The exocyst has been speculated to mediate the tethering of secretory vesicles to the plasma membrane. However, there has been no direct experimental evidence for this notion. An ectopic targeting strategy is used to provide experimental support for this model and investigate the regulators of exocyst assembly and vesicle targeting.
Abstract
During membrane trafficking, vesicular carriers are transported and tethered to their cognate acceptor compartments before soluble N-ethylmaleimide–sensitive factor attachment protein (SNARE)-mediated membrane fusion. The exocyst complex was believed to target and tether post-Golgi secretory vesicles to the plasma membrane during exocytosis. However, no definitive experimental evidence is available to support this notion. We developed an ectopic targeting assay in yeast in which each of the eight exocyst subunits was expressed on the surface of mitochondria. We find that most of the exocyst subunits were able to recruit the other members of the complex there, and mistargeting of the exocyst led to secretion defects in cells. On the other hand, only the ectopically located Sec3p subunit is capable of recruiting secretory vesicles to mitochondria. Our assay also suggests that both cytosolic diffusion and cytoskeleton-based transport mediate the recruitment of exocyst subunits and secretory vesicles during exocytosis. In addition, the Rab GTPase Sec4p and its guanine nucleotide exchange factor Sec2p regulate the assembly of the exocyst complex. Our study helps to establish the role of the exocyst subunits in tethering and allows the investigation of the mechanisms that regulate vesicle tethering during exocytosis.
INTRODUCTION
Exocytosis is essential for many basic biological processes, such as neurotransmission, epithelia formation, and cell migration. During the late stages of exocytosis, secretory vesicles are believed to be tethered to the plasma membrane before soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE)–mediated fusion. The exocyst complex, consisting of Sec3p, Sec5p, Sec6p, Sec8p, Sec10p, Sec15p, Exo70p, and Exo84p, is essential for exocytosis. The exocyst was first discovered by genetic and biochemical approaches in the budding yeast Saccharomyces cerevisiae and later found to be evolutionarily conserved in mammals (Novick et al., 1980; TerBush and Novick, 1995; TerBush et al., 1996; Ting et al., 1995; Hsu et al., 1996; Guo et al., 1999a). It was proposed more than a decade ago that the exocyst complex functions to target or tether post-Golgi secretory vesicles to the plasma membrane (Pfeffer, 1999; Waters and Pfeffer, 1999; Guo et al., 2000; He and Guo, 2009). However, the role of the exocyst in vesicle tethering has not been experimentally demonstrated. The current model of exocyst function in tethering is mostly based on the established protein–protein and protein–phospholipid interactions (Guo et al., 1999b; Matern et al., 2001; Boyd et al., 2004; He et al., 2007a; Liu et al., 2007; Zhang et al., 2008; Shen et al., 2013) and yeast mutation analyses (Finger and Novick, 1997; Finger et al., 1998; Wiederkehr et al., 2003). Despite numerous studies, the dynamic process of vesicle tethering remains elusive. A major reason that vesicle tethering at the plasma membrane is difficult to observe is that once the vesicles are tethered, they promptly fuse with the plasma membrane. Although membrane fusion can be blocked using the SNARE mutants, the vesicles frequently accumulate in the cytoplasm rather than being attached to the plasma membrane (Novick et al., 1980; Brennwald et al., 1994; Grote et al., 2000; Jantti et al., 2002). Moreover, the role of the exocyst is difficult to dissect or interpret in these mutant backgrounds due to the genetic complexity of multiple mutations. New assays are therefore called for to overcome these limitations.
A more definitive test for the role of a protein(s) in secretory vesicle targeting is to ectopically position the protein(s) to another location and examine whether the vesicles follow the protein(s) to that site. This principle has been followed in the past to establish the targeting mechanisms for a number of intracellular organelles such as nucleus and mitochondria. In this study, we developed an ectopic targeting assay in yeast and examined the role of the exocyst and its regulators in vesicle targeting and tethering.
RESULTS
Ectopic targeting of the exocyst subunits
The mitochondrial protein Tom20p associates with the outer membrane of mitochondria through its N-terminal transmembrane segment and exposes its C-terminus to the cytosol (Yamamoto et al., 2011). We engineered a Tom20-mCherry fusion in yeast expression vectors (Figure 1A). The Tom20-mCherry fusion protein was correctly targeted to mitochondria as indicated by its colocalization with a GFP-tagged mitochondria marker protein, Cit1p (Figure 1B). Using this vector, we cloned each of the eight exocyst subunits 3′ to the Tom20-mCherry fusion. When expressed in yeast, all of the exocyst fusion proteins were ectopically targeted to mitochondria, as shown by their colocalization with Cit1-GFP (Figure 1B and Supplemental Figure S1). We noticed that mitochondria tended to cluster in cells expressing Tom20-mCherry-exocyst subunits, which will be discussed later.
FIGURE 1:
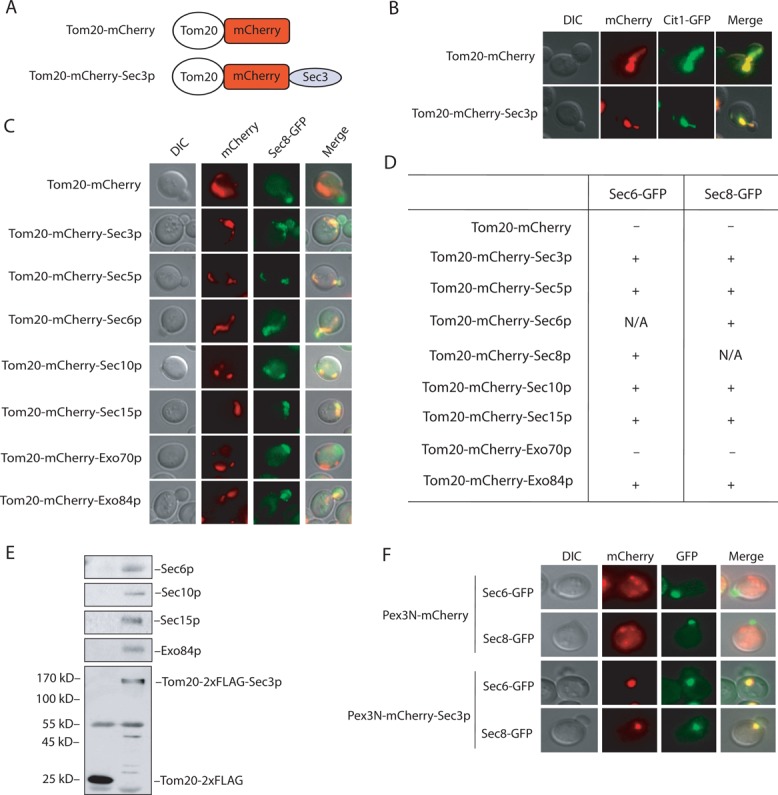
Ectopic targeting of the exocyst to mitochondria and peroxisomes. (A) The Tom20-mCherry and Tom20-mCherry-Sec3p fusions. See Materials and Methods for the construction of the plasmids. (B) Colocalization of Tom20-mCherry and Tom20-mCherry-Sec3p with Cit1-GFP, a marker protein for yeast mitochondria. (C) Sec8-GFP is recruited to mitochondria in cells expressing Tom20-mCherry–tagged exocyst subunits Sec3p, Sec5p, Sec6p, Sec10p, Sec15p, and Exo84p but not Exo70p. (D) Summary of Sec6-GFP and Sec8-GFP localization in cells expressing Tom20-mCherry–tagged exocyst subunits. +, located to mitochondria; –, not located to mitochondria; N/A, not applicable. (E) The exocyst complex is assembled at mitochondria in cells expressing Tom20-2xFLAG-Sec3p. Cells expressing Tom20-2xFLAG was used as a negative control. The exocyst complex was immunoprecipitated using the anti-FLAG antibody and the exocyst subunits (Sec6p, Sec10p, Sec15p, and Exo84p) coimmunoprecipitated with Tom20-2xFLAG-Sec3p. Molecular weights (in kilodaltons) are indicated to the left. (F) The exocyst can be ectopically targeted to peroxisomes. Sec6-GFP and Sec8-GFP were targeted to peroxisomes in cells expressing Pex3N-mCherry-Sec3p but not Pex3N-mCherry.
Using these constructs, we first examined the ability of each exocyst component to recruit other subunits of the complex to mitochondria. The yeast strain containing Sec8-GFP was first generated by chromosomal integration to the SEC8 locus. Plasmids containing Tom20-mCherry-exocyst fusions were individually transformed into the Sec8–green fluorescent protein (GFP)–expressing cells. Except for Tom20-mCherry-Exo70p, Tom20-mCherry tagged Sec3p, Sec5p, Sec6p, Sec10p, Sec15p, and Exo84p recruited Sec8-GFP to mitochondria. As a negative control, Sec8-GFP remained polarized to the bud tip in cells expressing Tom20-mCherry alone (Figure 1C). Besides Sec8-GFP, the same pattern was observed for the recruitment of Sec6-GFP (Figure 1D). It is not clear why Tom20-mCherry-Exo70p failed to recruit the other subunits, although its expression level was similar to those of the other fusion proteins (unpublished data). This issue will be discussed later.
Sec3p is localized to the bud tip, which is the site of active exocytosis and membrane expansion in yeast and was proposed to serve as a “landmark” protein (Finger et al., 1998; Boyd et al., 2004). Moreover, Sec3p interacts directly with phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) and the Rho family of small GTPases at the plasma membrane (Guo et al., 2001; Zhang et al., 2001, 2008; Baek et al., 2010). We therefore focused our experiments on ectopically targeted Sec3p in the following studies. In addition to microscopy, the ability of the ectopically expressed Sec3p to recruit other exocyst subunits to mitochondria was further tested biochemically. As shown in Figure 1E, Sec6p, Sec10p, Sec15p, and Exo84p were coimmunoprecipitated with Tom20-2xFLAG-tagged Sec3p using the anti-FLAG antibody. As a negative control, none of the exocyst components were coimmunoprecipitated with Tom20-2xFLAG. We also tested whether other subunits of the exocyst were capable of recruiting Sec3p. We found that all of the ectopically expressed exocyst subunits, except for Exo70p, recruited Sec3-GFP to mitochondria (Supplemental Figure S2).
To exclude the possibility that ectopic targeting of Sec3p was due to factors specific to mitochondria, we used peroxisomes as a targeting organelle. A Pex3N-mCherry vector, which allows the targeting of proteins to peroxisomes, was constructed. As shown in Figure 1F, Sec6-GFP and Sec8-GFP were targeted to peroxisomes in cells carrying Pex3N-mCherry-Sec3p but not for cells expressing Pex3N-mCherry. Taken together, our data show that the ectopic targeting system allows the assembly of the exocyst complex to surrogate organelles, and Sec3p is able to recruit the other exocyst subunits to these compartments.
Mistargeting of the exocyst results in defects in cell growth and secretion
The exocyst has been localized to the bud tip in yeast (TerBush and Novick, 1995; Finger et al., 1998; Guo et al., 1999a; Zajac et al., 2005). Using our newly developed assay, we tested whether mistargeting of the exocyst to mitochondria affected cell growth and exocytosis. First, growth of cells carrying different Tom20-mCherry-exocyst subunits was examined on plates using the standard yeast colony assay. As shown in Figure 2A, except for Exo70p and the Tom20-mCherry vector control, growth was inhibited in cells expressing the Tom20-mCherry-exocyst subunits. The growth defects of these cells could be rescued by the overexpression of SEC1 (encodes a SNARE regulator) and SEC4 (encodes the Rab small GTPase) using 2μ plasmids (Figure 2B). Because SEC1 and SEC4 have often been used to rescue secretion defects of the exocyst mutants, our results suggest that secretion in the exocyst mistargeted cells was affected. Next, we examined the secretion of 1,3-β-glucosyltransferase (Bgl2p), a cell wall–remodeling enzyme commonly used to assay for yeast exocytosis. As shown in Figure 2C, Bgl2p secretion was defective in cells carrying the Tom20-mCherry-exocyst plasmids (except for Exo70p). Similar to Bgl2p, secretion of another cargo protein, invertase, was also inhibited in these cells (Figure 2D).
FIGURE 2:
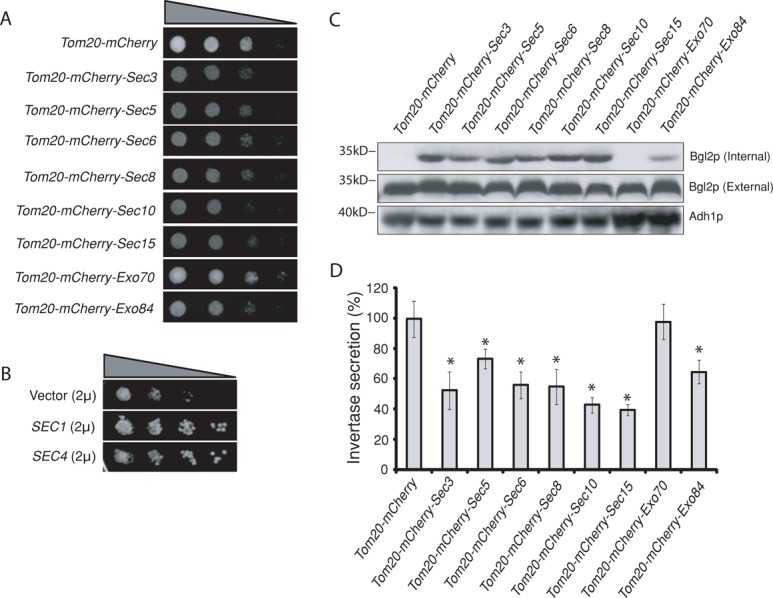
Ectopic targeting of the exocyst to mitochondria led to secretion defects. (A) Growth of cells expressing Tom20-mCherry–tagged exocyst subunits. The same amount of yeast cells was serially diluted at 1:10 ratio and then spotted on SC plates at 25°C. Except for Tom20-mCherry-Exo70, yeast cells expressing Tom20-mCherry–tagged exocyst subunits grew more slowly than control cells expressing Tom20-mCherry. (B) The growth defect of Tom20-mCherry-Sec3 cells can be suppressed by high-copy 2μ plasmids expressing either SEC1 or SEC4 gene. (C) Bgl2p secretion in cells expressing Tom20-mCherry–tagged exocyst subunits. Except for Tom20-mCherry-Exo70, yeast cells expressing Tom20-mCherry–tagged exocyst subunits showed accumulation of Bgl2p in cells (Internal) detected by Western blotting. Molecular weights (in kilodaltons) are indicated to the left. Alcohol dehydrogenase (Adh1p) was used as a loading control. (D) Except for Tom20-mCherry-Exo70, yeast cells expressing Tom20-mCherry–tagged exocyst subunits were defective in invertase secretion at 25°C. The percentage of external (secreted) relative to the total invertase is presented. Error bars represent SD (n = 3). *p < 0.01 as compared with cells expressing Tom20-mCherry.
The C-terminus of Sec3p mediates exocyst targeting
Sec3p contains two functional domains. Its N-terminus (amino acids 1–320) interacts with the Rho family of GTPases and PI(4,5)P2, which mediates the polarized localization of Sec3p at the plasma membrane (Guo et al., 2001; Zhang et al., 2001, 2008; Baek et al., 2010). The C-terminal region (amino acids 321–1336) of Sec3p binds to the exocyst subunit Sec5p (Guo et al., 2001). We fused the two domains of Sec3p to Tom20-mCherry (Tom20-mCherry-Sec3N and Tom20-mCherry-Sec3C) and expressed them in cells. Sec6-GFP and Sec8-GFP were recruited to mitochondria in cells carrying Tom20-mCherry-Sec3C but not in cells carrying Tom20-mCherry-Sec3N (Figure 3A). Cells expressing Tom20-mCherry-Sec3N grew similarly to those containing the control plasmid, whereas cells carrying Tom20-mCherry-Sec3C were defective in growth, similar to cells expressing Tom20-mCherry-Sec3p (Figure 3B). Consistent with the growth phenotype, Bgl2p secretion was also defective in these cells (Figure 3C). This result suggests that Sec3 C-terminal region recruits the exocyst subunits to mitochondria for exocyst assembly, which is consistent with the previous Sec3p domain mapping results (Guo et al., 2001; Zhang et al., 2008).
FIGURE 3:
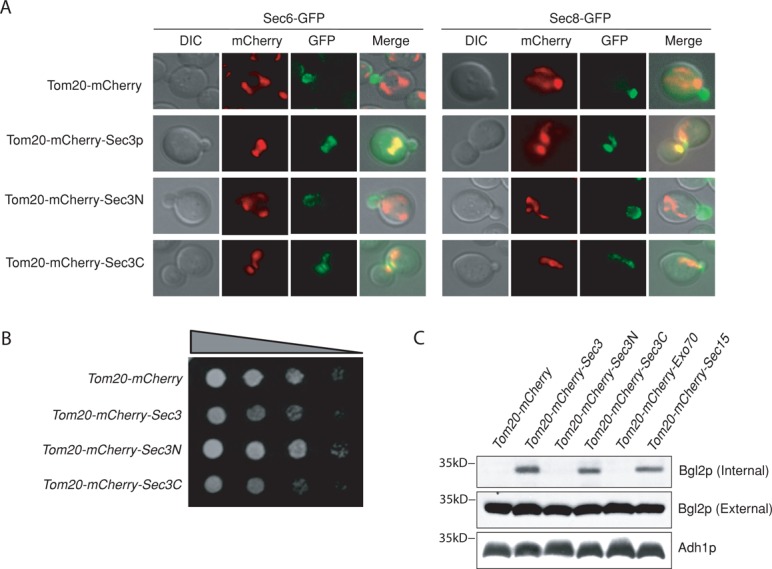
Ectopic expression of Sec3p is required for exocyst targeting and assembly at mitochondria. (A) Sec6-GFP and Sec8-GFP can be recruited to mitochondria in cells expressing Tom20-mCherry tagged Sec3C (amino acids [aa] 321–1336) but not Sec3N (aa 1–320). (B) Cells expressing Tom20-mCherry-Sec3p or -Sec3C grew more slowly than cells expressing Tom20-mCherry-Sec3N on a synthetic complete plate at 25°C. (C) Cells expressing Tom20-mCherry-Sec3 or -Sec3C showed accumulation of Bgl2p inside the cells as detected by Western blotting. Adh1p was used as a loading control. Molecular weights (in kilodaltons) are indicated to the left.
Tom20-mCherry-Sec3p recruits secretory vesicles to the mitochondria
Using the ectopic targeting assay, we examined whether secretory vesicles were recruited to mitochondria in cells expressing Tom20-mCherry-Sec3p. We first examined vesicles by immunofluorescence using the Rab protein Sec4p as a marker. In the Tom20-mCherry-Sec3p cells, while some Sec4p remained detectable at the bud, a portion of Sec4p colocalized with Tom20-mCherry-Sec3p at the mitochondria in 35.1% of the cells (n = 105; Figure 4A, left). We noted that not all of the Sec4p was recruited to mitochondria, which will be discussed later. As a control, all Sec4p proteins were localized to the bud in cells expressing Tom20-mCherry (>95% of the cells, n = 100). The secretory vesicles were also examined by following the cargo protein, Bgl2p, using immunofluorescence microscopy (Figure 4B, left). There was very little Blg2p detected inside the cells expressing Tom20-mCherry, consistent with the observation that Tom20-mCherry alone does not block secretion in cells shown earlier. In cells expressing Tom20-mCherry-Sec3p, however, Bgl2p was detectable at the mitochondria in 88.5% of the cells (n = 139). We also examined the localization of chitin synthase III (Chs3p), a protein required for cell wall remodeling at the mother–daughter junction. It was previously shown that Chs3-GFP was localized to mother–bud junction and some endosomal compartments (Chuang and Schekman, 1996), and the functional exocyst complex is required for its proper localization (Zanolari et al., 2011). As shown in Figure 4C, portions of Chs3-GFP were recruited to mitochondria in 89.7% of the cells (n = 108) expressing Tom20-mCherry-Sec3p, suggesting that Sec3p mediates the targeting of secretory vesicles. The fluorescence microscopy result was further supported by biochemistry experiments in which Sec4p, Bgl2p, and Chs3p were found to associate with mitochondria purified from yeast cells expressing Tom20-mCherry-Sec3p but not cells expressing Tom20-mCherry (Supplemental Figure S3).
FIGURE 4:
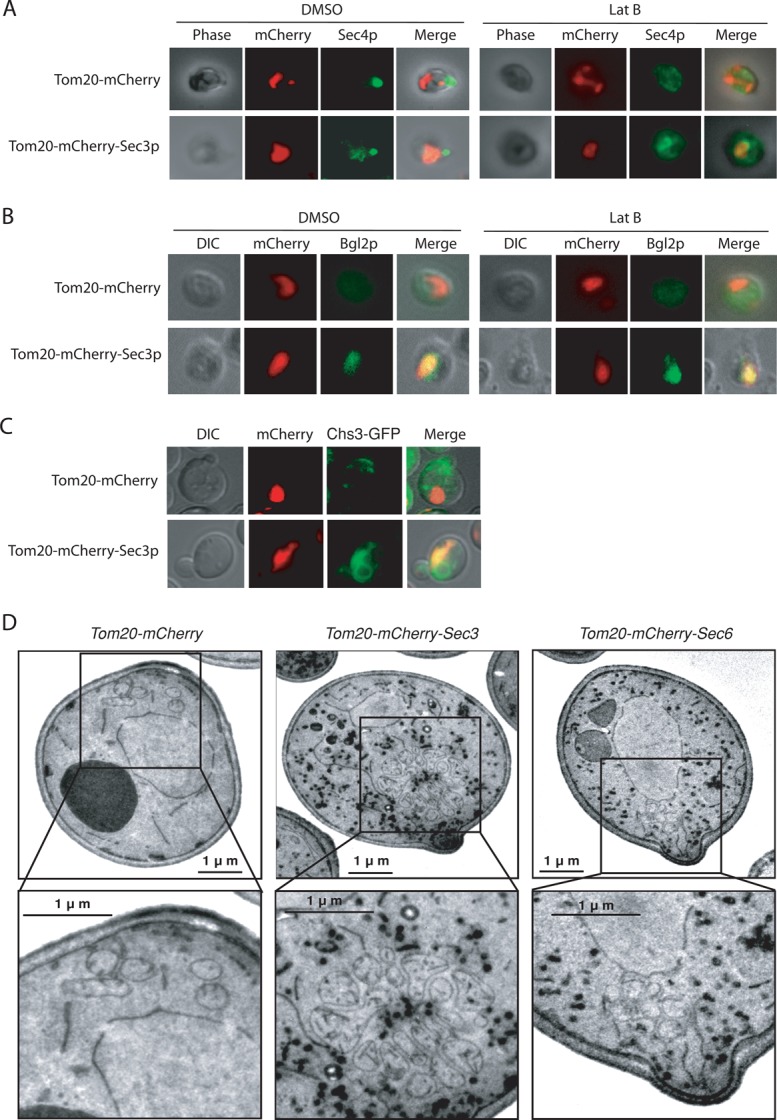
Ectopic recruitment of secretory vesicles to mitochondria. (A) Sec4p was examined by immunofluorescence microscopy using a rabbit polyclonal antibody. In the presence of DMSO, Sec4p was partially recruited to mitochondria in cells expressing Tom20-mCherry-Sec3p, with the rest localized to the bud. After latrunculin B treatment, however, Sec4p signal in the bud diminished. Concomitantly, significantly more Sec4p associated with mitochondria. As a control, in cells expressing Tom20-mCherry, Sec4p was localized to the bud in the presence of DMSO. After treatment with latrunculin B, Sec4p dispersed in the cytosol. (B) Bgl2p was detected by immunofluorescence microscopy using a rabbit polyclonal antibody. In cells expressing Tom20-mCherry, there was very little Blg2p accumulation in the presence of DMSO. After latrunculin B treatment, Bgl2p fluorescence became more detectable and was dispersed in the cytoplasm rather than associated with mitochondria. In cells expressing Tom20-mCherry-Sec3p, however, Bgl2p was detectable at the mitochondria in the presence of DMSO. After latrunculin B treatment, brighter Bgl2p signal was detected at mitochondria. (C) Chs3-GFP was recruited to mitochondria in cells expressing Tom20-mCherry-Sec3p but not in cells expressing Tom20-mCherry. (D) Thin-section electron microscopy analysis of cells expressing Tom20-mCherry, Tom20-mCherry-Sec3p, or Tom20-mCherry-Sec6p. Secretory vesicles (shown in dark staining) associated with mitochondria clustered in cells expressing Tom20-mCherry-Sec3p but not in cells expressing Tom20-mCherry or Tom20-mCherry-Sec6p. The selected areas are magnified and shown at the bottom. There was no vesicle accumulation in cells expressing Tom20-mCherry. Cells were fixed with permanganate, and the vesicles are shown in dark staining. Scale bar, 1 μm.
To further verify that secretory vesicles are tethered to the mitochondria in cells expressing Tom20-mCherry-Sec3p, thin-section electron microscopy was performed using protocols described previously (Perkins and McCaffery, 2007; Luo et al., 2013). In cells carrying Tom20-mCherry vector alone, vesicles were barely detectable at mitochondria. In cells expressing Tom20-mCherry-Sec3p, we observed attachment of vesicles of diameter 80–100 nm (the typical size of post-Golgi secretory vesicles) to mitochondria in 61.2% of the cells (n = 103; Figure 4D). The secretory vesicles and mitochondria do not appear to be perfectly colocalized because of their different sizes and organization (Figure 4D). However, the association of the two membrane entities is obvious. As a control, only 8.9% of the cells expressing Tom20-mCherry-Sec6p displayed vesicle association with mitochondria (n = 112). In cells expressing Tom20-mCherry-Sec3p, not all of the secretory vesicles were associated with mitochondria. We speculate that this is due to the number of the exocyst subunits, such as Sec8p and Sec10p, being limited in cells, and the fact that not all of the vesicles are fully equipped with the exocyst complex for tethering to mitochondria marked by Tom20-mCherry-Sec3p. The rest of the accumulated vesicles are in the cytoplasm without being tethered to any membrane compartment. We also noticed that mitochondria often clustered in cells expressing Tom20-mCherry-Sec3p. This is consistent with our fluorescence microscopy results showing that GFP-Cit1 fluorescence tended to concentrate at one or a few areas (Figure 1). It was previously reported that defects in vesicular trafficking affect mitochondria morphology in yeast, often making the mitochondria less “tubular” (Altmann and Westermann, 2005). However, these morphological changes in mitochondria do not interfere with the study of exocyst targeting to mitochondria. More important, previous studies demonstrated that defects in mitochondria morphology do not affect membrane trafficking (Prinz et al., 2000; Sesaki and Jensen, 2001; Fritz et al., 2003; Messerschmitt et al., 2003; Dimmer et al., 2005; Youngman et al., 2004).
The partial localization of vesicles to the mitochondria in the Tom20-mCherry-Sec3p–expressing cells (Figure 4A, left) suggests that a pathway parallel to Sec3p targets the vesicles to the bud. It was shown that vesicles are delivered along actin cables to the daughter cells in yeast (Pruyne et al., 1998; Schott et al., 1999; Santiago-Tirado et al., 2011). We therefore examined the localization of Sec4p in these cells with their actin cables disrupted. On latrunculin treatment, more cells (85.4%, n = 113) showed Sec4p localization to the mitochondria than did dimethyl sulfoxide (DMSO)–treated cells (35.1%, n = 105; Figure 4A, right). In cells expressing Tom20-mCherry, Bgl2p fluorescence became more detectable in the cytoplasm in latrunculin-treated cells than those treated with DMSO (Figure 4B). This is consistent with a previous study showing that disruption of actin led to random accumulation of secretory vesicles (Karpova et al., 2000). However, in cells expressing Tom20-mCherry-Sec3p, brighter Bgl2p signals were detected at mitochondria after latrunculin B treatment, and 67.2% of the cells (n = 122) show Bgl2p localization to the mitochondria (Figure 4B, right).
In cells expressing other Tom20-mCherry-exocyst subunits, Sec4p was dispersed in the cytosol rather than associated with the mitochondria (Supplemental Figure S4), suggesting that they are unable to recruit vesicles. Latrunculin treatment did not affect the recruitment of Sec8p to mitochondria in cells expressing Tom20-mCherry-Sec3p (Supplemental Figure S5).
Sec4p controls exocyst recruitment
Using the ectopic targeting assay, we examined exocyst assembly to Sec3p on mitochondria in a number of post-Golgi secretory pathway mutants. Sec8-GFP was expressed in wild-type, sec2-41, sec4-8, sec1-1, and sec9-4 cells carrying either Tom20-mCherry (as a vector control) or Tom20-mCherry-Sec3p. As shown in Figure 5, in cells carrying Tom20-mCherry-Sec3p, Sec8-GFP was recruited to mitochondria in all strains at the permissive temperature (25°C). At the restrictive temperature (37°C), Sec8-GFP failed to be recruited to mitochondria in the sec2-41 and sec4-8 cells. Because Sec2p is the guanine nucleotide exchange factor of Sec4p, this result further supports the role of Sec4p in regulating exocyst assembly and targeting. The mitochondria targeting of the Sec8-GFP in sec1-1 and sec9-4 strains expressing Tom20-mCherry-Sec3p was unaffected, which is consistent with the idea that Sec1p and SNARE proteins function downstream of the exocyst (Grote et al., 2000).
FIGURE 5:
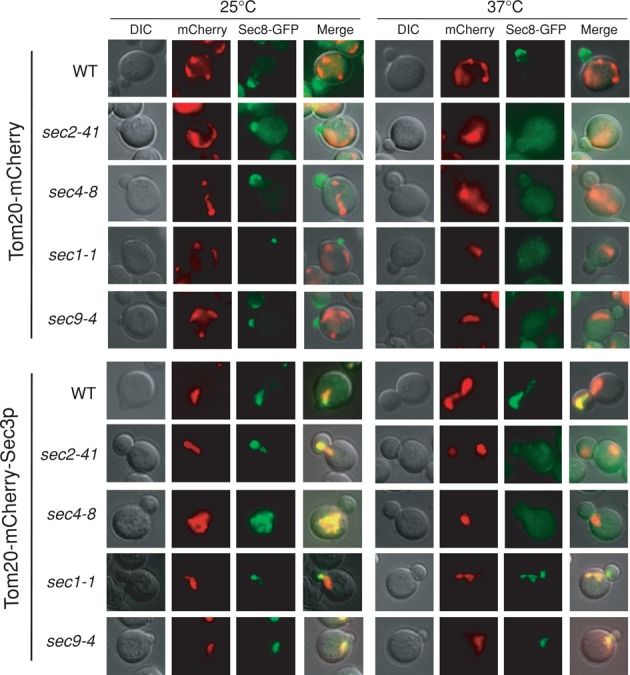
Sec2p and Sec4p control exocyst assembly at the mitochondria in cells expressing Tom20-mCherry-Sec3p. Localization of Sec8-GFP was examined in wild-type, sec1-1, sec2-41, sec4-8, and sec9-4 cells expressing Tom20-mCherry or Tom20-mCherry-Sec3p. In mutant cells expressing Tom20-mCherry, Sec8-GFP was located to the bud at the permissive temperature (25°C) and became diffused in the cytoplasm at the restrictive temperature (37°C). In cells expressing Tom20-mCherry-Sec3p, Sec8-GFP was recruited to mitochondria at 25°C. At 37°C, Sec8-GFP remained associated with mitochondria in wild-type, sec1-1, and sec9-4 cells but diffused in sec2-41 and sec4-8 cells.
DISCUSSION
Despite many studies, the role of the exocyst in vesicle tethering has remained hypothetical. Work on yeast and mammalian cells localized the exocyst to sites of active exocytosis (TerBush and Novick, 1995; Finger et al., 1998; Grindstaff et al., 1998; Guo et al., 1999a; Inoue et al., 2003; Zajac et al., 2005; Liu et al., 2007, 2009; Zhang et al., 2008; Rivera-Molina and Toomre, 2013). However, whether the exocyst actually tethers vesicle at the plasma membrane remains unclear. During exocytosis, due to the rapid transition from tethering to fusion, vesicle tethering at the plasma membrane is difficult to monitor by microscopic imaging. Using an ectopic targeting strategy, we are able to demonstrate that Sec3p was not only able to recruit the other subunits for exocyst complex assembly, but also, unlike the other subunits, recruit secretory vesicles. Our study provides experimental support for the role of Sec3p in vesicle targeting.
We used mitochondria as a surrogate organelle for the following reasons. First, mitochondria have been used to study organelle targeting because of their relative autonomy (Sengupta et al., 2009; Willett et al., 2013). Second, post-Golgi secretory vesicles targeted to mitochondria do not fuse with mitochondria due to the lack of cognate target-SNAREs there, thus allowing the observation of “tethering.” Thus the inability of vesicles to fuse with mitochondria is an advantage for this study. Mitochondria intersect with the endoplasmic reticulum at certain regions (Kornmann et al., 2009; Friedman et al., 2011). However, since the exocyst subunits are targeted only through the specific mitochondria protein Tom20p, they can only physically associate with mitochondria rather than the endoplasmic reticulum.
The observation that Tom20-tagged Exo70p was unable to recruit the other exocyst subunits to mitochondria is intriguing, as previous studies suggested the involvement of Exo70 in vesicle tethering at the plasma membrane (He et al., 2007a; Liu et al., 2007; Wu and Brennwald, 2010; Wu et al., 2010; Bendezu et al., 2012). It is possible that the tagging of Exo70p may affect its conformation. On the other hand, since Exo70p binds to PI(4,5)P2 and is a direct target of kinases and the Rho family of GTPases (Robinson et al., 1999; Inoue et al., 2003; He et al., 2007a; Hutagalung et al., 2009; Wu et al., 2010; Ren and Guo, 2012), “activation” by these regulators may be a prerequisite for Exo70 to participate in vesicle targeting and tethering. Indeed, in mammalian cells, the interaction of Exo70 with the rest of the exocyst is promoted by ERK1/2 phosphorylation in response to epidermal growth factor signaling (Ren and Guo, 2012).
Our study also revealed a difference between the recruitment of the exocyst subunits and the recruitment of secretory vesicles. Almost every exocyst subunit was capable of recruiting the other subunits to the mitochondria. Although a pool of the exocyst associates with the secretory vesicles (Guo et al., 1999b, Boyd et al., 2004; Shen et al., 2013), a significant amount of the exocyst in yeast is cytosolic, and a diffusion-based mechanism is probably sufficient for the assembly of the some of the exocyst complex in cells. This is consistent with previous biochemical analysis in yeast showing that most of the exocyst complex was soluble even in the absence of detergents (TerBush and Novick, 1995). However, for vesicle targeting, only Tom20-mCherry-Sec3p, but not other exocyst subunits, was able to reroute the secretory vesicles to mitochondria from sites of their physiological action (i.e., bud tip or mother–daughter cell junction in yeast). Although the other ectopically targeted exocyst subunits were able to recruit Sec3-GFP to mitochondria (Supplemental Figure S2), probably through diffusion, none of them could target vesicles there (Supplemental Figure S4). This result underscores a unique role that Sec3p plays in vesicle targeting. It was previously shown that Sec3p and a portion of Exo70p localized to the plasma membrane, whereas all of the other exocyst subunits, including some Exo70p, are associated with secretory vesicles, and the assembly of the holo-exocyst complex mediates vesicle tethering to the plasma membrane (Boyd et al., 2004). In the scenario of ectopic targeting, mitochondria-localized Sec3p can readily compete with the plasma membrane–localized endogenous Sec3p for the assembly of the exocyst complex for vesicle tethering. For the other ectopically targeted exocyst components such as Sec8p, however, since the secretory vesicles already has a copy of the endogenous Sec8p, it will not associate with Tom20-mCherry-Sec8p at the mitochondria. Thus we reason that the spatial segregation of Sec3p from the rest of the exocyst subunits in cells (plasma membrane vs. secretory vesicles) may provide a possible explanation for the unique vesicle-targeting function of Sec3p observed in our ectopic targeting assays.
It is also interesting to note that latrunculin treatment further enhanced vesicle targeting to mitochondria in the Tom20-mCherry-Sec3p–expressing cells. This observation suggests that the exocyst alone is probably capable of recruiting vesicles for SNARE-mediated fusion, whereas the actin cables may confer directionality and/or provide the necessary efficiency of vesicle delivery (polarized transport vs. slow diffusion) in a physiological setting such as polarized cell growth. The data are consistent with the previous observation that latrunculin treatment led to a moderate accumulation of vesicles in yeast cells in addition to their depolarized transport (Karpova et al., 2000). They are also consistent with the genetic analyses showing the synthetic lethality of sec3 mutants with mutants defective in myosin motors or actin cable formation (Bendezu and Martin, 2011; Bendezu et al., 2012; Guo lab, unpublished data).
Using the mitochondria-targeting system, we further found that the assembly of the exocyst was defective in the sec2 and sec4 mutants but not in mutants affecting SNARE assembly (sec1 and sec9). The Rab protein Sec4p has been proposed to recruit the exocyst to vesicles through its binding to Sec15p, and a previous glycerol gradient fractionation experiment showed decreased assembly of the exocyst complex in sec4 mutant cells (Guo et al., 1999b). Consistent with the previous work, our study using the ectopic targeting assay suggests that Sec4p regulates the assembly of the exocyst complex. It is possible that the interaction between Sec15p and Sec4p causes conformational changes of Sec15p, promoting its association with the rest of the exocyst components for complex assembly.
The exocyst is a member of the complex associated with tethering containing helical rods (CATCHR) family of proteins, which are believed to mediate vesicle tethering at various stages of membrane trafficking (Yu and Hughson, 2010; Brocker et al., 2010). The ectopic targeting strategy has been successfully used to study the targeting of Golgi vesicles by the conserved oligomeric Golgi complex in mammalian cells (Willett et al., 2013). The same ectopic targeting approach may also be applied in the future to test the other members of the CATCHR family in tethering.
MATERIALS AND METHODS
Yeast strains, plasmids, and procedures
Standard methods were used for yeast growth and genetic manipulations (Sherman, 2002). The yeast strains and plasmids used in this study are listed in Supplemental Tables S1 and S2. To construct the Tom20-mCherry mitochondria-targeting vector (pGV373), mCherry was first cloned into the BamHI/HindIII sites of the LEU2-based p415TEF (Mumberg et al., 1995) to generate pGV369. The open reading frame of Tom20 without stop codon was then amplified by PCR and cloned into the BamHI site of pGV369. The exocyst subunits were subcloned into pGV373 by the HindIII site. To construct the peroxisome-targeting vector (pGV377), the 5′ 135 base pairs of PEX3 were amplified by PCR and cloned into the BamHI site of pGV369. The Tom20-2xFlag vector (pGV379) was constructed by insertion of the Tom20-2xFlag sequence to the BamHI/HindIII sites of the p415TEF plasmid. The forward and reverse primers for the PCR reaction are 5′-TAGAACTAGTGGATCCATGTCCCAGTCGAACCCTAT-3′ and 5′-CGGTATCGATAAGCTTCTTATCGTCGTCATCTTTGTAATCCTTGTCATCGTCGTCCTTGTAGTCGTCATCGATATCGTTAGCTT-3′, respectively. The reverse primer contains 2xFlag sequence. The open reading frame of SEC3 was amplified by PCR and subcloned into the SalI site of pGV379.
Bgl2p secretion assays
Bgl2p secretion assays were performed as previously reported, with modifications (Harsay and Bretscher, 1995; He et al., 2007b). Briefly, yeast cells were grown in synthetic complete medium to early log phase at 25°C. We resuspended 10 OD600 units of cells in 300 μl of spheroplast solution buffer and incubated them at 37°C in a water bath for 30 min. The spheroplasts were pelleted, and 100 μl of supernatant was carefully transferred to a new tube as the external pool. The pellet (spheroplasts) was washed once with spheroplast wash buffer and then dissolved in 300 μl of lysate buffer. After centrifugation, 100 μl of supernatant was transferred to a new tube as the internal pool. Bgl2 was detected by Western blotting with an anti-Bgl2p polyclonal antibody.
Invertase secretion assay
Cells were grown to early log phase in synthetic complete (SC) medium overnight at 25°C. We collected 2.0 OD600 units of cells for each sample. Half of the sample was immediately pelleted, resuspended in 1 ml of ice-cold 10 mM NaN3 solution, and stored on ice. The other half was incubated in YP (1% yeast extract + 2% peptone) plus 0.1% glucose for 2 h at 25°C for invertase induction. Measurement of internal and external invertase activity was performed on all samples as described previously (He et al., 2007b). Statistical analyses were performed using the Student's t test.
Immunoprecipitation
For immunoprecipitation of Tom20-2xFLAG and Tom20-2xFLAG-Sec3, total proteins were extracted using the glass beads-beating method (Inagaki et al., 1999). Anti-FLAG beads (Clontech, Mountain View, CA) were used for immunoprecipitation. Typically, 50 OD600 units of a mid–log-phase yeast culture were harvested, and total protein was extracted using the glass beads method (Inagaki et al., 1999). Cell lysates were used for immunoprecipitation with anti-FLAG beads in immunoprecipitation buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM ethylene glycol tetraacetic acid [EGTA], 10% glycerol, and protease inhibitors [Roche, Branchburg, NJ]) at 4°C for 4 h with rotation. Beads were washed with washing buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, and 10% glycerol) three times. The beads were mixed with 30 μl of 1× SDS loading buffer and incubated at 95°C for 5 min. Protein samples were cooled on ice for several minutes and subjected to 10% SDS–PAGE. Western blotting was performed using the rabbit polyclonal antibodies against Sec6p, Sec10p, Sec15p, and Exo84p.
Fluorescence microscopy
Yeast strains were grown to early log phase in a synthetic complete medium overnight at 25°C. Samples were collected by centrifugation, and 2 μl of the suspension was used for fluorescence microscopy using a Leica (Buffalo Grove, IL) microscope (CTR6000) equipped with a Plan-Apochromat 100×, 1.40 numerical aperture oil immersion objective lens. Images were captured with a digital camera (DFC350FX; Leica) operated under LAS AF 1.5.1 software (Leica). Rabbit anti-Sec4p and anti-Bgl2p polyclonal antibodies were used at 1:2000 dilution for immunofluorescence microscopy using protocols described previously (Walch-Solimena et al., 1997). For F-actin disruption, cells were treated with 100 μM latrunculin B for 60 min before microscopy.
Electron microscopy
To visualize the tethering of secretory vesicles, electronic microscopy with permanganate fixation was performed according to the procedure described previously (Perkins and McCaffery, 2007). Yeast strains were grown in SC medium overnight to early log phase at 25°C. Cells were collected by centrifugation and resuspended in the fixation buffer (100 mM sodium cacodylate, pH 7.4, 3% glutaraldehyde, 5 mM CaCl2, and 5 mM MgCl2) for 1 h. The samples were washed with 100 mM sodium cacodylate (pH 7.4), embedded in ultra-low-temperature gelling agarose (Sigma-Aldrich, St. Louis, MO), and cut into small pieces. These blocks were postfixed in 4% KMnO4 for 1 h and then twice washed with double-distilled water. The samples were then treated with 0.5% sodium metaperiodate for 15 min and washed with double-distilled water. The samples were resuspended in 2% uranyl acetate, dehydrated through a graded series of ethanol (50–100%, ice cold), and washed twice with propylene oxide. The samples were resuspended in the mixture of propylene oxide and Spurr resin (Polysciences, Warrington, PA) in 1:1 ratio and rotated overnight, transferred to 100% Spurr resin, and left in a vacuum overnight. The next day, the resin was changed three times, and the samples were then rotated overnight. Subsequently, the samples were put in polyethylene BEEM embedding capsules (Polysciences) containing Spurr resin and placed in an 80°C oven for 24 h. Sections were cut, placed onto 400-mesh nickel grids, and poststained with lead citrate for 2 min. Cells were observed using a transmission electron microscope (model 1010; JEOL, Peabody, MA) at 100,000× magnification. The association of secretory vesicles with mitochondria was observed. Cells with three or more vesicles attached to a cluster of mitochondria were counted as positive for vesicle–mitochondria association.
Supplementary Material
Acknowledgments
We thank Liza A. Pon and Randy Schekman for the Git1-GFP and Chs3-GFP plasmids. We are grateful to Vladimir Lupashin and Adam Linstedt for helpful discussions on mitochondria targeting. This work was supported by a National Institutes of Health grant (R01 GM111128) to W.G.
Abbreviations used:
- CATCHR
complex associated with tethering containing helical rods
- DMSO
dimethyl sulfoxide
- GFP
green fluorescent protein
- GTPase
guanosine 5′-triphosphatase
- PI(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- SC
synthetic complete media
- SNARE
soluble N-ethylmaleimide–sensitive factor adaptor protein receptor
- YP
yeast extract peptone.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-04-0907) on September 17, 2014.
REFERENCES
- Altmann K, Westermann B. Role of essential genes in mitochondrial morphogenesis in Saccharomyces cerevisiae. Mol Biol Cell. 2005;16:5410–5417. doi: 10.1091/mbc.E05-07-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek K, Knodler A, Lee SH, Zhang X, Orlando K, Zhang J, Foskett TJ, Guo W, Dominguez R. Structure-function study of the N-terminal domain of exocyst subunit Sec3. J Biol Chem. 2010;285:10424–10433. doi: 10.1074/jbc.M109.096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezu FO, Martin SG. Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast. Mol Biol Cell. 2011;22:44–53. doi: 10.1091/mbc.E10-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezu FO, Vincenzetti V, Martin SG. Fission yeast Sec3 and Exo70 are transported on actin cables and localize the exocyst complex to cell poles. PLoS One. 2012;7:e40248. doi: 10.1371/journal.pone.0040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C, Hughes T, Pypaert M, Novick P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J Cell Biol. 2004;167:889–901. doi: 10.1083/jcb.200408124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. Sec9 Is a Snap-25-like component of a yeast Snare complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Brocker C, Engelbrecht-Vandre S, Ungermann C. Multisubunit tethering complexes and their role in membrane fusion. Curr Biol. 2010;20:R943–R952. doi: 10.1016/j.cub.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Chuang JS, Schekman RW. Differential trafficking and timed localization of two chitin synthase proteins Chs2p and Chs3p. J Cell Biol. 1996;135:1925–1925. doi: 10.1083/jcb.135.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmer KS, Jakobs S, Vogel F, Altmann K, Westermann B. Mdm31 and Mdm32 are inner membrane proteins required for maintenance of mitochondrial shape and stability of mitochondrial DNA nucleoids in yeast. J Cell Biol. 2005;168:103–115. doi: 10.1083/jcb.200410030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger FP, Hughes TE, Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92:559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- Finger FP, Novick P. Sec3p is involved in secretion and morphogenesis in Saccharomyces cerevisiae. Mol Biol Cell. 1997;8:647–62. doi: 10.1091/mbc.8.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Lackner L, West M, DiBenedetto J, Nunnari J, Voeltz G. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz S, Weinbach N, Westermann B. Mdm30 is an F-box protein required for maintenance of fusion-competent mitochondria in yeast. Mol Biol Cell. 2003;14:2303–2313. doi: 10.1091/mbc.E02-12-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Grote E, Carr CM, Novick PJ. Ordering the final events in yeast exocytosis. J Cell Biol. 2000;151:439–452. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Grant A, Novick P. Exo84p is an exocyst protein essential for secretion. J Biol Chem. 1999a;274:23558–23564. doi: 10.1074/jbc.274.33.23558. [DOI] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999b;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Sacher M, Barrowman J, Ferro-Novick S, Novick P. Protein complexes in transport vesicle targeting. Trends Cell Biol. 2000;10:251–255. doi: 10.1016/s0962-8924(00)01754-2. [DOI] [PubMed] [Google Scholar]
- Guo W, Tamanoi F, Novick P. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat Cell Biol. 2001;3:353–360. doi: 10.1038/35070029. [DOI] [PubMed] [Google Scholar]
- Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Xi F, Zhang J, TerBush D, Zhang X, Guo W. Exo70p mediates the secretion of specific exocytic vesicles at early stages of the cell cycle for polarized cell growth. J Cell Biol. 2007b;176:771–777. doi: 10.1083/jcb.200606134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Xi F, Zhang X, Zhang J, Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 2007a;26:4053–4065. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Ting AE, Hazuka CD, Davanger S, Kenny JW, Kee Y, Scheller RH. The mammalian brain rsec6/8 complex. Neuron. 1996;17:1209–1219. doi: 10.1016/s0896-6273(00)80251-2. [DOI] [PubMed] [Google Scholar]
- Hutagalung AH, Coleman J, Pypaert M, Novick PJ. An internal domain of Exo70p Is required for actin-independent localization and mediates assembly of specific exocyst components. Mol Biol Cell. 2009;20:153–163. doi: 10.1091/mbc.E08-02-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M, Schmelzle T, Yamaguchi K, Irie K, Hall MN, Matsumoto K. PDK1 homologs activate the Pkc1-mitogen-activated protein kinase pathway in yeast. Mol Cell Biol. 1999;19:8344–8352. doi: 10.1128/mcb.19.12.8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Chang L, Hwang J, Chiang SH, Saltiel AR. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- Jantti J, Aalto MK, Oyen M, Sundqvist L, Keranen S, Ronne H. Characterization of temperature-sensitive mutations in the yeast syntaxin 1 homologues Sso1p and Sso2p, and evidence of a distinct function for Sso1p in sporulation. J Cell Sci. 2002;115:409–420. doi: 10.1242/jcs.115.2.409. [DOI] [PubMed] [Google Scholar]
- Karpova TS, Reck-Peterson SL, Elkind NB, Mooseker MS, Novick PJ, Cooper JA. Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:1727–1737. doi: 10.1091/mbc.11.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue P, Artym VV, Mueller SC, Guo W. The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol Biol Cell. 2009;20:3763–3771. doi: 10.1091/mbc.E08-09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zuo X, Yue P, Guo W. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol Biol Cell. 2007;18:4483–4492. doi: 10.1091/mbc.E07-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Zhang J, Luca FC, Guo W. Mitotic phosphorylation of Exo84 disrupts exocyst assembly and arrests cell growth. J Cell Biol. 2013;202:97–111. doi: 10.1083/jcb.201211093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matern HT, Yeaman C, Nelson WJ, Scheller RH. The Sec6/8 complex in mammalian cells, Characterization of mammalian Sec3, subunit interactions, and expression of subunits in polarized cells. Proc Natl Acad Sci USA. 2001;98:9648–9653. doi: 10.1073/pnas.171317898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmitt M, Jakobs S, Vogel F, Fritz S, Dimmer KS, Neupert W, Westermann B. The inner membrane protein Mdm33 controls mitochondrial morphology in yeast. J Cell Biol. 2003;160:553–564. doi: 10.1083/jcb.200211113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D, Muller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Perkins EM, McCaffery JM. Conventional and immunoelectron microscopy of mitochondria. Methods Mol Biol. 2007;372:467–483. doi: 10.1007/978-1-59745-365-3_33. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport-vesicle targeting, tethers before SNAREs. Nat Cell Biol. 1999;1:E17–E22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- Prinz WA, Grzyb L, Veenhuis M, Kahana JA, Silver PA, Rapoport TA. Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Biol. 2000;150:461–474. doi: 10.1083/jcb.150.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne DW, Schott DH, Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J Cell Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- Ren J, Guo W. ERK1/2 regulate exocytosis through direct phosphorylation of the exocyst component Exo70. Dev Cell. 2012;22:967–978. doi: 10.1016/j.devcel.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Molina F, Toomre D. Live-cell imaging of exocyst links its spatiotemporal dynamics to various stages of vesicle fusion. J Cell Biol. 2013;201:673–680. doi: 10.1083/jcb.201212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NG, Guo L, Imai J, Toh-E A, Matsui Y, Tamanoi F. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol Cell Biol. 1999;19:3580–3587. doi: 10.1128/mcb.19.5.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Tirado FH, Legesse-Miller A, Schott D, Bretscher A. PI4P and Rab inputs collaborate in myosin-V-dependent transport of secretory compartments in yeast. Dev Cell. 2011;20:47–59. doi: 10.1016/j.devcel.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott D, Ho J, Pruyne D, Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J Cell Biol. 1999;147:791–807. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta D, Truschel S, Bachert C, Linstedt AD. Organelle tethering by a homotypic PDZ interaction underlies formation of the Golgi membrane network. J Cell Biol. 2009;186:41–55. doi: 10.1083/jcb.200902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesaki H, Jensen RE. UGO1 encodes an outer membrane protein required for mitochondrial fusion. J Cell Biol. 2001;152:1123–1134. doi: 10.1083/jcb.152.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Yuan H, Hutagalung A, Verma A, Kuummel D, Wu X, Reinisch K, McNew JA, Novick P. The synaptobrevin homologue Snc2p recruits the exocyst to secretory vesicles by binding to Sec6p. J Cell Biol. 2013;202:509–526. doi: 10.1083/jcb.201211148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41. doi: 10.1016/s0076-6879(02)50954-x. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting AE, Hazuka CD, Hsu SC, Kirk MD, Bean AJ, Scheller RH. rSec6 and rSec8, mammalian homologs of yeast proteins essential for secretion. Proc Natl Acad Sci USA. 1995;92:9613–9617. doi: 10.1073/pnas.92.21.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RN, Novick PJ. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Pfeffer SR. Membrane tethering in intracellular transport. Curr Opin Cell Biol. 1999;11:453–459. doi: 10.1016/s0955-0674(99)80065-9. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Du Y, Pypaert M, Ferro-Novick S, Novick P. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol Biol Cell. 2003;14:4770–4782. doi: 10.1091/mbc.E03-04-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett R, Kudlyk T, Pokrovskaya I, Schonherr R, Ungar D, Duden R, Lupashin V. COG complexes form spatial landmarks for distinct SNARE complexes. Nat Commun. 2013;4:1553. doi: 10.1038/ncomms2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Brennwald P. The function of two Rho Family GTPases is determined by distinct patterns of cell surface localization. Mol Cell Biol. 2010;30:5207–5217. doi: 10.1128/MCB.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Turner C, Gardner J, Temple B, Brennwald P. The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol Biol Cell. 2010;21:430–442. doi: 10.1091/mbc.E09-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Itoh N, Kawano S, Yatsukawa Y, Momose T, Makio T, Matsunaga M, Yokota M, Esaki M, Shodai T, et al. Dual role of the receptor Tom20 in specificity and efficiency of protein import into mitochondria. Proc Natl Acad Sci USA. 2011;108:91–96. doi: 10.1073/pnas.1014918108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman MJ, Hobbs AEA, Burgess SM, Srinivasan M, Jensen RE. Mmm2p, a mitochondrial outer membrane protein required for yeast mitochondrial shape and maintenance of mtDNA nucleoids. J Cell Biol. 2004;164:677–688. doi: 10.1083/jcb.200308012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- Zajac A, Sun X, Zhang J, Guo W. Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth. Mol Biol Cell. 2005;16:1500–1512. doi: 10.1091/mbc.E04-10-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanolari B, Rockenbauch U, Trautwein M, Clay L, Barral Y, Spang A. Transport to the plasma membrane is regulated differently early and late in the cell cycle in Saccharomyces cerevisiae. J Cell Sci. 2011;124:1055–1066. doi: 10.1242/jcs.072371. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bi E, Novick P, Du L, Kozminski KG, Lipschutz JH, Guo W. Cdc42 interacts with the exocyst and regulates polarized secretion. J Biol Chem. 2001;276:46745–46750. doi: 10.1074/jbc.M107464200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol. 2008;180:145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


