Abstract
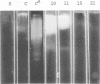
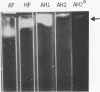
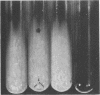
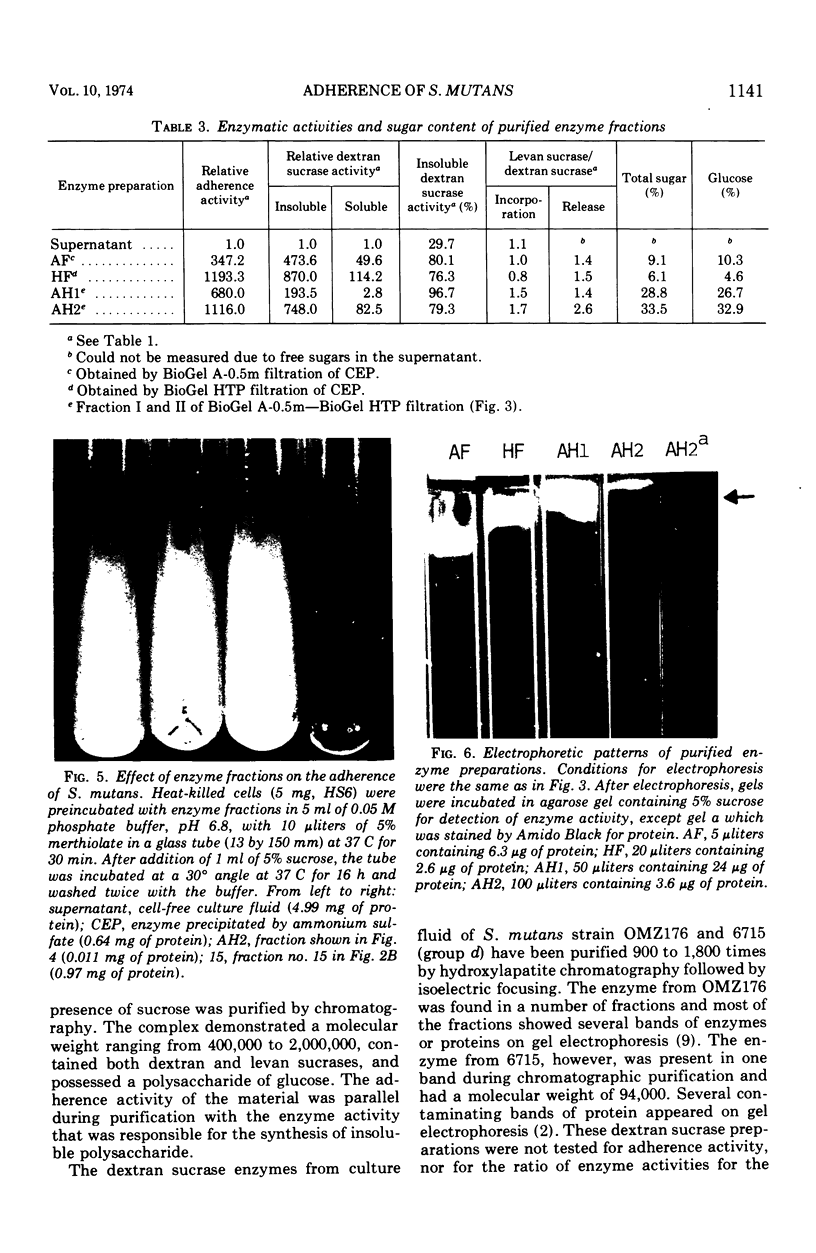
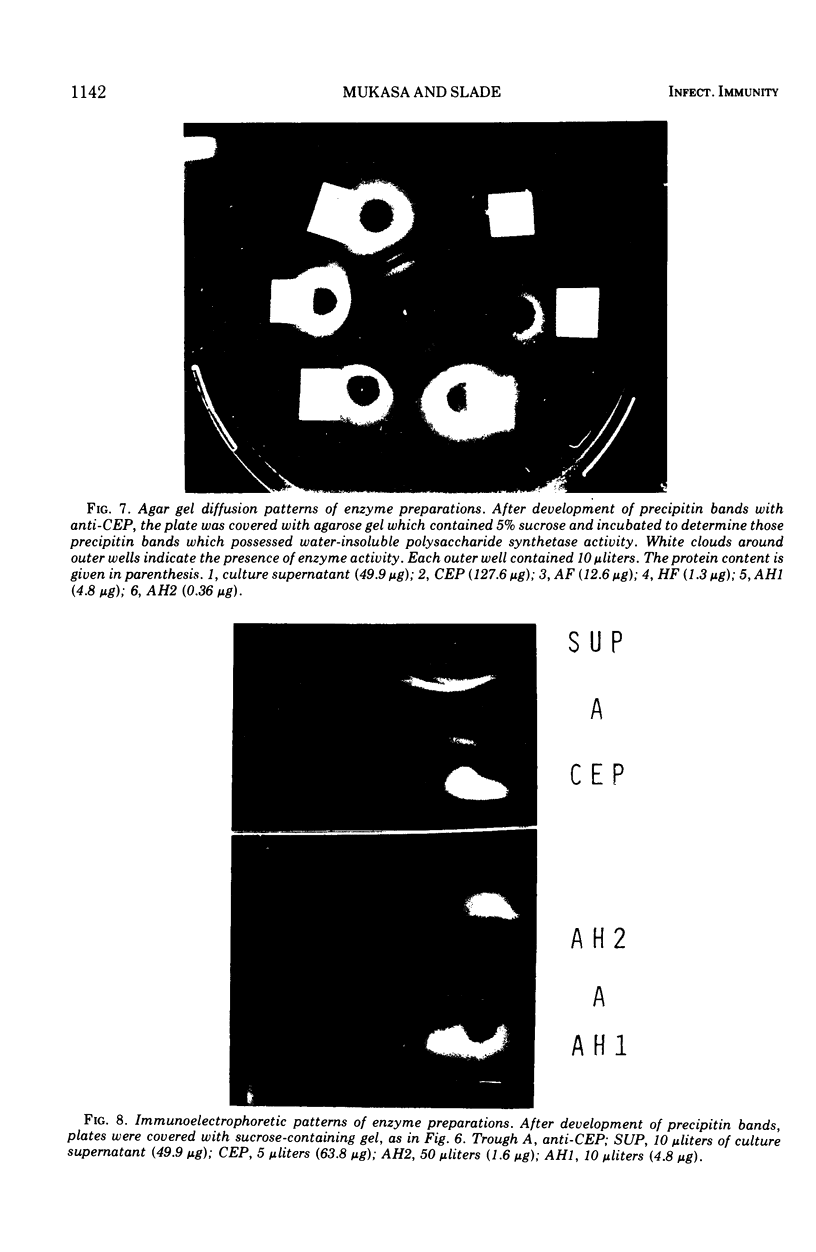
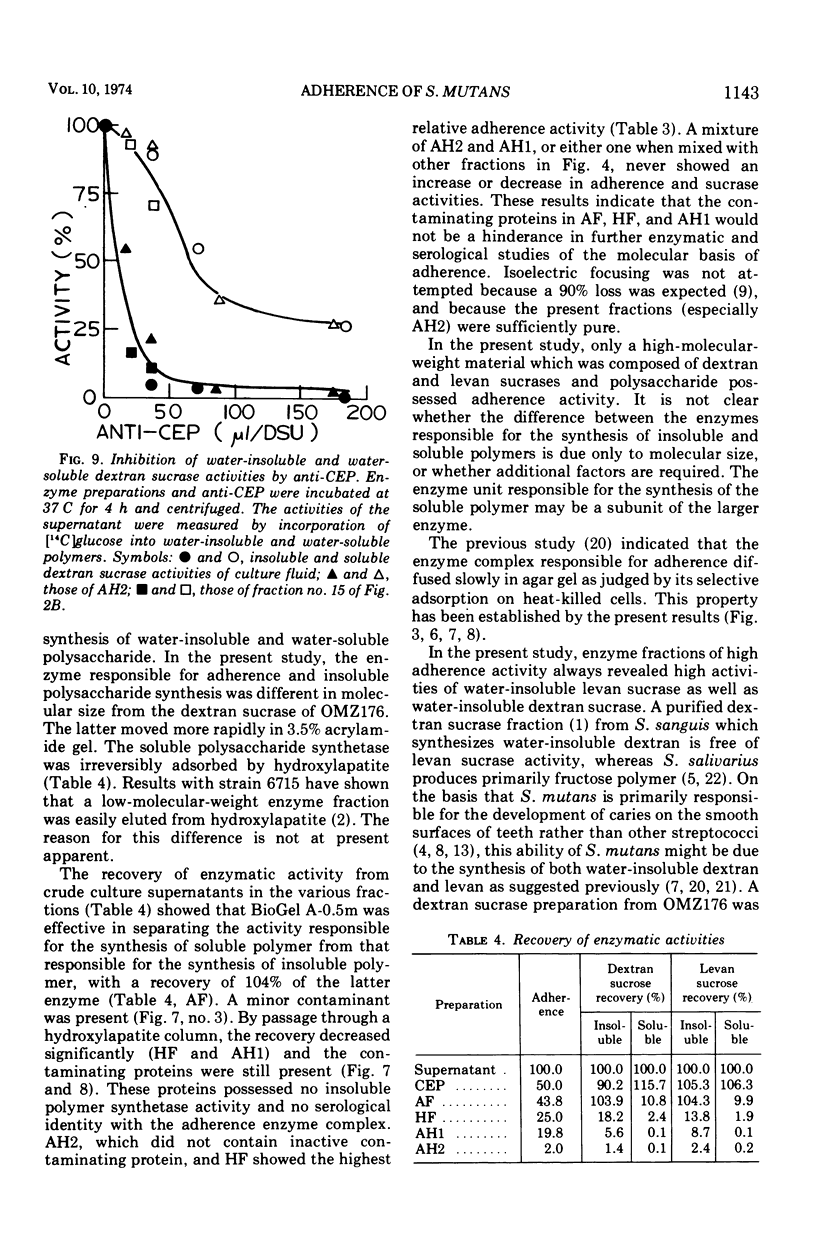
Enzymes which possess the ability to cause the adherence of Streptococcus mutans cells to a smooth glass surface were purified 1,100 times by chromatography on agarose gel followed by hydroxylapatite gel. During the purification procedures, the enzymes from strain HS6 (group a) were examined for the synthesis of water-soluble and water-insoluble polysaccharide and the ability to produce adherence. The enzyme preparations producing adherence of the S. mutans cells in the presence of sucrose possessed a molecular size of about 400,000 to 2,000,000 and were composed of approximately equivalent amounts of dextran and levan sucrases and 5 to 30% polysaccharide. The most highly purified preparation contained a negligible amount of contaminating protein as judged by polyacrylamide gel electrophoresis, immunoelectrophoresis, and gel diffusion. In these three tests, the location of the enzyme responsible for the synthesis of insoluble polymer was detected by embedding or covering the enzyme-containing gel with a layer of sucrose-containing agarose gel and observing the formation of insoluble polymer. During purification the ability of all fractions to produce adherence was parallel with the enzyme activity responsible for the synthesis of insoluble polysaccharide from sucrose. About two-thirds of the sucrase enzyme complex in the S. mutans culture fluid synthesized water-soluble polymer. This complex, obtained by filtration through agarose gel, was smaller in molecular size, lower in sugar content, and did not produce adherence, in contrast to the enzyme complex which possessed adherence activity. The inhibition of the enzyme complex synthesizing soluble polymer required more anti-synthetase serum than that required to inhibit the synthesis of water-insoluble polymer. It is not known whether the lack of adherence activity in this enzyme was due to its smaller size and lower sugar content or the absence of unknown factors which are essential for its activity. The carbohydrate in these enzyme preparations, composed of glucose, may represent a primer molecule and/or a remnant of the polymer synthesized by the enzyme. The enzyme activity was not inhibited by anti-dextran globulin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAD G., FEINGOLD D. S., HESTRIN S. The mechanism of polysaccharide production from sucrose. 3. Donor-acceptor specificity of levansucrase from Aerobacter levanicum. Biochem J. 1956 Oct;64(2):340–351. doi: 10.1042/bj0640340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Newbrun E., Krasse B. Purification and properties of dextransucrase from Streptococcus sanguis. Arch Oral Biol. 1969 May;14(5):469–478. doi: 10.1016/0003-9969(69)90140-x. [DOI] [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Garszczynski S. M., Edwards J. R. Synthesis of a broth levan by a cell-bound levansucrase from Streptococcus salivarius (SS2). Arch Oral Biol. 1973 Feb;18(2):239–251. doi: 10.1016/0003-9969(73)90144-1. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Presence of an invertase-like enzyme and a sucrose permeation system in strains of Streptococcus mutans. Caries Res. 1972;6(2):122–131. doi: 10.1159/000259784. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- KOEPSELL H. J., TSUCHIYA H. M. Enzymatic synthesis of dextran. J Bacteriol. 1952 Feb;63(2):293–295. doi: 10.1128/jb.63.2.293-295.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasse B., Carlsson J. Various types of streptococci and experimental caries in hamsters. Arch Oral Biol. 1970 Jan;15(1):25–32. doi: 10.1016/0003-9969(70)90142-1. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Adherence of Streptococcus mutans to dextran synthesized in the presence of extracellular dextransucrase. Infect Immun. 1974 Apr;9(4):764–765. doi: 10.1128/iai.9.4.764-765.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of cell-associated dextransucrase activity from glucose-grown cells of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):227–235. doi: 10.1128/iai.10.1.227-235.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuno T., Slade H. D. Composition and properties of a group A streptococcal teichoic acid. J Bacteriol. 1970 Jun;102(3):747–752. doi: 10.1128/jb.102.3.747-752.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno T., Slade H. D. Group a streptococcal polysaccharide antigens. Infect Immun. 1971 Mar;3(3):385–389. doi: 10.1128/iai.3.3.385-389.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E., Cowman R. A. Invertase activity in Streptococcus mutans and Streptococcus sanguis. Arch Oral Biol. 1973 Apr;18(4):525–531. doi: 10.1016/0003-9969(73)90073-3. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Chemical composition and immunological specificity of the streptococcal group O cell wall polysaccharide antigen. Infect Immun. 1972 May;5(5):707–714. doi: 10.1128/iai.5.5.707-714.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Structure and immunological specificity of the Streptococcus mutans group b cell wall antigen. Infect Immun. 1973 Apr;7(4):578–585. doi: 10.1128/iai.7.4.578-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbrun E., Carlsson J. Reaction rate of dextransucrase from Streptococcus sanguis in the presence of various compounds. Arch Oral Biol. 1969 May;14(5):461–468. doi: 10.1016/0003-9969(69)90139-3. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Loken A. E., Schmitt M. K. Use of specifically labeled sucrose for comparison of extracellular glucan and fructan metabolism by oral streptococci. Infect Immun. 1972 Feb;5(2):263–266. doi: 10.1128/iai.5.2.263-266.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprey P., Slade H. D. Immunochemistry of the streptococcal group R cell wall polysaccharide antigen. Infect Immun. 1972 Jan;5(1):91–97. doi: 10.1128/iai.5.1.91-97.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]