gastric adenocarcinomas from the same mice (Fig 2A and 2B). Next, we evaluated intestinal and mammary adenocarcinomas in mice created through the loss of the same set of genes using Villin-Cre and MMTV-Cre transgenes, respectively, to compare the tumor promoting effects of E-cadherin heterozygosity across the different target tissues. Intestinal adenocarcinoma-free survival was similar between Villin-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ and Villin-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mice [5.2 vs 5.4 months; Log-rank P=0.27] (Fig 2C). No distant metastases were observed in either genotype, except for one Villin-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mouse developing skin metastasis. E-cadherin was retained in intestinal adenocarcinomas formed in Villin-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (Fig 2A). MMTV-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ and MMTV-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mice were also not different in the mammary carcinoma-free survival [10.4 vs 12.1 months; Log-rank P=0.73] and lung metastasis [33.3% (3/9) vs 35.7% (5/14), respectively; P for Chi-square test=0.91] (Fig 2C). Histologically, MMTV-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ tumors were invasive ductal carcinomas with a squamous component (Fig S4). Mammary adenocarcinomas arising in MMTV-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice also retained E-cadherin (Fig 2A), perhaps accounting for why lobular carcinomas were not observed in this model as has been reported in K14cre;Trp53F/F;Cdh1F/F [22]. These results clearly demonstrate that E-cadherin loss is important for the development of gastric adenocarcinomas, but not for the development of intestinal or mammary adenocarcinomas.
It was surprising to us that the Cdh1 promoter was not significantly methylated in gastric adenocarcinomas formed in our Cdh1 heterozygous mice. In human diffuse-type gastric cancers, the CDH1 promoter hypermethylation has been identified in up to 80% of patients. To gain further insight into the clinical relevance of this mouse tumor data, we examined the E-cadherin mutation profiles of 13 young (≤ 40 years old) Korean gastric cancer patients (Table S3). Three of 13 (23.1%) patients demonstrated very low levels of E-cadherin immunostaining and mRNA expression (Fig 3E and 3F), and one of them harbored a germline missense CDH1 mutation (c.1018A>G (p.T340A)) that was previously reported in a hereditary diffuse gastric cancer kindred [23]. Promoter hypermethylation of the CDH1 gene was not identified in any of the three E-cadherin-negative patients (Fig 3G). Thus, transcriptional repression of CDH1 was possibly due to epigenetic changes other than promoter methylation or other regulatory factors that may be mechanisms for E-cadherin inactivation in humans as in our mutant mice.
Smad4 cooperates with E-cadherin in constraining the development of gastric adenocarcinoma by inhibiting cell cycle progression
No neoplastic lesions were found at the time of necropsy of Pdx-1-Cre;Trp53F/F;Cdh1F/+ mice (Fig 4A). These results suggest that loss of Smad4 is required for the development of gastric adenocarcinoma in Pdx-1-Cre;Trp53F/F;Cdh1F/+ mice [Log-rank P=0.005 and stratified log-rank P=0.009] (Fig 4A). Since all of the Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice analyzed were found to develop E-cadherin-negative gastric adenocarcinomas, tumors from those mice were compared with tumors from Pdx-1-Cre;Trp53F/F;Cdh1F/F mice. Six mice in the Pdx-1-Cre;Trp53F/F;Cdh1F/F cohort (n=15) developed gastric adenocarcinomas with a median tumor-free survival of 9.4 months for this group (Fig 4A), but did not develop distant metastases. This phenotype is consistent with that of the previously reported Atp4b-Cre;Trp53F/F;Cdh1F/F mice [10]. Gastric adenocarcinoma-free survival was significantly longer in Pdx-1-Cre;Trp53F/F;Cdh1F/F mice than in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice [median, 9.4 vs 8.0 months; Log-rank P=0.007 and stratified log-rank P=0.007], demonstrating the role of Smad4 in constraining gastric cancer progression (Fig 4A). Gastric adenocarcinomas formed in Pdx-1-Cre;Trp53F/F;Cdh1F/F mice were less invasive than those arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (Fig 4B). Gastric adenocarcinomas formed in Pdx-1-Cre;Trp53F/F;Cdh1F/F mice exhibited lower Ki-67 positivity than Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice [median, 42.8% vs 71.7%, respectively; P<0.001], suggesting that Smad4 constrains tumor progression through the inhibition of the cell cycle (Fig 4C and 4D).
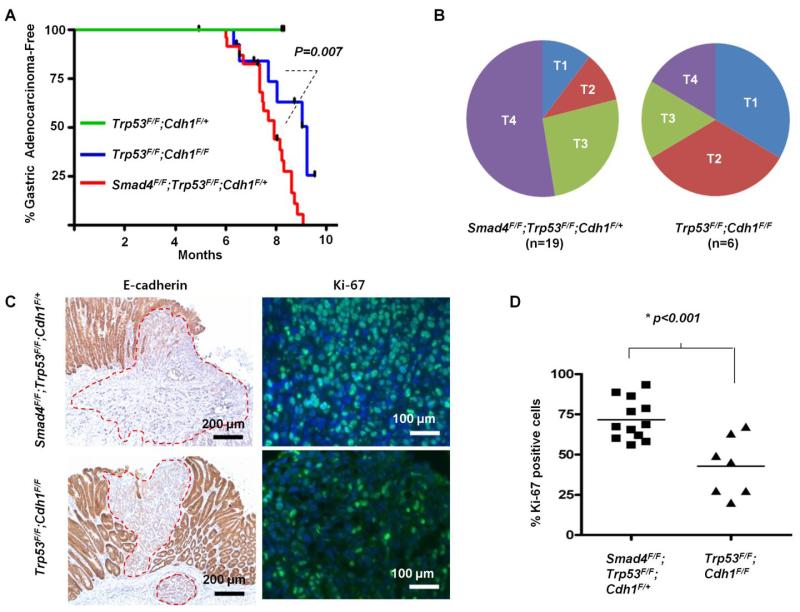
Fig 4.
(A) Gastric adenocarcinoma-free survival of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (n=25) compared with Pdx-1-Cre;Trp53F/F;Cdh1F/F mice (n=15) and with Pdx-1-Cre;Trp53F/F;Cdh1F/+ mice (n=7) (B) Depth of invasion between gastric adenocaricnomas arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice and Pdx-1-Cre;Trp53F/F;Cdh1F/F mice at 7 to 9 months of age (T1; mucosa and submucosa, T2; muscularis propria, T3; subserosa, T4; serosa and adjacent organ). (C) Representative E-cadherin immunohistochemical and Ki-67 immunofluoresence staining of gastric adenocarcinomas arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ and Pdx-1-Cre;Trp53F/F;Cdh1F/F. Dotted red lines indicate tumor margins. (D) The percentage of Ki-67-positive cells in gastric adenocarcinomas arising in Pdx-1-Cre;Trp53F/F;Cdh1F/F mice was lower than that of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice.
Loss of E-cadherin and Smad4 cooperate to promote lung metastasis through the accumulation of nuclear β-catenin
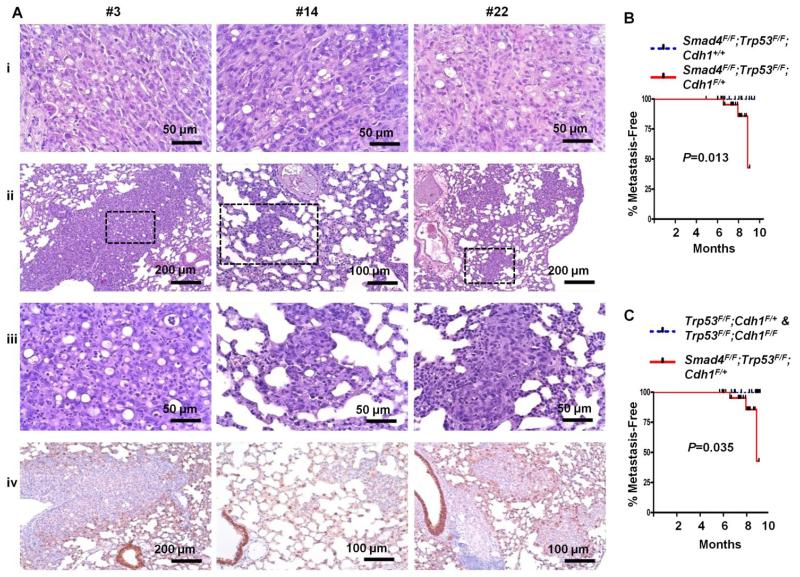
Importantly, three of 21 Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice with gastric adenocarcinomas (14.2%) developed lung metastases. The metastatic lesions had similar cytologic features to the primary gastric tumors, including the lack of E-cadherin immunostaining (Fig 5A). Prompted by the GSEA analyses showing the enrichment of Lef1 target genes (involved in the Wnt signaling pathway) in the gastric cancers arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (Table S2), we sought to evaluate β-catenin (a regulator of Wnt signaling) by immunostaining the mutant mouse tumors. Nuclear β-catenin accumulation was identified at the invasive front of gastric adenocarcinomas from Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice, suggesting the association of nuclear β-catenin accumulation with tumor progression [P for t-test between the invasive front and tumor center<0.001] (Fig S5) [24]. β-catenin target genes, such as MMP7 and vimentin, were focally overexpressed among nuclear β-catenin -positive carcinoma cells at the invasive front, suggesting an epithelial-to-mesenchymal transition (EMT) (Fig S5 and 5C). Ki-67 immunostaining was decreased at the invasive front of these mice, compared with the tumor center; Fig S5).
Fig 5.
(A) H&E (Aii, Aiii) and E-cadherin immunostaining (Aiv) of lung metastases of gastric carcinomas arising in three Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (#3, #14, and #22). Corresponding primary tumors are shown in (Ai). (B) Metastasis-free survival of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ compared with Pdx-1-Cre; Smad4F/F;Trp53F/F;Cdh1+/+ mice. (C) Metastasis-free survival of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ compared with Pdx-1-Cre;Trp53F/F;Cdh1F/F and Pdx-1-Cre; Trp53F/F;Cdh1F/+ mice.
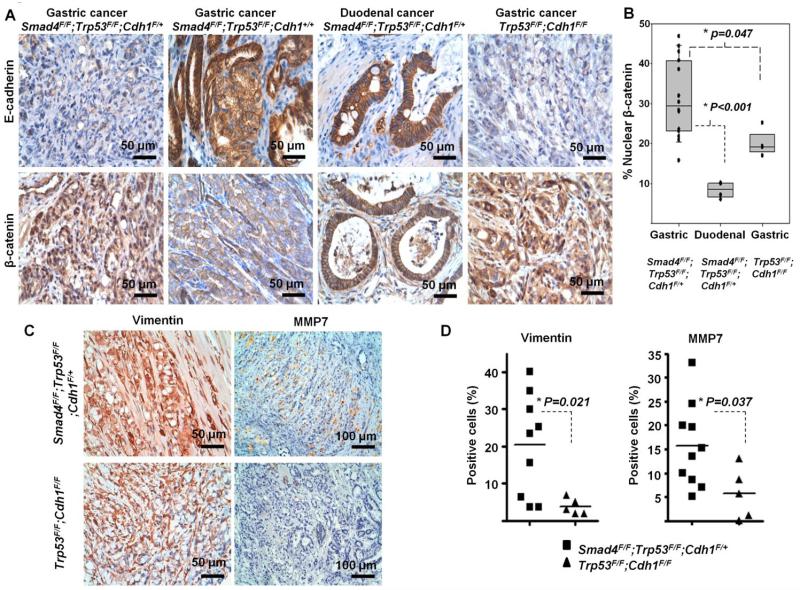
A gastric carcinoma from a Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mouse did not demonstrate nuclear β-catenin staining (Fig 6A). Duodenal carcinomas from Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (n=4), with intact E-cadherin expression, also demonstrated significantly lower nuclear β-catenin positivity at the invasive front than gastric carcinomas from mice with the same genotype (n=14) [median, 8.5% vs 29.3%; P for t-test<0.001] (Fig 6B). Invasive fronts of these tumors with E-cadherin expression did not express vimentin (data not shown). Thus, complete loss of E-cadherin may be a prerequisite for nuclear β-catenin accumulation across the different epithelial tumor types. The Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mouse with a gastric adenocarcinoma had no distant metastases [Log-rank P value for metastasis-free survival between Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ and Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+=0.013] (Fig 5B). Given the low nuclear β-catenin positivity of the duodenal adenocarcinomas from Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice which did not metastasize (Fig 6B), these results suggest the metastatic propensity of Pdx-1-Cre;Trp53F/F;Cdh1F/+ mice may be attributable, at least in part, to the activation of the β-catenin signaling pathway following E-cadherin loss.
Fig 6.
(A) Representative immunohistochemistry findings for E-cadherin and β-catenin at the invasive front of tumors across genotypes. (B) Percentage of nuclear β-catenin-postive cells at the invasive front of gastric adenocarcinomas from Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ cohort (n=14) and Pdx-1-Cre;Trp53F/F;Cdh1F/F cohort (n=4) and duodenal carcinomas from Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (n=4). Box indicates interquartile range with median. (C) Vimentin and MMP7 immunohistochemistry at the invasive front of gastric adenocarcinomas between Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ and Pdx-1-Cre;Trp53F/F;Cdh1F/F mice. (D) Percentage of Vimentin- and MMP7-positive cells in gastric adenocarcinomas arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (n=10) and Pdx-1-Cre;Trp53F/F;Cdh1F/F mice (n=5).
Since no distant metastases were identified in mice with intact Smad4 (Pdx-1-Cre;Trp53F/F;Cdh1F/+ and Pdx-1-Cre;Trp53F/F;Cdh1F/F) [Log-rank P=0.035] (Fig 5C), we investigated possible mechanisms for the role of Smad4 in suppressing metastasis. Gastric adenocarcinomas arising in Pdx-1-Cre;Trp53F/F;Cdh1F/F (n=4) demonstrated nuclear β-catenin accumulation at the invasive front, but not as frequently as gastric adenocarcinomas from Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (n=14), consistent with a prior report [10] [P for t-test= 0.047] (Fig 6B). Gastric cancers formed in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice demonstrated higher expression of vimentin and MMP7 at the invasive front than those arising from Pdx-1-Cre; Trp53F/F;Cdh1F/+ mice [t-test P values for the positivity, 0.021 and 0.037 for vimentin and MMP7, respectively] (Fig 6C and 6D). MMP8 expression also tended to be higher in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice (Fig S6).
To further evaluate the effect of Smad4 loss on β-catenin (Ctnnb1) activity, we established primary cultured cell lines from gastric adenocarcinomas arising from Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ and Pdx-1-Cre;Trp53 F/F;Cdh1F/F mice. Consistent with our in vivo results, Ctnnb1 mRNA expression was higher in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ cells than in Pdx-1-Cre;Trp53F/F;Cdh1F/F cells [P<0.001] (Fig 7A). Similar results were obtained when Smad4 was overexpressed in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ cells. Ctnnb1 mRNA was significantly low in the Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ cells stably expressing Smad4 similar to the endogenous level (designated as Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+–Smad4), compared with vector only-transfected Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ cells [P=0.006] (Fig 7B). We wished to examine how Smad4 repressed β-catenin expression by determining whether repression was induced through the TGF-β or BMP signaling pathways, since Smad4 can mediate both pathways. Among BMP family members, BMP2 was selected for subsequent functional experiments based on its putative role as a gastric tumor suppressor [25, 26, 27] and the activity in our cell lines. Our results demonstrated that BMP2 significantly suppressed β-catenin expression when Smad4 was expressed, while TGF-β did not have these effects, and that BMP2 modestly augmented Smad4-induced Ctnnb1 downregulation (Fig 7A and B). BMP2-induced repression of β-catenin expression was reversed by treatment with noggin, a BMP antagonist (Fig 7A and B). Concordantly, Tcf/Lef reporter activity was lower in Smad4 expressing cell lines than in Smad4-null cells, and BMP2 treatment suppressed Tcf/Lef reporter activity in Smad4 expressing cells (Fig 7C and 7D). The specificities of the responses to BMP2 and TGF-β in these experiments are depicted in Fig S7. These findings suggest that canonical BMP signaling through Smad4 mediates transcriptional repression of Ctnnb1 and that Ctnnb1 is overexpressed with the loss of Smad4.
Fig 7.
Inhibition of β-catenin by Smad4/BMP pathway. (A and B) Ctnnb1 mRNA expression in primary cultured cells according to Smad4 status. (A) Real-time RT-PCR for Ctnnb1 mRNA in primary cultured gastric adenocarcinomas cells from Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ and Pdx-1-Cre;Trp53F/F;Cdh1F/F mice. Expression levels of Ctnnb1 after treatment with 100 ng/ml of BMP2, 400 ng/ml of noggin (a BMP antagonist) and 5 ng/ml of TGF-β1 for 16 hours. (Bi) Real-time RT-PCR for Ctnnb1 mRNA in Smad4 overexpressing stable transfectants of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ primary cells (Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+-Smad4) vs. vector only-transfected Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ primary cells (Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+-Mock). (Bii) Smad4 Western blot analyses on (Bi). (C and D) β-catenin reporter activity in (A and B). Tcf/Lef reporter activity was lower in Smad4 expressing cell lines (Pdx-1-Cre;Trp53F/F;Cdh1F/F and Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+-Smad4) than in Smad4-null cells. (E) Migratory activity of primary cultured cells from gastric adenocarcinomas arising from Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ (n=4) and Pdx-1-Cre;Trp53F/F;Cdh1F/F mice (n=2). (F) Migratory activity of two independent primary cultured cell lines from gastric adenocarcinomas arising from Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice, after knockdown of β-catenin using two different short hairpin RNAs. Western blot analysis showed the efficiency of β-catenin knockdown. (G) Representative image for shCtnnb1-1-tranfected Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ cell line 2 (spindle-like shape) is shown with scrambled control-transfected cells (epithelial, cuboidal shape). (H) β-catenin knockdown-induced changes in mRNA expression of Cdh2, MMP7 and EMT-activating transcription factors in (G). Asterisks (*) denote the statistical significance (P<0.05). Bars indicate standard errors of the mean. Representative data for repeated experiments are shown.
We determined that Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ cells demonstrated higher migratory activity than Pdx-1-Cre;Trp53 F/F;Cdh1F/F cells using the Boyden chamber assay [P<0.001] (Fig 7E and S8). This was consistent with our in vivo results demonstrating increased metastatic propensity of the Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice compared to Pdx-1-Cre;Trp53 F/F;Cdh1F/F mice. In order to determine whether the increased migration was due to increased β-catenin expression, we performed β-catenin knockdown experiments on two independent Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ cell lines. Knockdown of β-catenin, using two different short hairpin RNAs, significantly inhibited migratory activity of both Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ cell lines [P<0.001] (Fig 7F), while it did not affect the monolayer growth rate of these cells (Fig S9). Additionally, knockdown of β-catenin in the Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ cell lines led to a mesenchymal-to-epithelial morphological switch (Fig 7G), with downregulation of EMT-activating transcription factors including Twist1, but with unchanged, almost undetectable level of Cdh1 expression (Fig 7H and S10). These results collectively suggest that the loss of E-cadherin and Smad4 expression cooperate to promote lung metastasis partly through the β-catenin activation.
Discussion
Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice described in this study are unique in several aspects. In contrast to previously reported spontaneous murine gastric tumors, which are of parietal cell or neuroendocrine lineage [10, 28-30], gastric adenocarcinomas formed in these mice are of the mucus-secreting gastric epithelial cell origin. In addition, tumor suppressor genes most commonly inactivated in human gastric adenocarcinomas were targeted to be knocked-out in the gastroduodenal epithelium of this model [2-5]. While Smad4 is inactivated in 40% of human gastric cancers [4], functional roles of Smad4 in gastric cancer progression have not been fully evaluated using in vivo models. This study provides functional evidence for the role of Smad4 in suppressing gastric cancer progression. Although this study focused on gastric adenocarcinomas, it also demonstrates a role of Smad4 in suppressing duodenal carcinomas. While Pdx-1-Cre;Trp53F/F;Cdh1F/F mice developed no duodenal carcinomas, the additional loss of Smad4 significantly promoted duodenal carcinomas (Table S1), confirming that Smad4 loss in the intestinal epithelium promotes carcinogenesis [31]. Possibly, if Pdx-1-Cre; Smad4F/F;Trp53F/F;Cdh1+/+ mice did not die of duodenal obstruction, they might have developed gastric cancers at later time points.
The specific role of E-cadherin loss in the development of diffuse-type gastric adenocarcinomas was clearly documented by this cross-tissue tumorigenesis study. These results validate and extend a prior study suggesting a role for E-cadherin in the development of gastric cancer [10, 32]. In contrast to breast cancers arising in humans with germline E-cadherin mutation, mammary adenocarcinomas arising in MMTV-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice retained E-cadherin expression, and were not more aggressive than tumors arising in MMTV-Cre;Smad4F/F;Trp53F/F;Cdh1+/+ mice. Thus, the selection pressure for E-cadherin loss may be relatively low in the context of the mouse mammary gland, compared with the stomach. This is consistent with the lower lifetime risk for breast cancer than for stomach cancer in germline E-cadherin mutation carriers [33]. Studies are ongoing in our laboratory to identify gastric tissue-specific epigenetic events or signaling pathway activation leading to E-cadherin loss that may be promoted by loss of Smad4 or p53.
Notably, 14.3% of gastric adenocarcinomas arising in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice metastasized to the lung, which is a highly unique finding for a genetically engineered mouse model for gastric cancers. To date, only a few genetically-engineered mouse models of gastric cancer have been reported, and none of them develop distant metastases [10, 28-30, 34]. After Helicobacter inoculation, insulin-gastrin (INS-GAS) transgenic mice develop invasive gastric carcinomas with a frequency of 75% [28], but without distant metastases. Thirty percent of mice deficient in pS2 trefoil factor develop multifocal intraepithelial or intramucosal carcinomas, but no distant metastases [29]. Gp130F/F mice lacking in the SHP2-binding site on the IL-6 family receptor gp130 develop gastric cancer with submucosal invasion only [30]. The K19-C2mE mice expressing COX-2 and microsomal prostaglandin E synthase-1 in the stomach develop dysplastic tumors in the gastric epithelium [34]. Atp4b-Cre;Cdh1F/F;Trp53F/F mice develop diffuse-type gastric cancers which frequently metastasize to lymph nodes but not to the distant visceral organs [10]. Thus, gastric cancer metastasis may require a concerted action of key molecular events, including losses of E-cadherin and Smad4, as demonstrated in this study.
The metastatic phenotype of our mutant mice provides a unique opportunity to dissect the roles of E-cadherin and Smad4 in gastric cancer progression, and in relation to EMT. Without exposure to carcinogens, the gastric epithelium of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice lost E-cadherin expression and developed adenocarcinomas that progressed through EMT, suggesting the importance of EMT in gastric cancer progression. Nuclear β-catenin accumulation, suggesting activation of the canonical Wnt/ β-catenin pathway, was accompanied by EMT phenotype such as vimentin overexpression [24]. Given that this phenotype was not observed in gastrointestinal tumors of our mutant mice that retained E-cadherin, the unique metastatic phenotype of Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice may be attributable in part to the activation of the β-catenin signaling pathway following E-cadherin loss [35]. Our study also provides functional evidences for the causal role of β-catenin for the migration process related to metastasis of gastric cancer, consistent with data in the literature suggesting that β-catenin promotes the metastatic progression of breast and colorectal cancers by transcriptional activation of vimentin, MMP7, fibronectin, and other prometastatic genes [35-38].
The anti-metastatic role of Smad4 in gastric cancer, which has not been previously demonstrated in vivo, is in line with previous data for colorectal and prostate cancers [39, 40]. Notably, nuclear β-catenin accumulation at the invasive front was more prominent in our Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice than in Pdx-1-Cre;Trp53F/F;Cdh1F/F, which is partly consistent with a report by Shimada, et al. that Atp4b-Cre;Cdh1−/−;Trp53−/− mice do not overexpress β-catenin [10]. In addition, our Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ cells demonstrated enhanced β-catenin activity and migratory activity compared with Pdx-1-Cre;Trp53F/F;Cdh1F/+ cells. Thus, our study is the first to demonstrate the Smad4 loss-induced β-catenin activation in gastric cancer, and is consistent with a prior report that Smad4 mediates canonical BMP signaling by repressing the transcription of β-catenin in SW480 colon cancer cells [41]. Therefore, inhibition of β-catenin may be a converging node for the anti-metastatic signaling pathways driven by E-cadherin and Smad4 in Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ mice. Other possible mechanisms for the anti-metastatic role of Smad4 may include suppression of proliferation, leading to proliferative dormancy [40].
Taken together, we conclude that loss of E-cadherin and Smad4 cooperate to promote the development and metastatic progression of p53-null diffuse-type gastric carcinoma. By performing gastroduodenal epithelium-specific knockout of one allele of Cdh1 and both alleles of p53 and Smad4, which are frequently inactivated in human gastric cancers, we have created a genetically-engineered mouse model that develops distant metastasis. Gastric adenocarcinomas that formed in this genetic context, but not intestinal or mammary adenocarcinomas, lose E-cadherin expression and undergo EMT. These results closely recapitulate human diffuse-type gastric cancers, and sharpen our understanding of the interaction between E-cadherin and Smad4, two commonly inactivated tumor suppressors in gastric cancer. Additionally, the Pdx-1-Cre;Smad4F/F;Trp53F/F;Cdh1F/+ animal model will be extremely useful for preclinical studies given its similarities with human diffuse-type gastric carcinoma and its metastatic propensity.
Supplementary Material
Acknowledgements
The work was supported by the Proteogenomic Research Program through the National Research Foundation of Korea and the Converging Research Center Program (2013K000429) funded by the Korean Ministry of Education, Science and Technology; by National Cancer Center Grant 1210051; and by the National Institutes of Health intramural program, Center for Cancer Research, National Cancer Institute, Bethesda, MD.
Study sponsor has no roles in the study design in the collection, analysis, and interpretation of data.
Footnotes
Disclosure:
Authors have nothing to disclose.
References
- 1.Alberts S, Cervantes A, van de Velde C. Gastric cancer: epidemiology, pathology, and treatment. Ann Oncol. 2003;14:31–6. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 2.Maesawa C, Tamura G, Suzuki Y, Ogasawara S, Sakata K, Kashiwaba M, et al. The sequential accumulation of genetic alterations characteristic of the colorectal adenoma-carcinoma sequence does not occur between gastric adenoma and adenocarcinoma. J Pathol. 1995;176:249–258. doi: 10.1002/path.1711760307. [DOI] [PubMed] [Google Scholar]
- 3.Schmierer B, Hill CS. TGF beta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 4.Wang LH, Kim SH, Lee JH, Choi YL, Kim YC, Park TS, et al. Inactivation of SMAD4 tumor suppressor gene during gastric carcinoma progression. Clin Cancer Res. 2007;13(1):102–10. doi: 10.1158/1078-0432.CCR-06-1467. [DOI] [PubMed] [Google Scholar]
- 5.Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, et al. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845–3852. [PubMed] [Google Scholar]
- 6.Machado JC, Oliveira C, Carvalho R, Soares P, Berx G, Caldas C, et al. E-cadherin gene (CDH1) promoter methylation as the second hit in sporadic diffuse gastric carcinoma. Oncogene. 2001;20:1525–1528. doi: 10.1038/sj.onc.1204234. [DOI] [PubMed] [Google Scholar]
- 7.Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, et al. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002;161(5):1881–91. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A, et al. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998;12(1):107–19. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Brodie SG, Yang X, Im YH, Parks WT, Chen L, et al. Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene. 2000;19(15):1868–74. doi: 10.1038/sj.onc.1203504. [DOI] [PubMed] [Google Scholar]
- 10.Shimada S, Mimata A, Sekine M, Mogushi K, Akiyama Y, Fukamachi H, et al. Synergistic tumour suppressor activity of E-cadherin and p53 in a conditional mouse model for metastatic diffuse type gastric cancer. Gut. 2012;61(3):344–53. doi: 10.1136/gutjnl-2011-300050. [DOI] [PubMed] [Google Scholar]
- 11.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 12.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29(4):418–25. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 13.Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002 Jul;115(1-2):53–62. doi: 10.1016/s0925-4773(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Li C, Herrera PL, Deng CX. Generation of Smad4/Dpc4 conditional knockout mice. Genesis. 2002;32(2):80–1. doi: 10.1002/gene.10029. [DOI] [PubMed] [Google Scholar]
- 15.Larsson LI, Madsen OD, Serup P, Jonsson J, Edlund H. Pancreatic-duodenal homeobox 1-role in gastric endocrine patterning. Mech Dev. 1996;60:175–184. doi: 10.1016/s0925-4773(96)00609-0. [DOI] [PubMed] [Google Scholar]
- 16.el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39(3):186–93. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 17.Qiao XT, Ziel JW, McKimpson W, Madison BB, Todisco A, Merchant JL, et al. Prospective identification of a multi-lineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133(6):1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrechek ER, Hardy WR, Siegel PM, Rudnicki MA, Cardiff RD, Muller WJ. Amplification of the neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis. Proc Natl Acad Sci U S A. 2000;97(7):3444–9. doi: 10.1073/pnas.050408497. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung KM, Elashoff RM, Afifi AA. Censoring issues in survival analysis. Annu Rev Public Health. 1997;18:83–104. doi: 10.1146/annurev.publhealth.18.1.83. [DOI] [PubMed] [Google Scholar]
- 20.Allard MA, Bachet JB, Beauchet A, Julie C, Malafosse R, Penna C, et al. Linear quantification of lymphoid infiltration of the tumor margin: a reproducible method, developed with colorectal cancer tissues, for assessing a highly variable prognostic factor. Diagn Pathol. 2012;7:156. doi: 10.1186/1746-1596-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho SB, Takamura K, Anway R, Shekels LL, Toribara NW, Ota H. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig Dis Sci. 2004;49(10):1598–606. doi: 10.1023/b:ddas.0000043371.12671.98. [DOI] [PubMed] [Google Scholar]
- 22.Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10(5):437–49. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira C, Bordin MC, Grehan N, Huntsman D, Suriano G, Machado JC, et al. Screening E-cadherin in gastric cancer families reveals germline mutations only in hereditary diffuse gastric cancer kindred. Hum Mutat. 2002;19(5):510–7. doi: 10.1002/humu.10068. [DOI] [PubMed] [Google Scholar]
- 24.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, et al. Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–61. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirai YT, Ehata S, Yashiro M, Yanagihara K, Hirakawa K, Miyazono K. Bone morphogenetic protein-2 and -4 play tumor suppressive roles in human diffuse-type gastric carcinoma. Am J Pathol. 2011;179(6):2920–30. doi: 10.1016/j.ajpath.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen XZ, Miyake S, Akiyama Y, Yuasa Y. BMP-2 modulates the proliferation and differentiation of normal and cancerous gastric cells. Biochem Biophys Res Commun. 2004;316(1):100–6. doi: 10.1016/j.bbrc.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Itoh K, Kataoka H, Sasaki M, Tanida S, Oshima T, Ogasawara N, Ohara H, Nakao H, Joh T. Bone morphogenetic protein 2 induced differentiation toward superficial epithelial cells in the gastric mucosa. J Gastroenterol. 2006;41(11):1064–75. doi: 10.1007/s00535-006-1899-6. [DOI] [PubMed] [Google Scholar]
- 28.Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118(1):36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 29.Lefebvre O, Chenard MP, Masson R, Linares J, Dierich A, LeMeur M, et al. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996;274(5285):259–62. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- 30.Judd LM, Alderman BM, Howlett M, Shulkes A, Dow C, Moverley J, et al. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology. 2004;126(1):196–207. doi: 10.1053/j.gastro.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 31.Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92(5):645–56. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 32.Humar B, Blair V, Charlton A, More H, Martin I, Guilford P. E-cadherin deficiency initiates gastric signet-ring cell carcinoma in mice and man. Cancer Res. 2009;69(5):2050–6. doi: 10.1158/0008-5472.CAN-08-2457. [DOI] [PubMed] [Google Scholar]
- 33.Pharoah PD, Guilford P, Caldas C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348–1353. doi: 10.1053/gast.2001.29611. [DOI] [PubMed] [Google Scholar]
- 34.Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131(4):1086–95. doi: 10.1053/j.gastro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P, et al. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res. 2003;63(10):2658–64. [PubMed] [Google Scholar]
- 36.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155(4):1033–8. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gradl D, Kühl M, Wedlich D. The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol. 1999;19(8):5576–87. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, et al. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999;96(4):1603–8. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Halder SK, Kashikar ND, Cho YJ, Datta A, Gorden DL, et al. Antimetastatic role of Smad4 signaling in colorectal cancer. Gastroenterology. 2010;138(3):969–80. doi: 10.1053/j.gastro.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang J, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470(7333):269–73. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman TJ, Smith JJ, Chen X, Washington MK, Roland JT, Means AL, et al. Smad4-mediated signaling inhibits intestinal neoplasia by inhibiting expression of β-catenin. Gastroenterology. 2012;142(3):562–571. doi: 10.1053/j.gastro.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.