Abstract
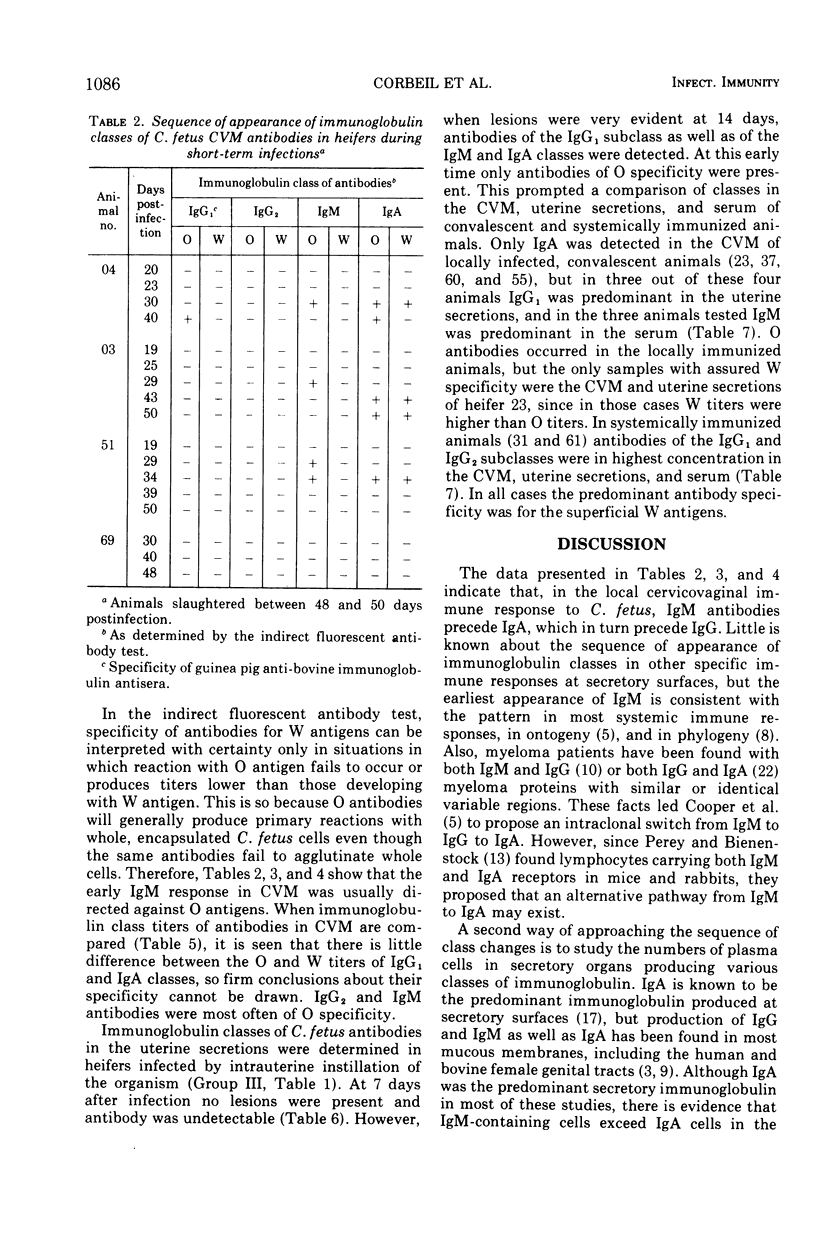
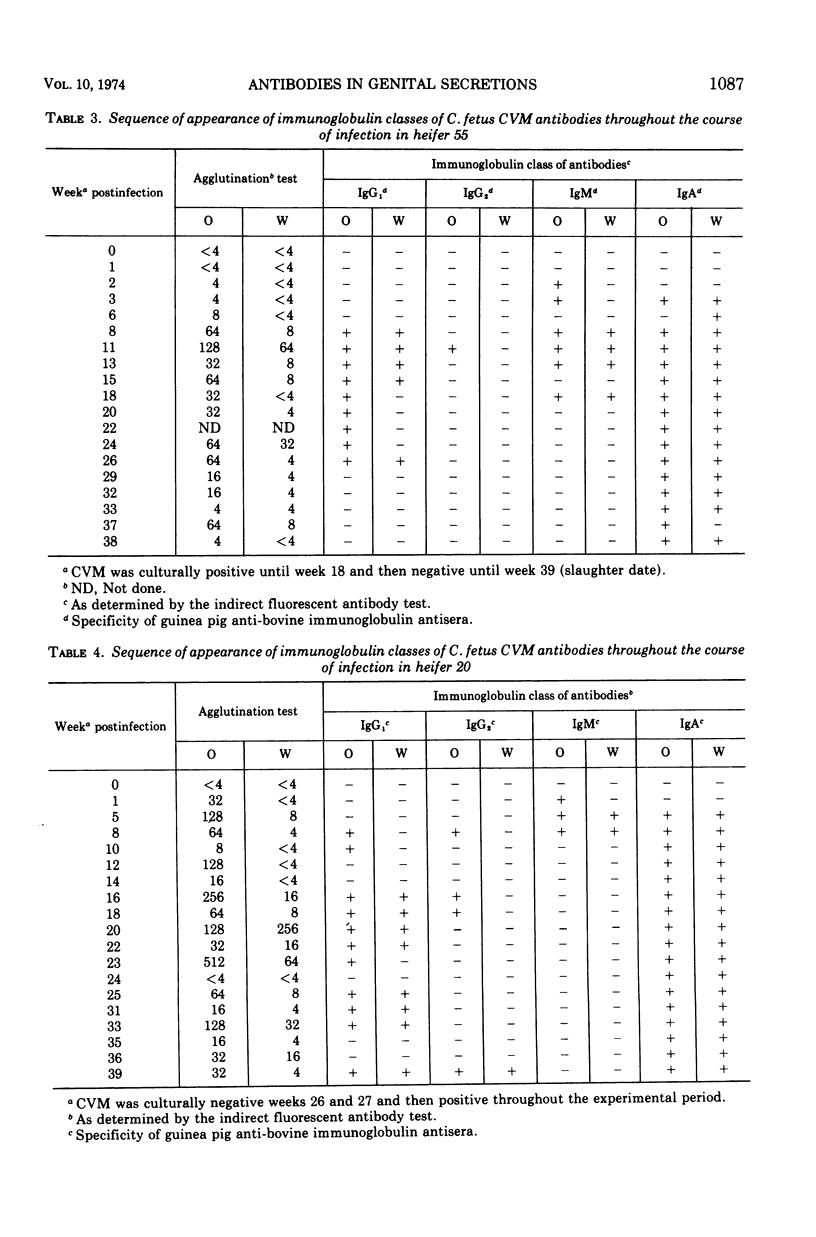
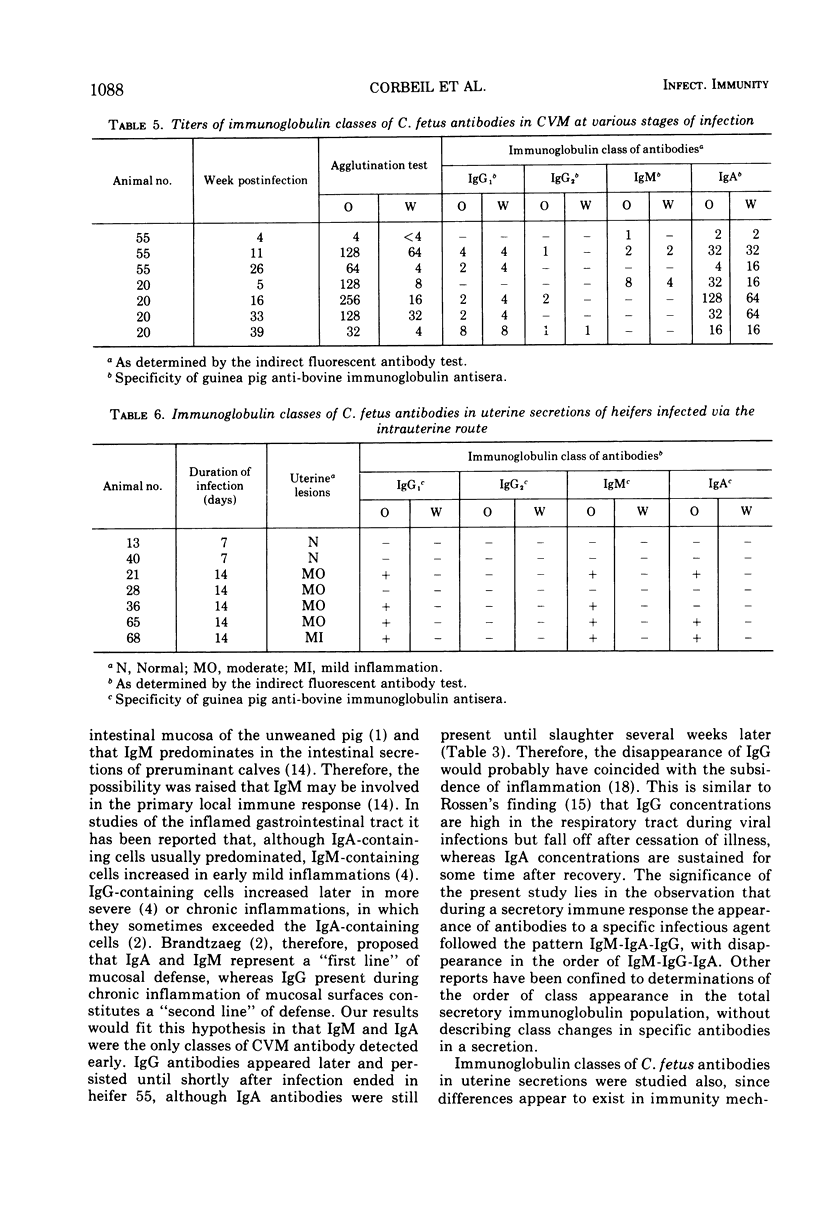
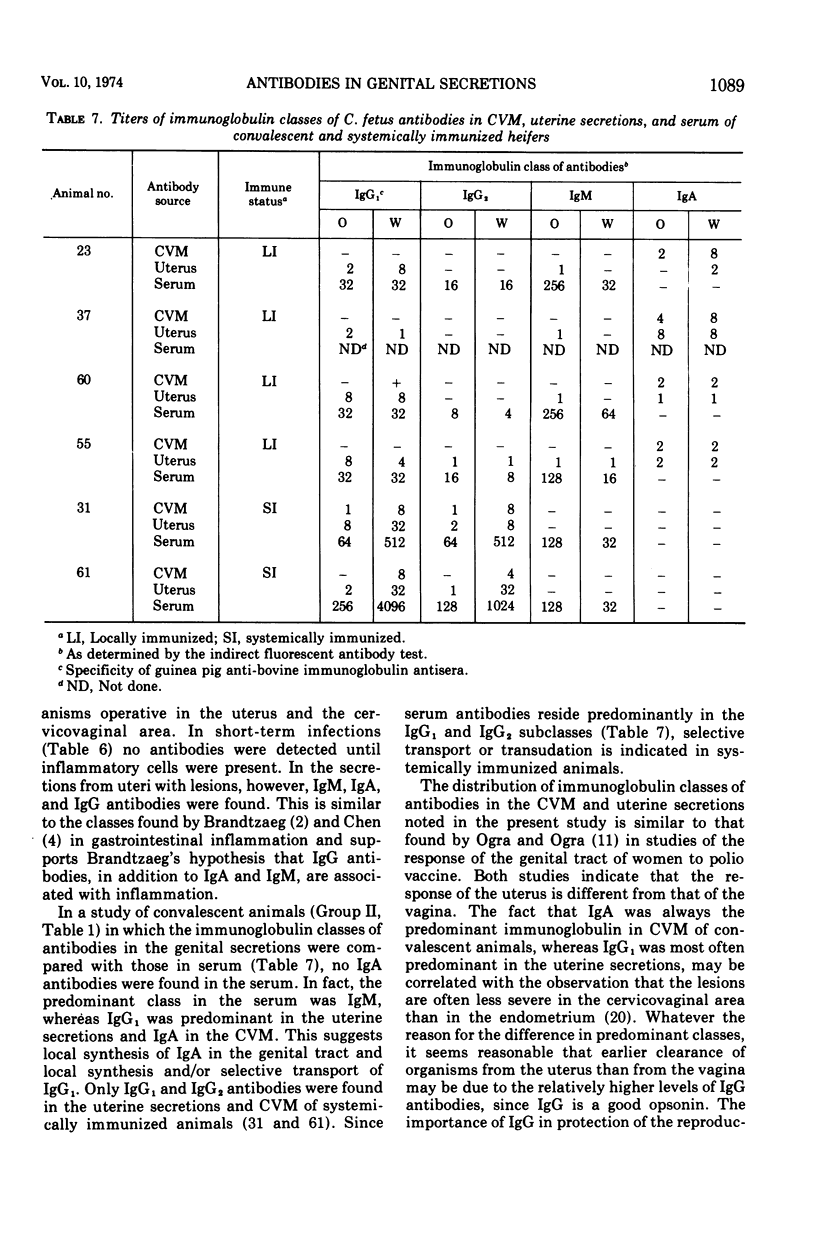
The immunoglobulin classes of antibodies to Campylobacter (Vibrio) fetus in cervicovaginal mucus (CVM) were determined by the indirect fluorescent antibody test at sequential periods, since the order of class appearance has not been established for specific secretory immune responses. In the local immune response to C. fetus immunoglobulin M (IgM) antibodies appeared first, immunoglobulin A (IgA) antibodies next, and immunoglobulin G (IgG) last. IgM antibodies were quite transient, but IgG antibodies remained longer, and those of the IgA class persisted until the end of the experimental period (up to 10 months). Since differences appear to exist between immune mechanisms at cervicovaginal and uterine sites, as well as between immune responses induced by local and systemic immunizations, the immunoglobulin classes of antibodies in uterine secretions were compared with the classes in CVM and serum. Uterine antibodies arose coincidently with uterine lesions in heifers slaughtered after short periods of infection. In convalescent animals only IgA antibodies were found in CVM, whereas the predominant class of antibodies in the uterine secretions was IgG1 in three of four animals studied. Only IgG antibodies were detected in CVM and uterine secretions of systemically immunized animals. These findings could account for faster clearance of C. fetus from the uterus than from the cervicovaginal area in locally infected animals and for failure of colonization in systemically immunized animals, because IgG antibodies are good opsonins and IgA antibodies are not. IgA antibodies do immobilize C. fetus, however, so they could prevent recolonization of the uterus in cervicovaginal carriers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen W. D., Porter P. The relative distribution of IgM and IgA cells in intestinal mucosa and lymphoid tissues of the young unweaned pig and their significance in ontogenesis of secretory immunity. Immunology. 1973 Mar;24(3):493–501. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Structure, synthesis and external transfer of mucosal immunoglobulins. Ann Immunol (Paris) 1973 Aug;124(3):417–438. [PubMed] [Google Scholar]
- Butler J. E., Maxwell C. F., Pierce C. S., Hylton M. B., Asofsky R., Kiddy C. A. Studies on the relative synthesis and distribution of IgA and IgG1 in various tissues and body fluids of the cow. J Immunol. 1972 Jul;109(1):38–46. [PubMed] [Google Scholar]
- Chen S. T. Cellular sites of immunoglobulins. II. The relative proportions of mucosal cells containing IgG, IgA, and IgM, and light polypeptide chains of kappa and lambda immunoglobulin in human appendices. Acta Pathol Jpn. 1971 Feb;21(1):67–83. [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., Kincade P. W. A two-stage model for development of antibody-producing cells. Clin Exp Immunol. 1972 May;11(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Schurig G. D., Duncan J. R., Corbeil R. R., Winter A. J. Immunoglobulin classes and biological functions of Campylobacter (Vibrio) fetus antibodies in serum and cervicovaginal mucus. Infect Immun. 1974 Sep;10(3):422–429. doi: 10.1128/iai.10.3.422-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddie D. S., Schulkind M. L., Robbins J. B. The isolation and biologic activities of purified secretory IgA and IgG anti-Salmonella typhimurium "O" antibodies from rabbit intestinal fluid and colostrum. J Immunol. 1971 Jan;106(1):181–190. [PubMed] [Google Scholar]
- Grey H. M. Phylogeny of immunoglobulins. Adv Immunol. 1969;10:51–104. doi: 10.1016/s0065-2776(08)60415-0. [DOI] [PubMed] [Google Scholar]
- Nisonoff A., Fudenberg H. H., Wilson S. K., Hopper J. E., Wang A. C. Individual antigenic specificity in immunoglobulins: relationship to biosynthesis. Fed Proc. 1972 Jan-Feb;31(1):206–209. [PubMed] [Google Scholar]
- Ogra P. L., Ogra S. S. Local antibody response to poliovaccine in the human female genital tract. J Immunol. 1973 May;110(5):1307–1311. [PubMed] [Google Scholar]
- Pedersen K. B., Aalund O., Nansen P., Adler H. C. Immunofluorescent immunoglobulin differentiation of vibrio fetus antibodies from bovine cervico-vaginal secretions. Acta Vet Scand. 1971;12(2):303–306. doi: 10.1186/BF03547763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perey D. Y., Biennenstock J. Effects of bursectomy and thymectomy on ontogeny of fowl IgA, IgG, and IgM. J Immunol. 1973 Aug;111(2):633–637. [PubMed] [Google Scholar]
- Schurig G. D., Hall C. E., Burda K., Corbeil L. B., Duncan J. R., Winter A. J. Persistent genital tract infection with Vibrio fetus intestinalis associated with serotypic alteration of the infecting strain. Am J Vet Res. 1973 Nov;34(11):1399–1403. [PubMed] [Google Scholar]
- Thomasi T. B., Jr Secretory immunoglobulins. N Engl J Med. 1972 Sep 7;287(10):500–506. doi: 10.1056/NEJM197209072871008. [DOI] [PubMed] [Google Scholar]
- Wilkie B. N., Winter A. J. Bovine vibriosis: the distribution and specificity of antibodies induced by vaccination and infection and the immunofluorescent localization of the organism in infected heifers. Can J Comp Med. 1971 Oct;35(4):301–312. [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- Wolfenstein-Todel C., Franklin E. C., Rudders R. A. Similarities of the light chains and the variable regions of the heavy chains of the IgG2 lambda and IgA1 lambda myeloma proteins from a single patient. J Immunol. 1974 Mar;112(3):871–876. [PubMed] [Google Scholar]
- Zipursky A., Brown E. J., Bienenstock J. Lack of opsonization potential of 11S human secretory A. Proc Soc Exp Biol Med. 1973 Jan;142(1):181–184. doi: 10.3181/00379727-142-36983. [DOI] [PubMed] [Google Scholar]


