Abstract
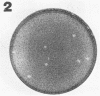
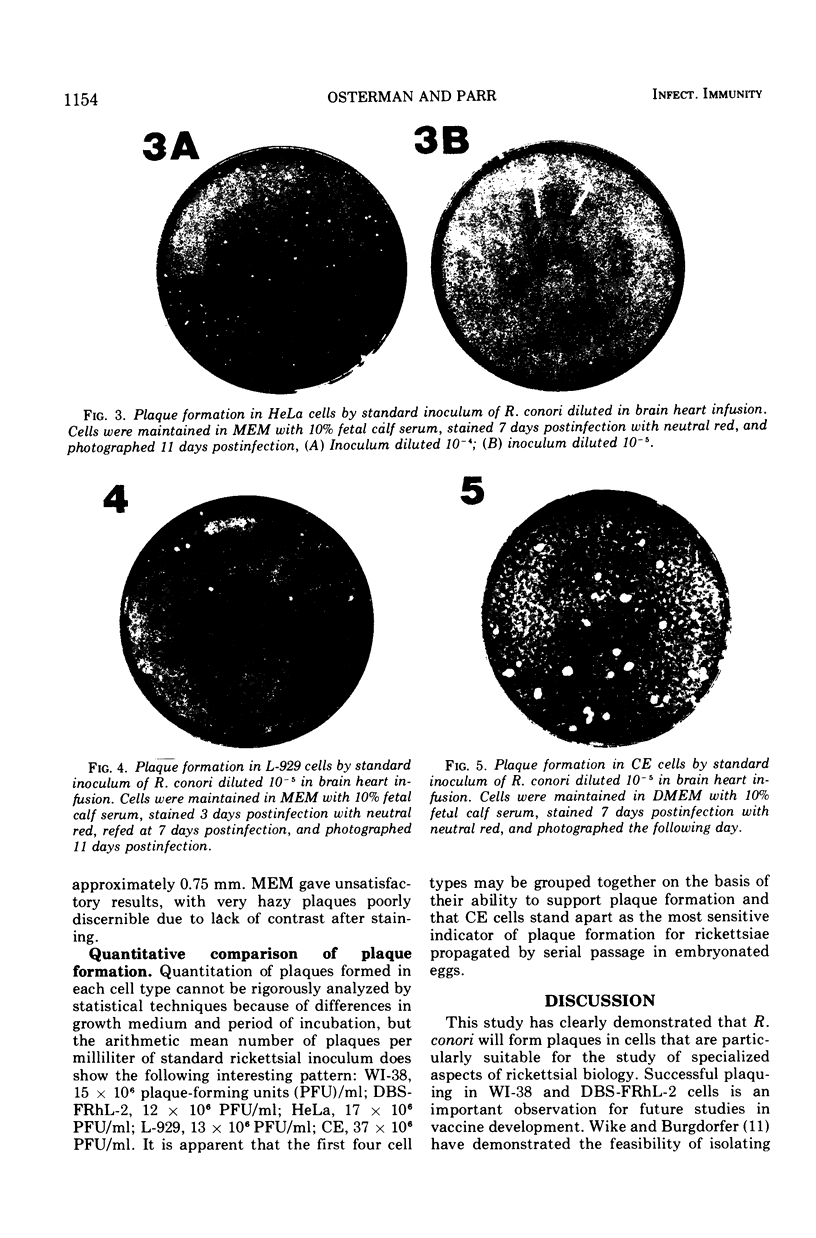
Mammalian cells particularly suitable for the study of specialized aspects of rickettsial biology were tested for their ability to support plaque formation by Rickettsia conori. The detection of plaques was substantially influenced by the combination of growth medium and cell type used. Large plaques (2.0 to 3.0 mm in diameter) occurred by 8 days postinfection in WI-38 and DBS-FRhL-2 cells supported by medium 199. Smaller plaques (0.5 to 1.0 mm in diameter) were seen in L-929 and HeLa cells at 8 to 11 days postinfection and were more discernible in cells supported with Eagle minimal essential medium. Chicken embryo cells maintained in Dulbecco's modified Eagle medium exhibited large spherical plaques with a diameter of approximately 1.5 mm by 8 days postinfection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E. S., Penman S. Regulation of protein synthesis in mammalian cells. V. Further studies on the effect of actinomycin D on translation control in HeLa cells. J Mol Biol. 1973 Oct 25;80(2):243–254. doi: 10.1016/0022-2836(73)90170-8. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Kordová N. Plaque assay of rickettsiae. Acta Virol. 1966 May;10(3):278–278. [PubMed] [Google Scholar]
- Marcovici M., Prier J. E. Enhancement of St. Louis arbovirus plaque formation by neutral red. J Virol. 1968 Mar;2(3):178–181. doi: 10.1128/jvi.2.3.178-181.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Petricciani J. C., Hopps H. E., Lorenz D. E. Subhuman primate diploid cells: possible substrates for production of virus vaccines. Science. 1971 Dec 3;174(4013):1025–1027. doi: 10.1126/science.174.4013.1025. [DOI] [PubMed] [Google Scholar]
- Weinberg E. H., Stakebake J. R., Gerone P. J. Plaque assay for Rickettsia rickettsii. J Bacteriol. 1969 May;98(2):398–402. doi: 10.1128/jb.98.2.398-402.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Newman L. W., Grays R., Green A. E. Metabolism of Rickettsia typhi and Rickettsia akari in irradiated L cells. Infect Immun. 1972 Jul;6(1):50–57. doi: 10.1128/iai.6.1.50-57.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Burgdorfer W. Plaque formation in tissue cultures by Rickettsia rickettsi isolated directly from whole blood and tick hemolymph. Infect Immun. 1972 Nov;6(5):736–738. doi: 10.1128/iai.6.5.736-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Ormsbee R. A., Tallent G., Peacock M. G. Effects of various suspending media on plaque formation by rickettsiae in tissue culture. Infect Immun. 1972 Oct;6(4):550–556. doi: 10.1128/iai.6.4.550-556.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Tallent G., Peacock M. G., Ormsbee R. A. Studies of the rickettsial plaque assay technique. Infect Immun. 1972 May;5(5):715–722. doi: 10.1128/iai.5.5.715-722.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]