Abstract
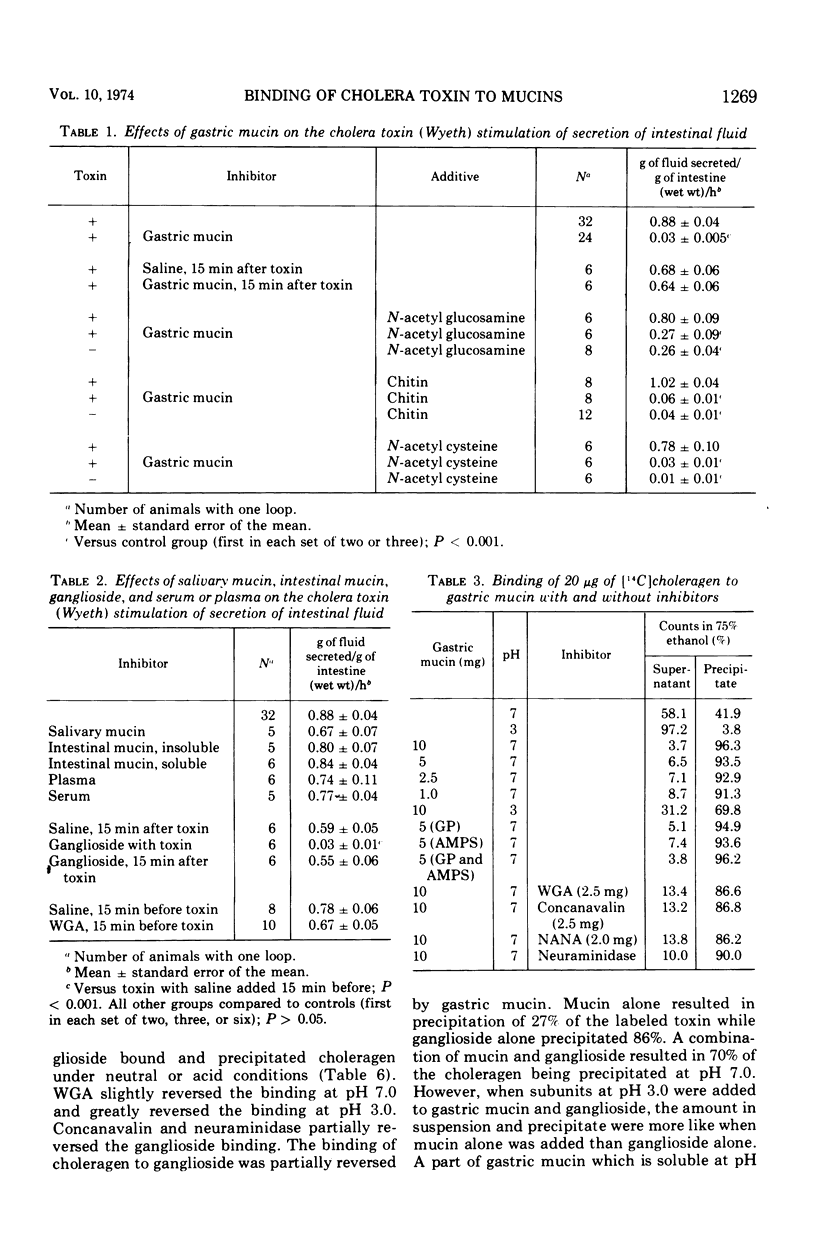
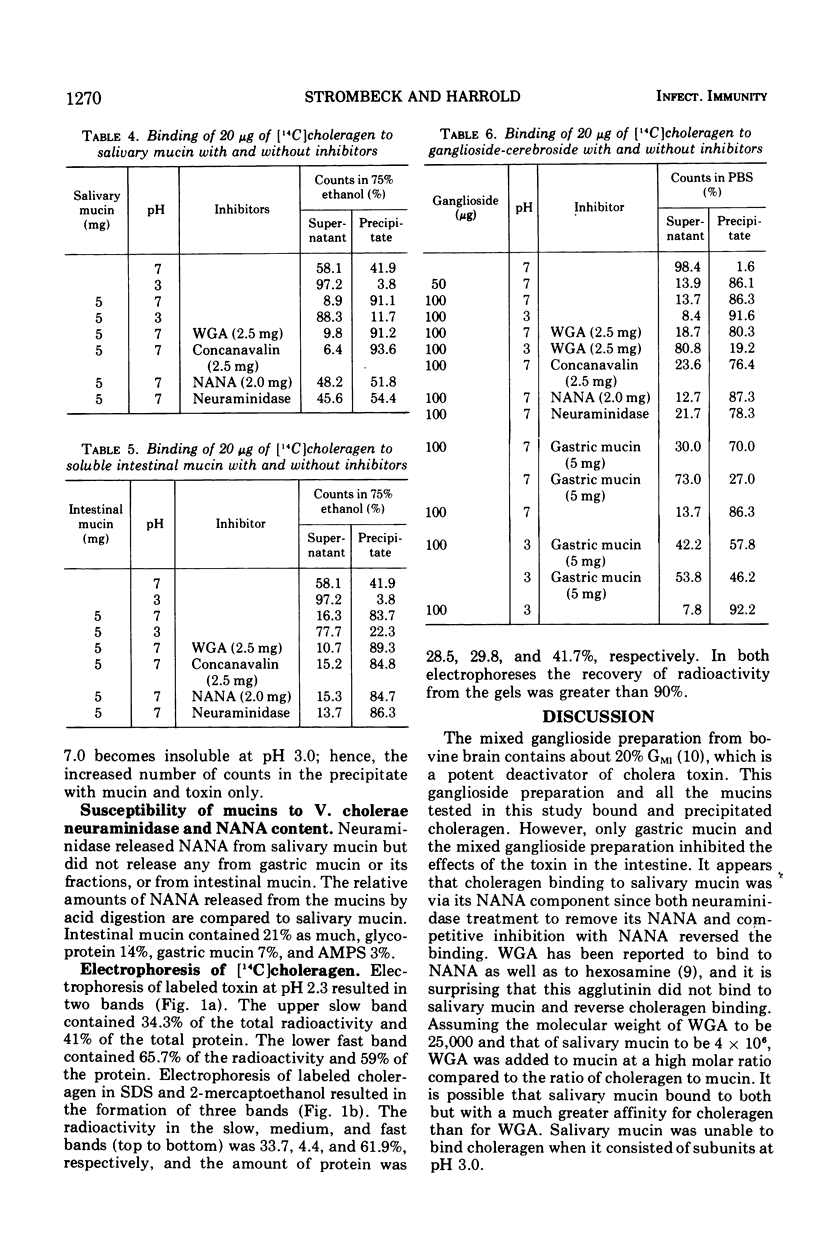
The effects of mucin on cholera toxin induced intestinal secretion was studied in the rat small intestine. Gastric mucin inhibited secretion when premixed with cholera toxin on an equal dry-weight basis. Neither salivary nor intestinal mucin inhibited the toxin's action in the intestine. Inhibition by gastric mucin was not reversed by a mucolytic agent or by hexoses or agents that bind hexoses. Plasma and serum did not inhibit the effects of cholera toxin in the intestine. Choleragen was labeled with 14C and its binding to mucins and ganglioside was studied. Gastric mucin was shown to bind to choleragen, but less binding was observed when choleragen was dissociated into subunits by acid pH. Salivary and intestinal mucins bound intact choleragen but not the subunits at acid pH. Salivary mucin binding was reversed by N-acetyl neuraminic acid and neuraminidase. Ganglioside bound choleragen in both the intact and dissociated forms. Binding to the dissociated form was reversed by wheat germ agglutinin. Gastric mucin and ganglioside competed for binding to choleragen with the binding of intact choleragen greater for ganglioside and the affinity of the subunits greater for gastric mucin. Electrophoresis of labeled choleragen showed uniform labeling of the subunits dissociated by acid pH, but a major part of cholera toxin was found not to be labeled when fractionated with sodium dodecyl sulfate and 2-mercaptoethanol.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bella A., Jr, Kim Y. S. Rat small intestinal mucin: isolation and characterization of a water-soluble mucin fraction. Arch Biochem Biophys. 1972 Jun;150(2):679–689. doi: 10.1016/0003-9861(72)90086-0. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I., Hollenberg M. D. Affinity chromatography and structural analysis of Vibrio cholerae enterotoxin-ganglioside agarose and the biological effects of ganglioside-containing soluble polymers. Biochemistry. 1973 Oct 9;12(21):4253–4264. doi: 10.1021/bi00745a033. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., LaRue M. K., LoSpalluto J. J. Properties of the cholera exo-enterotoxin: effects of dispersing agents and reducing agents in gel filtration and electrophoresis. Infect Immun. 1972 Dec;6(6):934–944. doi: 10.1128/iai.6.6.934-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J. F., Jabbal I., Forstner G. G. Goblet cell mucin of rat small intestine. Chemical and physical characterization. Can J Biochem. 1973 Aug;51(8):1154–1166. doi: 10.1139/o73-152. [DOI] [PubMed] [Google Scholar]
- Greenaway P. J., LeVine D. Binding of N-acetyl-neuraminic acid by wheat-germ agglutinin. Nat New Biol. 1973 Feb 7;241(110):191–192. doi: 10.1038/newbio241191a0. [DOI] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leitch G. J. Intestinal epithelium brush border and microsome chemistry. I. Crude cholera toxin effects. Exp Mol Pathol. 1972 Oct;17(2):187–197. doi: 10.1016/0014-4800(72)90068-8. [DOI] [PubMed] [Google Scholar]
- Snary D., Allen A. Studies on gastric mucoproteins. The isolation and characterization of the mucoprotein of the water-soluble mucus from pig cardiac gastric mucosa. Biochem J. 1971 Aug;123(5):845–853. doi: 10.1042/bj1230845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strombeck D. R. The production of intestinal fluid by cholera toxin in the rat. Proc Soc Exp Biol Med. 1972 May;140(1):297–303. doi: 10.3181/00379727-140-36444. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



