Abstract
Protein phosphorylation is very common post-translational modification, catalyzed by kinases, for signaling and regulation. Phosphotyrosines frequently target SH2 domains. The spleen tyrosine kinase (Syk) is critical for tyrosine phosphorylation of multiple proteins and for regulation of important pathways. Phosphorylation of both Y342 and Y346 in Syk linker B is required for optimal signaling. The SH2 domains of Vav1 and PLC-γ both bind this doubly phosphorylated motif. Here we used a recently developed method to calculate the effects of Y342 and Y346 phosphorylation on the rate constants of a peptide from Syk linker B binding to the SH2 domains of Vav1 and PLC-γ. The predicted effects agree well with experimental observations. Moreover, we found that the same doubly phosphorylated peptide binds the two SH2 domains via distinct mechanisms, with apparent rigid docking for Vav1 SH2 and dock-and-coalesce for PLC-γ SH2.
Keywords: transient complex, association rate constant, association mechanism, dock-and-coalesce, electrostatic interactions, phosphorylation
Introduction
Protein phosphorylation is a common post-translational modification, catalyzed by kinases, for signaling and regulation1. As a result of phosphorylation or dephosphorylation of hydroxyl-containing residues (serine, threonine, and tyrosine), enzymes can become activated or inactivated2. The phosphorylated residues may create docking sites for signal-transduction proteins, exemplified by those containing the Src homology 2 (SH2) domain3.
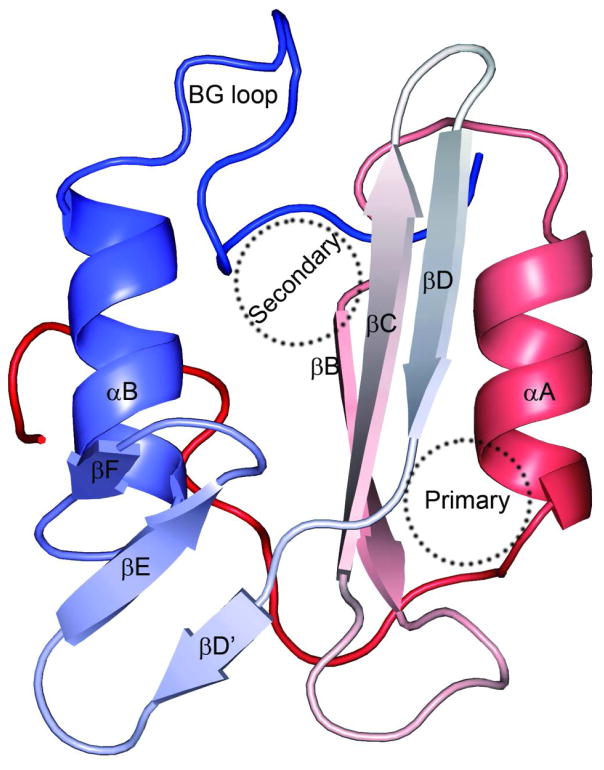
SH2 domains specifically recognize phosphotyrosine (pY). They were first identified in the oncoproteins Src and Fps4. They contain approximately 100 amino acids, comprised of two α-helices and seven β-strands5, 6. As shown in Fig. 1, the two helices (αA and αB) sandwich a central β-sheet formed by strands βB, βC, and βD. An SH2 domain harbors two pY binding sites, referred to as primary and secondary and located on opposite sides of the central β-sheet. The primary site, positioned between the central β-sheet and αA, typically binds peptides with a single pY, which can interact with two highly conserved residues, an arginine and a histidine, at the βB5 and βD4 positions, respectively. Tyrosine phosphorylation on the peptides could increase the binding affinities by several orders of magnitude7. For peptides with two pY residues, the secondary site, situated between the central β-sheet and αB, is also engaged, resulting in enhanced binding affinity and higher sequence specificity8.
Figure 1.
Structure of an SH2 domain (as found in PDB entry 2FCI; peptide not displayed), displayed as ribbon with color varying from red at the N-terminus to blue at the C-terminus. The primary and secondary sites for phosphotyrosine binding are indicated by dash circles.
The spleen tyrosine kinase (Syk) has multiple proteins as substrates and is essential in B cell signaling9. Murine Syk contains three conserved tyrosine residues, Y317, Y342, and Y346, in linker B, which connects the second SH2 domain and the catalytic domain. Phosphorylation of Y317 negatively regulates the function of Syk10, whereas phosphorylation of both Y342 and Y346 is required for optimal signaling11. The SH2 domains of Vav1 and PLC-γ can both bind to the doubly phosphorylated Syk linker B (referred to as pYpY hereafter), with pY342 in the primary site and pY346 in the secondary site8, 12. The dissociation constants of pYpY are 7.4 and 0.07 μM, respectively, for Vav1 SH2 and PLC-γ SH28, 12. For Vav1 SH2, the binding affinity is reduced by 10-fold when Y342 is unphosphorylated (abbreviated as YpY) and by 2-fold when Y346 is unphosphorylated (abbreviated as pYY)12. The binding affinity of pYY for PLC-γ SH2 is 6-fold lower8. For both systems, from the experimental measurement8, 12, we can see that the decreases in affinity come mostly from the decreases of association rate constant (ka), while the dissociation rate constants (kd) are only modestly affected.
What is the physical basis for the enhancements in association rate constants (and binding affinities) by tyrosine phosphorylation? A direct consequence of phosphorylation is the addition of one to two negative charges (depending on the pH) to each tyrosine, which may introduce favorable electrostatic interactions with the SH2 domains. Electrostatic interactions have indeed been suggested to make significant contributions to the binding affinities of pY-containing peptides for SH2 domains13. For example, it was found that addition of a phosphate to the YEEI peptide results in a ~104-fold increase in binding affinity7.
We and others have found that, for stereospecific protein association, electrostatic attraction can significantly enhance association rate constants, by as much as three or more orders of magnitude14–21. To calculate the association rate constant, we envisioned that association proceeds by first reaching via translational and rotational diffusion a transient complex, in which the two subunits have near-native separation and relative orientation but have yet to form most of the native interactions. Thereafter the two subunits undergo conformational rearrangement and further tightening to form the native complex. Assuming that the second sub-step is fast, the overall association rate constant can be predicted as15, 22
| (1) |
where ka0 is the “basal” rate constant for reaching to transient complex by unbiased diffusion, ΔGel* is the electrostatic interaction energy in the transient complex, and kBT is thermal energy. Our method for ka calculations has been automated18 and is accessible as the TransComp web server at http://pipe.sc.fsu.edu/transcomp/. The basal rate constant is mainly determined by the shape of the interface between the two subunits in the native complex, and ΔGel* is dictated by the degree of charge complementarity across the interface. By separating the two contributing factors to ka, we gain better understanding on how the magnitudes of ka are modulated in different protein complexes.
In this study, we used TransComp to calculate the effects of Y342 and Y346 phosphorylation on the rate constants of a peptide (DTEVY342ESPY346ADPE) from Syk linker B binding to the SH2 domains of Vav1 and PLC-γ. The predicted effects agree well with the experimental observations9, 13. Moreover, we found that the same doubly phosphorylated peptide binds the two SH2 domains via distinct mechanisms. The binding with Vav1 SH2 can be characterized as rigid docking, whereby contacts with both the primary and secondary sites are formed nearly all at once to produce the native complex. In contrast, binding to PLC-γ SH2 proceeds via a dock-and-coalesce mechanism, whereby docking of pY342 to the primary site is followed by coalescing of pY346 and the flanking residues around the secondary site, leading to eventual insertion of pY346 into the secondary site.
Results
Syk linker B peptide binding to Vav1 SH2
TransComp calculations for association rate constants use the structures of the native complexes as input18. Each ka calculation consists of three steps: generation of transient-complex ensemble; determination of the basal rate constant ka0 for reaching the transient complex; and evaluation of the electrostatic interaction free energy ΔGel* in transient complex. For pYpY binding to Vav1 SH2, we used the structure in Protein Data Bank (PDB) entry 2LCT12 as input. The output from the server is shown in Fig. 2. The transient complex was identified by mapping the interaction energy surface in the 6-dimensional space of relative translation and relative rotation in and around the native-complex energy well. As illustrated in Fig. 2D, in the native-complex energy well, the subunits form a large number of contacts (Nc) but have little freedom in the relative rotation angle (χ). Outside the native-complex energy well, the two subunits lose most of the contacts but gain nearly complete rotational freedom. The transient complex is located at the rim of the native-complex energy well (Fig. 2D), identified by the mid-point of the transition from restricted rotation to complete rotational freedom (Fig. 2C). Representative configurations of the transient complex are shown in Fig. 3A.
Figure 2.
Output of the TransComp run for pYpY binding to Vav1 SH2. (A) Vav1 SH2 is shown as electrostatic surface accompanied by a ribbon representation of pYpY to indicate the binding site. Blue and red indicate positive and negative electric potentials, respectively. (B) The representations of Vav1 SH2 and pYpY are reversed and the view is rotated around vertical axis by 180°. (C) The Nc versus σχ curve. (D) Scatter plot of Nc versus χ for clash-free configurations. The native complex and the transient complex are indicated by a green circle and blue line, respectively.
Figure 3.
Representative transient-complex configurations for the (A) Vav1 SH2-pYpY and (B) PLC-γ SH2-pYpY systems. Seven poses of pYpY with color varying from red at the N-terminus to blue at the C-terminus are shown. pYpY in the native complex is also shown in yellow as reference.
Successful completion of a TransComp run is a sign that the complex is formed via rigid docking, whereby the inter-subunit contacts in the native complex are formed nearly all at once18, 23. This binding mechanism for forming the Vav1 SH2-pYpY complex is further described below.
The rate constants (ka) and their contributing factors (ka0 and ΔGel*) for the binding of Vav1 SH2 with the Syk linker B peptide in different phosphorylation forms are listed in Table 1 (net charge of phosphotyrosine is −1). The basal rate constant ka0 is largely dictated by the shape and size of binding interface, with more convoluted larger interfaces corresponding to lower ka0 whereas flatter and smaller interface corresponding to higher ka018, 24. For the doubly phosphorylated peptide, a basal rate constant of 0.49 × 106 M−1s−1 and an electrostatic interaction energy of −3.34 kcal/mol in the transient complex were found, resulting in an association rate constant of 1.36 × 108 M−1s−1. The significant attractive electrostatic interaction between the subunits comes from strong complementarity between the cationic binding site on Vav1 SH2 and the anionic pYpY peptide, as shown in Fig. 2A, B.
Table 1.
Calculated and experimental results for the binding kinetics of Vav1 SH2 with the Syk linker B peptide in different phosphorylation forms (ionic strength = 120 mM). Each phosphorylation is treated with single protonated phosphate group (net charge of −1).
| Peptides/SH2 | ka0 (106 M−1s−1) | ΔGel* (kcal/mol) | ka (106 M−1s−1) | Fold decrease | Experimental fold decrease12 |
|---|---|---|---|---|---|
| pYpY | 0.49 | −3.34 | 136 | ||
| YpY | 0.49 | −1.64 | 7.78 | 17.5 | 17.3 |
| pYY | 0.49 | −2.65 | 42.5 | 3.2 | 2.1 |
| YY | 0.49 | −0.93 | 2.37 | 57.4 | |
| βD3 K→Qa | 0.49 | −2.57 | 37.6 | 3.6 |
Binding of pYpY to Vav1 SH2 βD3 K→Q mutant
We investigated the contributions of Y342 and Y346 phosphorylation to the Vav1 SH2-pYpY association rate constant by calculating the ka values for the singly phosphorylated (YpY and pYY and unphosphorylated (YY) forms. Following our previous studies18, 19, we assumed that a change in the phosphorylation state of a tyrosine residue, just like a mutation, does not affect the location of the transient complex. Hence the basal rate constant remained the same and only ΔGel* was re-calculated for YpY, pYY, and YY, using the transient complex determined for pYpY binding. With Y342 unphosphorylated, ΔGel* changed from −3.34 kcal/mol to −1.64 kcal/mol, resulting in a 17.5-fold decrease in ka. In comparison, with Y346 unphosphorylated, ΔGel* changed to −2.65 kcal/mol, resulting in a 3.2-fold decrease in ka.
Experimentally, the decreases in ka for binding YpY and pYY were 17.3- and 2.1-fold, respectively12. These results agree well with our calculations, suggesting that the contributions of Y342 and Y346 phosphorylation to the Vav1 SH2-pYpY association rate constant can be largely accounted for by the electrostatic attraction between the added phosphate groups on the peptide and the cationic residues on the binding site of Vav1 SH2. We note that the magnitudes of the experimental associate rate constants, determined by surface plasmon resonance (SPR), were much lower than those of our calculations. As noted previously16, 25, compared to methods such as stopped-flow spectroscopy that operate in solution, SPR has confounding effects such as mass transport and surface immobilization, which can be especially severe for fast association, a scenario implicated here by our calculation for the Vav1 SH2-pYpY system and recognized for other SH2-phosphotyrosyl systems25. Nevertheless SPR can still be useful for measuring changes in ka for related systems.
The above calculation was performed with phosphotyrosine in singly charged phosphate group. We also performed calculation with phosphotyrosine in doubly charged phosphate group as shown in Table S1. For pYpY, the attractive electrostatic interaction is increased by the doubly charged phosphotyrosine from −3.34 kcal/mol to −4.61 kcal/mol, and the association rate constant is 9.58 × 108 M−1s−1 (from 1.36 × 108 M−1s−1, 7-fold increase). With Y342 unphosphorylated (YpY), ΔGel* changed from −4.61 kcal/mol to −1.97 kcal/mol, resulting in a 71.0-fold decrease in ka. In comparison, with Y346 unphosphorylated (pYY), ΔGel* changed to −3.70 kcal/mol, resulting in a 3.9-fold decrease in ka. A 71.0-fold decrease in ka, resulting from unphophorylation of Y342, is overestimated, compared to the experimental observation (17.3-fold decrease). Therefore, a singly charged state of phosphotyrosine here seems to be more reasonable.
Our calculations showed that, when both Y342 and Y346 are unphosphorylated, ΔGel* further decreased in magnitude to −0.93 kcal/mol (corresponding to a 60-fold decrease in ka). This ΔGel* is nearly equal to what is expected when the effects of separately dephosphorylating the two tyrosines are added. This additive effect is consistent with the wide separation between the two phosphate groups (distance between the two phosphorus atoms ~ 18 Å) and correspondingly that between the primary and secondary sites in the native complex. It is unknown whether the YY peptide still has measurable binding affinity for Vav1 SH2. If so our calculated ka stands to be tested. We also calculated the effect of mutating a conserved lysine (at βD3) around the secondary site, and found that binding of pYpY to this K→Q mutant has ΔGel* = −2.57 kcal/mol, resulting in a 3.6-fold decrease in ka. This result also remains to be tested.
Very recently Chen et al.26 determined the structure of the Vav1 SH2-YpY complex (PDB entry 2MC1). Using this structure we repeated the ka calculation for the Vav1 SH2-YpY system. The results were very similar to those obtained above by using the transient complex determined for the Vav1 SH2-pYpY system: the basal rate constant was modest higher (by 1.8-fold) while ΔGel* was virtually unchanged. Our calculated results thus appear to be robust.
The transient complex ensemble is determined by the input complex structure. The first model of the NMR structure of Vav1 SH2-pYpY complex was used for the above calculation. To test sensitivity of the different models on calculation results, we performed the calculation on the all 20 models of the NMR structure. The calculation results are shown in Table S2. Of 20 models, calculations of 10 models completed successfully, while the rest failed because of bad fit of the Nc versus σχ curve, rather than big gap. Generally, a calculation failed because of bad fit of the Nc versus σχ curve could result from poor structure, while a big gap could mean the association process is a multiple-step process. Calculation on models of low energy, i.e. first ten models, completed successfully, while most of last ten models failed. It should be noted that the Nc* = 16 of model 3 is too big to have enough poses of transient complex in the ensemble, which led to very low value of ka0, thus ka. For rest of the nine successful calculations, the values of ka0 range from 0.028 × 106 M−1s−1 to 2.14 × 106 M−1s−1; the values of ΔGel* range from −1.89 kcal/mol to 4.48 kcal/mol; and the values of ka range from 11.5 × 106 M−1s−1 (~0.1-fold of model 1) to 560 × 106 M−1s−1 (~ 4.3 fold of model 1).
Syk linker B peptide binding to PLC-γ SH2
Using the structure for the PLC-γ SH2-pYpY complex (PDB entry 2FCI8) as input, we carried out a TransComp run for this system. However, this TransComp run was aborted, due to a large gap of 14 in the sampled Nc values. Such a large Nc gap showed that the formation of the PLC-γ SH2-pYpY native complex could not be modeled as rigid docking18. It has been found that8 for pYpY and pYY binding to PLC-γ SH2, chemical shift perturbations showed negligible differences for residues around the primary site but significant differences for residues around the secondary site. It indicates pYpY has similar interactions and orientations in the primary site as that of pYY, but different in the secondary site. Consistent with this finding,, compared to the situation when a peptide with a single pY is bound to the primary site (PDB entry 2PLD27), PLC-γ SH2 upon binding pYpY undergoes extensive conformational change around the secondary site (Fig. S1). The BG loop moves outward, allowing the αB helix to closely approach the central β-sheet and bound the secondary site (Fig. S1A). Moreover, two sidechains, from βD3 K56 and αB9 Y84, extend over the secondary site to cover the bound pY346 (Fig. S1B). The doubly phosphorylated pYpY peptide and the singly phosphorylated peptide from the platelet-derived growth factor receptor (referred to as pY1021) have similar interactions in the primary binding site, but the C-terminal portion of pY1021 is displaced from the secondary site (Fig. S1A).
We hypothesized that the binding of pYpY to PLC-γ SH2 involves two steps. The first is the docking of pY342 to the primary site. The resulting complex will be referred to as the docked intermediate. The second is the coalescence of the C-terminal portion of pYpY around the secondary site and the eventual insertion of pY346 into the secondary site. This dock-and-coalesce mechanism was found to be widely followed by the binding of intrinsically disordered proteins (IDPs) to structured targets18, 28. Accordingly, we built a homology model for the docked complex using the structure of the PLC-γ SH2-pY1021 complex27 as template (Fig. S2). In the model for the docked complex, pY342 is inserted into the primary site, but pY346 points away from the SH2 domain, which does not have a well-formed secondary site (Fig. S2C).
Using the model for the docked complex as input, a TransComp run completed successfully. The output is shown in Fig. S3. Representative configurations in the transient complex are shown in Fig. 3B. The calculated ka0, ΔGel*, and ka results for the docking step are listed in Table 2 (net charge of phosphotyrosine is −1). Assuming that the coalescing step is fast (relative to the undocking step), these calculations yield the overall rate constant for the full binding of the peptide to the primary and secondary sites. For the binding of pYpY, the basal rate constant was 0.39 × 105 M−1s−1 and the electrostatic interaction energy in the transient complex was −3.84 kcal/mol, resulting in a ka of 2.38 × 107 M−1s−1. When either Y342 (YpY) or Y346 (pYY) or both (YY) are unphosphorylated, weakened electrostatic attraction resulted in decreases in the association rate constant by 4.6-, 2.5-, and 12.6-fold, respectively. Experimentally it was observed that the association rate constant of pYY was smaller than that of pYpY by 6-fold8. We also calculated the effect of the PLC-γ SH2 βD3 K→Q mutation on the docking rate constant of pYpY and predicted a 5.9-fold decrease. Experimentally the mutation was found to result in slight increase (1.5-fold) in the overall binding rate constant. A better model for the docked complex perhaps can reduce the discrepancy for the K→Q mutant.
Table 2.
Calculated and experimental results for the binding kinetics of PLC-γ SH2 with the Syk linker B peptide in different phosphorylation forms (ionic strength = 155 mM). Each phosphorylation is treated with single protonated phosphate group (net charge of −1).
| Peptides/SH2 | ka0 (105 M−1s−1) | ΔGel* (kcal/mol) | ka (106 M−1s−1) | Fold decrease | Experimental fold decrease8 |
|---|---|---|---|---|---|
| pYpY | 0.39 | −3.84 | 23.8 | ||
| YpY | 0.39 | −2.91 | 5.22 | 4.6 | |
| pYY | 0.39 | −3.26 | 9.40 | 2.5 | 6 |
| YY | 0.39 | −2.30 | 1.89 | 12.6 | |
| βD3 K→Qa | 0.39 | −2.75 | 4.01 | 5.9 | 0.6 |
Binding of pYpY to PLC-γ SH2 βD3 K→Q mutant
Distinct mechanisms for binding to Vav1 and PLC-γ SH2 domains
Our TransComp calculations suggested that the doubly phosphorylated Syk B linker peptide binds to Vav1 SH2 and PLC-γ SH2 via distinct mechanisms (Fig. 4). Apparently, the pYpY peptide can rapidly sample near-native conformations and, upon approaching Vav1 SH2, forms appropriate inter-subunit contacts at both the primary and secondary sites; additional fast conformational rearrangement of both the peptide and the SH2 domain finally results in the native complex (Fig. 4A).
Figure 4.
Two distinct binding mechanisms. (A) pYpY rapidly samples near-native conformations and then docks to the primary and secondary sites of Vav1 SH2 at once nearly as a rigid body. (B) pYpY binds to the primary and secondary sites of PLC-γ SH2 in a sequential manner. First a docking step anchors pY342 to the primary site. In the subsequent coalescing step, the C-terminal portion of the peptide and the elements around the would-be secondary site undergo conformational rearrangement, leading to the insertion of pY346 into the secondary site.
In contrast, when binding to PLC-γ SH2, pYpY forms near-native contacts at the primary and secondary sites not all at once but sequentially (Fig. 4B). pY342 first docks to the primary site while the secondary site has yet to be properly formed. Once anchored on the PLC-γ SH2, both the C-terminal portion of the peptide and the structural elements around the future secondary site undergo extensive conformational changes. These include outward movement of the BG loop and squeeze in of the αB helix toward the central β-sheet. The result is the proper formation of the secondary site and insertion of pY346 into this site.
Discussion
Roles of electrostatic interactions in SH2 recognition
For binding to Vav1 SH2 and PLC-γ SH2, phosphorylation of a single tyrosine was found to result in an increase in binding affinity of up to 10-fold8, 12. For other systems, much greater effects have been reported7. An increase in affinity can be achieved by either an increase in the association rate constant, or a decrease in the dissociation rate constant, or a combination. For binding to both Vav1 SH2 and PLC-γ SH2, the contributions of tyrosine phosphorylation have been linked mostly to increases in association rate constant, with dissociation rate constants only modestly affected8, 12. Our calculations here have now further established that the increases in association rate constant are due to the additional electrostatic attraction with the SH2 domains afforded by the phosphate groups on the tyrosines. This conclusion is in line with observations on many other protein-protein complexes, where electrostatic attraction has been implicated in elevating the probability of the two subunits being in near-native separation and orientation, leading to significant enhancement of association rates14–21, 29, 30.
At the primary site, the conserved βB5 arginine has been shown to be important for the electrostatic interaction with a pY7. In the secondary site of both Vav1 SH2 and PLC-γ SH2, βD3 lysine is in close contact with pY346 of the Syk B linker peptide8, 12. Mutation of this lysine to a neutral residue glutamine would eliminate the electrostatic interaction with pY346 and possibly neighboring anionic residues on the peptide. In the case of Vav1 SH2, our calculations predicted that the βD3 K→Q mutation would decrease the association rate constant by 3.6-fold. Though this prediction is yet to be tested experimentally, it appears reasonable, since it is similar to the effect of dephosphorylating pY346 (3.2-fold decrease in ka) and the latter result is consistent with experiment12. In the case of PLC-γ SH2, the βD3 K→Q mutation was found experimentally to increase ka by 1.5-fold8 whereas our calculations predicted a decrease in ka by 5.9-fold. These calculations were based on assuming the dock-and-coalesce mechanism and used a modeled structure for the docked intermediate; the latter, as suggested above, could contribute to the discrepancy from experiment. Further discussion is given below.
Dock-and-coalesce mechanism
Our calculations identified two distinct mechanisms for the Syk linker B peptide pYpY’s binding to Vav1 SH2 and PLC-γ SH2. In the former case, pYpY rapidly samples near-native conformations and then docks to the primary and secondary sites at once nearly as a rigid body. In the latter case, pYpY binds to the primary and secondary sites in a sequential manner. First pY342 docks to the primary site, and then the C-terminal portion of the peptide and the elements around the would-be secondary site undergo conformational rearrangement, leading to the insertion of pY346 into the secondary site. The former rigid docking mechanism is common for the association between globular proteins that do not undergo significant conformational changes18, 23, whereas the latter dock-and-coalesce mechanism is common for the binding of IDPs to structured targets18, 28.
The distinct binding mechanisms have some experimental support. For Vav1 SH2, the chemical shift perturbations of residues around the primary and secondary sites were found to be identical upon binding pYpY, YpY, and pYY26. This observation can be easily explained if the Vav1 SH2-pYpY complex is stabilized by a cooperative set of interactions that spans both the primary and secondary sites, such that dephosphorylation of either one of the two phosphotyrosines does not sufficient weakening of the cooperative set. This is consistent with the proposed rigid-docking mechanism. In contrast, for pYpY and pYY binding to PLC-γ SH2, chemical shift perturbations showed negligible differences for residues around the primary site but significant differences for residues around the secondary site8. This observation is consistent with the proposed dock-and-coalesce mechanism, in which the docking of pY342 into the primary site facilitates subsequent conformational search. Depending on whether Y346 is phosphorylated, the additional coalesced structure in the SH2-peptide interface is different.
Our ka calculations for peptide binding to PLC-γ SH2 were based on the dock-and-coalesce mechanism and used a modeled structure for the docked intermediate. Moreover, we assumed that the docking step is rate-limiting or, equivalently, the coalescing step is much faster than the undocking step. If the docking step is only partially rate-limiting, then we could underestimate the effect of a mutation that slows down the coalescing step and overestimate the effect of a mutation that speeds up the coalescing step. Our calculations apparently underestimated the effect of dephosphorylating pY346 (calculated 2.5-fold decrease in ka versus an experimental 6-fold decrease8). It is indeed reasonable to expect that dephosphorylating pY346 would slow down the coalescing step. On the other hand, we overestimated the adverse effect of the βD3 K→Q mutation (calculated 5.9-fold decrease in ka versus a slight 1.5-fold increase by experiment8). Upon pYpY binding in the wild-type SH2, the sidechain of βD3 K56 (along with that of αB9 Y84) extends over the secondary site to cover the bound pY346 (Fig. S1B). This could be a slow event that is eliminated by the K→Q mutation, a scenario that is consistent with the observation that the mutation increases the dissociation rate constant by 10-fold8. It is thus conceivable that the K→Q mutant has a faster coalescing step than the wild-type SH2.
What is the advantage of dock-and-coalesce mechanism? In a case where the proper formation of a secondary site involves significant conformational rearrangement, anchoring of the peptide to the primary site enables the cooperation of the elements around the secondary site and the cognate residues on the peptide in their search for the native structure, leading to acceleration of the overall binding process. Further increase in binding affinity can be produced in the coalescing step by covering up the ligand bound to the secondary site, as in the PLC-γ SH2-pYpY system through the extension of the βD3 K56 and αB9 Y84 sidechains over the bound pY346 (Fig. S1B).
Conclusions
We have used TransComp to calculate the rate constants for the binding of Vav1 SH2 and PLC-γ SH2 with a Syk-derived peptide in different phosphorylation forms. The calculations allowed for the isolation of the contributions of electrostatic interactions in SH2 recognition. Moreover, we identified distinct mechanisms for recruiting the doubly phosphorylated peptide to the two SH2 domains: rigid docking for Vav1 SH2 and dock-and-coalesce for PLC-γ SH2. Applications to other binding processes regulated by phosphorylation will further advance our quantitative and mechanistic understanding of this important posttranslational modification.
Methods
TransComp calculations
The procedure for TransComp calculations has been described previously15, 18. Here we briefly summarize the three constituent steps. The transient complex is identified through mapping the interaction energy landscape in and around the native-complex energy well. Starting from the structure of the native complex, the two subunits are translated and rotated, and configurations that are clash-free are saved. For each clash-free configuration, the number of inter-subunit contacts (Nc) is calculated. As the two subunits move away from the native complex, Nc decreases but the rotational freedom, as measured by the standard deviation (σχ) of the relative rotation angle (χ) among the clash-free configurations at a given Nc, increases (Fig. 2 and Fig. S3). The dependence of σχ on Nc is fitted to a function used for modeling reversible two-state protein denaturation. The midpoint of this fit, where Nc is denoted as Nc*, defines the transient complex. That is, configurations with Nc = Nc*, make up the transient-complex ensemble.
The basal rate constant ka0 is calculated from force-free Brownian dynamics simulations according to an algorithm developed previously31. Each Brownian trajectory starts from a configuration inside the native-complex energy well (i.e., Nc > Nc*) and is propagated in the translational and rotational space. At each step during the simulation, if Nc > Nc* then the subunits are allowed to form the native complex; if successful the trajectory is then terminated. The survival fraction of the Brownian trajectories as a function of time is used to calculate ka0.
To calculate the electrostatic interaction energy ΔGel*, 100 configurations are randomly chosen from the transient-complex ensemble, using the APBS program32 and the results averaged.
All our calculations were done through the TransComp web server at http://pipe.sc.fsu.edu/transcomp/. The inputs, including the structure of the native complex and the ionic strength, are described below.
pYpY binding to Vav1 SH2
Here we used the first model of NMR structure (PDB entry 2LCT) of the Syk-derived doubly phosphorylated peptide pYpY (DTEVY342ESPY346ADPE) bound to Vav1 SH212. We acetylated the N-terminus and amidated the C-terminus of the peptide and optimized the positions of hydrogen atoms by energy minimization (Amber with Amber99SB force field33). The resulting structure in PQR format (with Amber charges and Bondi radii) was input to the TransComp server, along an ionic strength of 120 mM (experimental condition of Ref. 12). The partial charges for phosphotyrosine (singly protonated phosphate, −1 net charge) were from Homeyer et al.34.
pYpY binding to PLC-γ SH2
An initial, aborted TransComp run used the first model of NMR structure (PDB entry 2FCI8) for the PLC-γ SH2-pYpY native complex as input. In subsequent calculations, we used a docked intermediate modeled on the PLC-γ SH2-pY1021 complex (PDB 2PLD27) (Fig. S2). After aligning Y342 of pYpY to the only tyrosine of pY1021 (Fig. S2A), a homology model for the PLC-γ SH2-pYpY docked intermediate was generated by the Modeller program35. Subsequent steps were similar to those described in the last subsection, except that the ionic strength was 155 mM (experimental condition of Ref. 8).
Supplementary Material
Acknowledgments
This work is supported by National Institutes of Health Grant GM058187.
References
- 1.Pawson T, Scott JD. Protein phosphorylation in signaling--50 years and counting. Trends Biochem Sci. 2005;30:286–290. doi: 10.1016/j.tibs.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Graves JD, Krebs EG. Protein phosphorylation and signal transduction. Pharmacol Ther. 1999;82:111–121. doi: 10.1016/s0163-7258(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 3.Pawson T. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell. 2004;116:191–203. doi: 10.1016/s0092-8674(03)01077-8. [DOI] [PubMed] [Google Scholar]
- 4.Sadowski I, Stone JC, Pawson T. A Noncatalytic Domain Conserved among Cytoplasmic Protein-Tyrosine Kinases Modifies the Kinase Function and Transforming Activity of Fujinami Sarcoma-Virus P130gag-Fps. Mol Cell Biol. 1986;6:4396–4408. doi: 10.1128/mcb.6.12.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waksman G, Shoelson SE, Pant N, Cowburn D, Kuriyan J. Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: crystal structures of the complexed and peptide-free forms. Cell. 1993;72:779–790. doi: 10.1016/0092-8674(93)90405-f. [DOI] [PubMed] [Google Scholar]
- 6.Liu BA, Engelmann BW, Nash PD. The language of SH2 domain interactions defines phosphotyrosine-mediated signal transduction. FEBS Lett. 2012;586:2597–2605. doi: 10.1016/j.febslet.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw JM, Mitaxov V, Waksman G. Investigation of phosphotyrosine recognition by the SH2 domain of the Src kinase. J Mol Biol. 1999;293:971–985. doi: 10.1006/jmbi.1999.3190. [DOI] [PubMed] [Google Scholar]
- 8.Groesch TD, Zhou F, Mattila S, Geahlen RL, Post CB. Structural basis for the requirement of two phosphotyrosine residues in signaling mediated by Syk tyrosine kinase. J Mol Biol. 2006;356:1222–1236. doi: 10.1016/j.jmb.2005.11.095. [DOI] [PubMed] [Google Scholar]
- 9.Sada K, Takano T, Yanagi S, Yamamura H. Structure and function of Syk protein-tyrosine kinase. J Biochem. 2001;130:177–186. doi: 10.1093/oxfordjournals.jbchem.a002970. [DOI] [PubMed] [Google Scholar]
- 10.Keshvara LM, Isaacson CC, Yankee TM, Sarac R, Harrison ML, Geahlen RL. Syk- and Lyn-dependent phosphorylation of Syk on multiple tyrosines following B cell activation includes a site that negatively regulates signaling. J Immunol. 1998;161:5276–5283. [PubMed] [Google Scholar]
- 11.Hong JJ, Yankee TM, Harrison ML, Geahlen RL. Regulation of signaling in B cells through the phosphorylation of Syk on linker region tyrosines - A mechanism for negative signaling by the Lyn tyrosine kinase. J Biol Chem. 2002;277:31703–31714. doi: 10.1074/jbc.M201362200. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Martin VA, Gorenstein NM, Geahlen RL, Post CB. Two closely spaced tyrosines regulate NFAT signaling in B cells via Syk association with Vav. Mol Cell Biol. 2011;31:2984–2996. doi: 10.1128/MCB.05043-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grucza RA, Bradshaw JM, Mitaxov V, Waksman G. Role of electrostatic interactions in SH2 domain recognition: salt-dependence of tyrosyl-phosphorylated peptide binding to the tandem SH2 domain of the Syk kinase and the single SH2 domain of the Src kinase. Biochemistry (Mosc) 2000;39:10072–10081. doi: 10.1021/bi000891n. [DOI] [PubMed] [Google Scholar]
- 14.Gabdoulline RR, Wade RC. Protein-protein association: investigation of factors influencing association rates by Brownian dynamics simulations. J Mol Biol. 2001;306:1139–1155. doi: 10.1006/jmbi.2000.4404. [DOI] [PubMed] [Google Scholar]
- 15.Alsallaq R, Zhou HX. Electrostatic rate enhancement and transient complex of protein-protein association. Proteins. 2008;71:320–335. doi: 10.1002/prot.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schreiber G, Haran G, Zhou H-X. Fundamental aspects of protein-protein association kinetics. Chem Rev. 2009;109:839–860. doi: 10.1021/cr800373w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou HX. Rate theories for biologists. Q Rev Biophys. 2010;43:219–293. doi: 10.1017/S0033583510000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin S, Pang XD, Zhou HX. Automated Prediction of Protein Association Rate Constants. Structure. 2011;19:1744–1751. doi: 10.1016/j.str.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang XD, Qin SB, Zhou HX. Rationalizing 5000-fold differences in receptor-binding rate constants of four cytokines. Biophys J. 2011;101:1175–1183. doi: 10.1016/j.bpj.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant BJ, Gheorghe DM, Zheng W, Alonso M, Huber G, Dlugosz M, McCammon JA, Cross RA. Electrostatically biased binding of kinesin to microtubules. PLoS Biol. 2011;9:e1001207. doi: 10.1371/journal.pbio.1001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang XD, Zhou KH, Qin SB, Zhou HX. Prediction and dissection of widely-varying association rate constants of actin-binding proteins. PLoS Comput Biol. 2012;8 doi: 10.1371/journal.pcbi.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou HX, Wlodek ST, McCammon JA. Conformation gating as a mechanism for enzyme specificity. Proc Natl Acad Sci U S A. 1998;95:9280–9283. doi: 10.1073/pnas.95.16.9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou HX, Bates PA. Modeling protein association mechanisms and kinetics. Curr Opin Struct Biol. 2013 doi: 10.1016/j.sbi.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang XD, Zhou KH, Qin SB, Zhou HX. Prediction and Dissection of Widely-Varying Association Rate Constants of Actin-Binding Proteins. PLoS Comp Biol. 2012;8 doi: 10.1371/journal.pcbi.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladbury JE, Lemmon MA, Zhou M, Green J, Botfield MC, Schlessinger J. Measurement of the binding of tyrosyl phosphopeptides to SH2 domains: a reappraisal. Proc Natl Acad Sci U S A. 1995;92:3199–3203. doi: 10.1073/pnas.92.8.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CH, Piraner D, Gorenstein NM, Geahlen RL, Post CB. Differential recognition of syk-binding sites by each of the two phosphotyrosine-binding pockets of the Vav SH2 domain. Biopolymers. 2013;99:897–907. doi: 10.1002/bip.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pascal SM, Singer AU, Gish G, Yamazaki T, Shoelson SE, Pawson T, Kay LE, Forman-Kay JD. Nuclear magnetic resonance structure of an SH2 domain of phospholipase C-gamma 1 complexed with a high affinity binding peptide. Cell. 1994;77:461–472. doi: 10.1016/0092-8674(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhou HX, Pang X, Lu C. Rate constants and mechanisms of intrinsically disordered proteins binding to structured targets. Physical chemistry chemical physics : PCCP. 2012;14:10466–10476. doi: 10.1039/c2cp41196b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemsath L, Dvorsky R, Fiegen D, Carlier MF, Ahmadian MR. An electrostatic steering mechanism of Cdc42 recognition by Wiskott-Aldrich syndrome proteins. Mol Cell. 2005;20:313–324. doi: 10.1016/j.molcel.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad M, Gu W, Helms V. Mechanism of fast peptide recognition by SH3 domains. Angew Chem Int Ed Engl. 2008;47:7626–7630. doi: 10.1002/anie.200801856. [DOI] [PubMed] [Google Scholar]
- 31.Zhou HX. Kinetics of diffusion-influenced reactions studied by Brownian dynamics. J Phys Chem. 1990;94:8794–8800. [Google Scholar]
- 32.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc Natl Acad Sci U S A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C. Comparison of multiple amber force fields and development of improved protein backbone parameters. Proteins. 2006;65:712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homeyer N, Horn AH, Lanig H, Sticht H. AMBER force-field parameters for phosphorylated amino acids in different protonation states: phosphoserine, phosphothreonine, phosphotyrosine, and phosphohistidine. J Mol Model. 2006;12:281–289. doi: 10.1007/s00894-005-0028-4. [DOI] [PubMed] [Google Scholar]
- 35.Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen M-y, Pieper U, Sali A. Comparative protein structure modeling using MODELLER. Curr Protoc Protein Sc. 2007;Chap 2(Unit 2.9) doi: 10.1002/0471140864.ps0209s50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.