Abstract
This review presents a brief overview of the γ-aminobutyric acid (GABA) system in the developing and mature central nervous system (CNS) and its potential connections to pathologies of the CNS. γ-aminobutyric acid (GABA) is a major neurotransmitter expressed from the embryonic stage and throughout life. At an early developmental stage, GABA acts in an excitatory manner and is implicated in many processes of neurogenesis, including neuronal proliferation, migration, differentiation, and preliminary circuit-building, as well as the development of critical periods. In the mature CNS, GABA acts in an inhibitory manner, a switch mediated by chloride/cation transporter expression and summarized in this review. GABA also plays a role in the development of interstitial neurons of the white matter, as well as in oligodendrocyte development. Although the underlying cellular mechanisms are not yet well understood, we present current findings for the role of GABA in neurological diseases with characteristic white matter abnormalities, including anoxic-ischemic injury, periventricular leukomalacia, and schizophrenia. Development abnormalities of the GABAergic system appear particularly relevant in the etiology of schizophrenia. This review also covers the potential role of GABA in mature brain injury, namely transient ischemia, stroke, and traumatic brain injury/post-traumatic epilepsy.
Keywords: γ-aminobutyric acid receptors, chloride transporters, anoxic-ischemic injury, periventricular leukomalacia, schizophrenia, stroke
INTRODUCTION
γ-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the adult brain. In tandem with the excitatory neurotransmitter glutamate, GABA modulates the inhibitory-excitatory balance necessary for proper brain function in mature brains (Markram et al. 2004; Xu et al. 2011; Koós and Tepper, 1999; Takesian and Hensch 2013). There are two main types of GABA receptors, the ionotropic GABAA receptor and the metabotropic GABAB receptor. In the adult brain, GABA acts primarily through activation of the fast hyperpolarizing GABAA receptors. When GABA binds to these receptors at the post-synaptic site, the ion channel opens and chloride (Cl−) diffuses into the cell along its concentration gradient, thus hyperpolarizing the post-synaptic mature neuron (Luján et al. 2005; Blednov et al 2014). Fast ionotropic GABAA receptors are are ligand-gated chloride ion channels comprised of α, β, γ and δ subunits in a heteropentameric structure (Macdonald and Olsen 1994; Blednov et al. 2014). There is additional diversity within subunit families, contributing to further heterogeneity of the GABAA receptor (Macdonald and Olsen 1994). GABAA receptors with unique subunit compositions are distributed differentially in the mature brain. Receptors containing α1 and γ2 subunits localize in the synaptic cleft whereas receptors containing α4, α5, α6, and δ subunits localize extrasynaptically/perisynaptically. Extrasynaptic GABAA receptors are high-affinity GABA receptors implicated in tonic inhibition, whereas synaptic GABAA receptors are those involved in fast, phasic inhibition (Lee and Maguire 2014).
A second type of ionotropic GABA receptor, previously named GABAC, has been identified broadly in the central nervous system and particularly in the vertebrate retina (Boue-Grabot et al. 1998). It has a homopentameric structure composed solely of 3 ρ subunits. This receptor displays insensitivity to the GABAA antagonist bicuculline but its relatively similar structure and function, similar degree of variation in subunit pharmacology as that found among GABAA subunit types, and potential to partner with other GABAA subunits has led the IUPHAR Nomeclature committee to recommend its classification as a GABAA receptor subclass, GABAA-ρ (Barnard et al. 1998).
In contrast to the ionotropic GABAA receptors, GABAB receptors are composed of two subunits, GABAB1 and GABAB2. GABAB receptors are responsible for the later and slower component of inhibitory transmission (Couve et al. 2000). GABAB receptors are found both pre and post synaptically (Misgeld et al. 1995). Activation of these receptors is coupled to K+ and/or Ca2+ channels via a G-protein mediated pathway or in a membrane delimited manner (Misgeld et al. 1995; Lujan et al. 2005; Owens and Kriegstein 2002). In the pathway involving second messengers, binding of GABA results in release of associated G protein subunits, which then diffuse and activate various intracellular signal cascades and ultimately lead to either activation of postsynaptic K+ channels or inhibition of presynaptic Ca2+ channels (Couve et al. 2000). Presynaptically, this decrease in Ca2+ conductance, leads to reduced neurotransmitter release (Misgeld et al. 1995). Some evidence also exists for G-protein mediated postsynaptic inhibition of voltage dependent Ca2+ channels (Harayama et al. 1998).
The following sections present an overview of the GABAergic system in the developing and mature CNS and their potential roles in neurological diseases.
I. GABA and GABAergic neurons in the developing brain
1. Development of GABAergic neurons
It is generally accepted that GABAergic neurons develop early in the cortical anlage during embryonic development, whereas glutamatergic activity arises later (Del Rio et al. 1992; Chen et al. 1995). GABA-immunocytochemistry on rodent coronal and sagittal brain sections of embryonic days 12 through 19 (E12–19), as well as several postnatal points up to postnatal day 21 (P21), revealed that GABA-immunopositive cells first appeared at embryonic day 12 (E12). Most of the GABA-immunopositive cells seen were localized in the primitive plexiform layer at E12 and the subventricular and ventricular zones by E13 (Del Rio et al. 1992). Staining of neocortical sections taken from progressively more developed embryonic brains revealed GABA-immunoreactive cells present in the preplate, subventricular and ventricular proliferative zones by E12, subplate, marginal zone, and intermediate zones by E14, and cortical plate by E16. At E16 the density of GABAergic cells in the proliferative zones had markedly decreased (Haydar et al. 2000). GABAergic neurons were also detected in the neocortical mouse brain as early as E10 at the pial surface of the neoepithelial wall (Haydar et al. 2000).
Electrophysiological recordings of cultured mouse embryonic neurons support the model of an early-developing GABAergic system and a later-developing glutamatergic system. Whole-cell voltage clamp recording detected a GABA-evoked inward current in neurons cultured from E15 embryos. 30 µM GABA was sufficient to evoke this response in cultured neurons, 5–10 hours after plating. Furthermore, all cells at all stages responded to GABA (Chen et al. 1995). In contrast, response of cells to glutamate was not seen until the sixth day in culture, suggesting that glutamatergic sensitivity develops later than GABAergic. However, in humans, there is evidence that important developments of the GABAergic system occur during the latter half of gestation and into the first few years of infancy (Xu et al. 2011). In fact, it is possible that the human GABAergic system does not fully mature until postnatal adolescence (Kilb 2012).
In the rodent spinal cord, GABA-immunoreactive (GABA-ir) neurons develop in a specific pattern, namely rostral-caudally and along a ventral-dorsal gradient. Immunohistochemistry analysis on spinal cord sections of E9.5 to P0 mice revealed GABA-immunoreactivity first appeared at E11.5, at the highest brachial levels. By E12.5, GABA-ir somata were detected at all levels of the spinal cord, though localized to the ventral gray matter. By E13.5, sparse GABA-ir somata were seen in the dorsal marginal zones. The most dramatic shift came at E15.5, at which point GABA-ir somata appeared in all future gray matter of the spinal cord, with GABA-ir fibers spreading in the future ventral white matter. At lumbar levels, white matter of the spinal cord had densely GABA-labeled marginal zones. At E17.5, GABA-ir patterns were more dorsal overall, with GABA-ir fibers within the entire surrounding white matter. By P0, there was an overall decrease in the density of labeling. Most GABA-ir cells were localized dorsally. Thus, development of GABA-immunoreactive cells in the rodent spinal cord spreads in a specific pattern; the dramatic shift in density from ventral to dorsal areas occurred between E13.5 and E17.5 at both brachial and lumbar levels (Allain et al. 2004).
A substantial body of evidence indicates that cortical interneurons originate in the subcortical telencephalon and migrate through white matter into the overlying cortex (Marín and Rubenstein 2001; Letinic et al. 2002; Robinson et al. 2006; Petanjek et al. 2008). Studies in rodent and chicken models reveal that nearly all GABAergic interneurons involved in local cortical circuitry are likely to originate in the subpallium (ventral telencephalon) in particular and migrate tangentially to the cortex (Anderson et al. 1997; Marin and Rubenstein 2001; Anderson et al. 2002). This tangential migration into the neocortex appears to be dependent upon transcription factors distal-less homeobox 1 (DLX-1) and distal-less homeobox 2 (DLX-2), as mutant mice lacking Dlx-1 and Dlx-2 exhibit little or no tangential cell migration into the neocortex (Anderson et al. 1997). In contrast, glutamatergic-fated projection neurons appear to undergo radial migration from their origin in the pallium (Tan et al. 1998; Marin and Rubenstein 2001). Of note however, GABAergic interneurons of the rodent cerebellum appear to arise in the neuroepithelium of embryonic cerebellar primordium, then migrate into the prospective white matter where they receive environmental signals that induce phenotypically diverse differentiation. In this way, the cerebellar interneuron population appears to develop from a single and unique source of progenitor cells (Leto et al. 2009).
In contrast to rodent models, primate cortical interneuron may arise from areas in the telencephalon other than the subpallium. There is evidence that in humans, the population of cortical interneurons is comprised of two populations expressing different proteins and originating in different areas. Interestingly, 65% of neocortical GABAergic neurons express the protein Mash1 and most likely are born in the ventricular/subventricular zone of the dorsal telencephalon. 35% of neocortical GABAergic neurons do not express Mash1 and originate from the ganglionic eminences, proliferative zones of the subpallium (Letinic et al. 2002). The finding that neocortical GABAergic neurons arise from the dorsal telencephalon as well as the subpallium may be a property of higher primates, as this finding has been supported by studies in the cynomolgus monkey (Petanjek et al. 2009).
Interneurons are most likely the first neurons to generate network-driven activity in the developing brain (Ben-Ari 2004). Interneurons have been shown to mature earlier than pyramidal cells in the rat hippocampus—indeed, evidence points to an established interneuronal network that is active even at embryonic day 18 (E18), a time point at which most other neurons generate no synaptic currents (i.e. are nonfunctional). Patch-clamp recording of pyramidal neurons and interneurons in the same hippocampal slices taken from E18 rat embryos showed that 88% of pyramidal neurons expressed no spontaneous or evoked post-synaptic currents, whereas 65% of interneurons were already functional (Hennou et al. 2002). In the hippocampus, these interneuron-driven post-synaptic currents are the main generators for giant GABAergic potentials (GGPs) that play an important role in enhancing synaptic efficiency between excitatory neurons in a Hebbian manner. This effect was demonstrated in synapses between mossy fibers and CA3 pyramidal neurons (Kasyanov et al. 2004). Thus, interneuron generated network-driven patterns modulate the proper development of synapses among cortical neurons and likely play a role in priming unorganized silent neurons for the shift into functional circuits (Ben-Ari et al. 2004). Furthermore, these synapse-enhancing GGPs in the hippocampus, alternatively known as giant depolarizing potentials (GDPs), appear to be under control of an inwardly-directed cationic pacemaker current (Ih) in interneurons (Strata et al. 1997). In this way, interneurons are also implicated in pacemaker properties in the developing brain.
2. Chloride transporters dictate GABA-mediated early excitatory actions in the developing brain
It is widely appreciated that the GABAergic system plays an important role in the developing brain even prior to synaptogenesis. At the earliest stages of neuronal development, GABA, acting at GABAA receptors, produces an excitatory response that facilitates many of the early development effects attributed to GABA instead of an inhibitory response as seen in the mature brain (Ben-Ari et al. 1994). An increasingly large body of evidence points to a critical role for cation-chloride transporters in modulating this time-dependent differential response, via regulation of intracellular chloride concentration levels ([Cl−]i). Two major cation-chloride transporters, the inwardly directed Na-K-Cl cotransporter (NKCC1) and the Cl− extruding K-Cl cotransporter (KCC2) dictate intracellular [Cl−]i and thus GABA actions.
NKCC1 is largely responsible for the high intracellular [Cl−]i that allows GABA to have an excitatory effect in the developing brain (Sun and Murali 1999; Yamada et al. 2004; Pfeffer et al.2009). Electrophysiological recording of the reversal potential for GABA-induced currents (EGABA) can be used to calculate the [Cl−]i using the Nernst Equation; in this way, it is determined that a more positive EGABA correlates with high [Cl−]i. Single-cell patch clamp recording on rat neocortical slices revealed that EGABA was significantly more positive in immature cells containing NKCC1 mRNA than in immature cells that did not (Yamada et al. 2004). Patch-clamp recording of NKCC1 knockout (Nkcc1−/−) neurons showed much less of a positive shift in EGABA than in wild type neurons, indicating a decrease in [Cl−]i attributable to lack of NKCC1 (Pfeffer et al. 2009). NKCC1 activity, as measured by NKCC1-dependent K+ influx, shows upregulation of activity in the first postnatal week (Sun and Murali 1999). In mouse hippocampal sections, neuronal expression of NKCC1 was shown to be high at P0 and to subsequently decrease from P1 to P15, as measured by levels of NKCC1 mRNA (Pfeffer et al. 2009). Similarly, Western blot analysis of rat cortical neurons in vitro and in vivo showed substantial expression of NKCC1 at P0 and maintenance of steady expression by P2 (Sun and Murali 1999). Taken together, these results shows that NKCC1 expression is high in early development and contributes to a high [Cl−]i that facilitates GABA-mediated excitatory actions in the early developing and postnatal brain.
The so called “GABA shift” is a fascinating change in the effect of GABA from depolarizing action in the developing brain to hyperpolarizing action in the adult brain. The mechanistic picture revealed thus far indicates that as cortical development progresses, intracellular [Cl−]i decreases and EGABA becomes more negative, allowing GABA to become increasingly inhibitory. This increase in intracellular [Cl−]i and subsequent GABA shift is mediated by KCC2 activity (Rivera et al. 1999; Owens and Kriegstein 2002). KCC2 is a Cl10 extruding K+ /Cl− co-transporter localized only in neurons (Williams et al. 1999). To show that KCC2 action is coupled to GABAergic activity, P11–P13 rat hippocampal slices were treated with antisense-A form phosphorothionate-protected antisense oligodeoxynucleotide (antisense-A PODN) which down-regulates KCC2 protein expression. Measurements of EGABA in these slices showed no significant hyperpolarization, whereas control slices were always hyperpolarized, indicating that KCC2 expression is necessary for inhibitory hyperpolarization (Rivera et al. 1999). Furthermore, patch-clamp measurements of E18.5 spinal cord motoneurons in KCC2 knockout mice (kcc2−/−) revealed that a loss of KCC2 led to a significant increase in [Cl−]i and depolarizing action of GABA (Hübner et al. 2001). Furthermore, KCC2 mRNA expression appeared in a developmentally time-dependent manner, with barely detectable levels at P0 but increased levels by P9 (Rivera et al. 1999). This finding is consistent with the timeframe of the GABA shift.
To round out the picture, glutamate has been shown to have an inhibitory effect on this early excitatory activity of the GABAergic system via metabotropic glutamate receptors, thus providing a mechanism for excitatory-inhibitory balance in the early neocortex (Van den Pol et al. 1998). Similarly, there is evidence that activity at GABAB receptors in the developing (E18) rat hypothalamus modulates the excitatory responses mediated by GABAA receptors, namely by depressing the rise in intracellular Ca2+, thus further providing a mechanism for excitatoryinhibitory balance during development (Obrietan and Van den Pol 1998).
3. GABA in neuronal proliferation and migration
Studies have shown GABA to have a variable trophic effect in different proliferative zones of the developing embryonic cortex, and it appears to play a role in the proliferation of neuronal progenitor cells (Wang and Kriegstein 2009; Jovanovic and Thomson 2011). In the early stages of development, GABA acting at GABAA receptors in an excitatory manner has been shown to inhibit DNA synthesis in cortical progenitor cells and inhibit thus the total number of these cells. The likely mechanism for this inhibition is depolarization-induced Ca2+ influx, which in turn may affect phases of the cell cycle that are highly calcium-sensitive and thus decrease DNA synthesis (LoTurco et al. 1995).
Recently, a more complicated picture of GABA’s effects on progenitor cell proliferation has been suggested. Exposing mouse embryonic brain sections with GABA showed a differential effect on proliferation of immature progenitor cells of the subventricular and ventricular zones. Exposing embryonic brain sections with GABA increased the rate of proliferation and size of neural progenitors in the ventricular zone, acting via GABAA receptors, but conversely decreased the rate of proliferation of neural progenitors in the subventricular zone (Haydar et al. 2000). This finding suggests that distinct populations of neural progenitors might respond to GABA differently.
The early excitatory action of GABA in the developing brain is also critical for proper morphological development of neurons. Eliminating this early excitatory action by prematurely expressing the Cl− extruding transporter KCC2 resulted in a significant reduction in neurite length as well as number and length of dendritic processes (Cancedda et al. 2007). Release of GABA from local interneurons in the hippocampus induces maturation of intermediate progenitor cells into mature granular cells (Wang and Kriegstein 2009). The GABAA receptor-specific agonist muscimol was shown to inhibit the proliferative effect of basic fibroblast growth factor (bFGF) on cortical neuron progenitor cells of the rat brain. In an interesting reciprocal relationship, bFGF increased the number of neurons expressing GABAA receptors, possibly providing a feedback pathway for modulating bFGF induced cell proliferation (Antonopoulos et al. 1997).
Populations of differentiated cortical neurons and immature ventricular zone neurons also have a differential migratory response to high or low concentrations of GABA. Micromolar GABA appears to increase the migration of glutamate decarboxylase (GAD) positive cortical neurons in the rat brain and act via a G-protein coupled mechanism, whereas femtomolar GABA increased motility in GAD-negative ventricular zone neurons and appeared to act via a GABAA receptor-mediated mechanism (Behar et al. 1998). Tangential migration also appears to be modulated by the action of GABA at GABAB receptors, as evidenced by results obtained from in vitro embryonic organotypic culture of rat brain slices. Blocking these GABAB receptors with a specific antagonist CGP52432 in culture led to the accumulation of GABAergic interneurons in the ventricular/subventricular zones (VZ/SVZ) of the cortex and a parallel decrease in the number of these migrating neurons in the cortex. The accumulated population of neurons also displayed morphological differences compared to controls, with significantly shorter leading processes than cells in control slices (López-Bendito et al. 2003).
4. GABA in proper cortical network development and plasticity
Proper cortical network development and function has been shown to rely on appropriate modulation of inhibitory control. When the efficacy of the cortical GABAergic inhibitory system is decreased even just slightly in juvenile (14–18 day) rats, long-latency field potentials are expressed. Cortical sections from juvenile rats that were incubated in a bath solution containing the GABAA receptor antagonist bicuculline methiodide (BMI) displayed such long-latency field potentials upon orthodromic stimulation of underlying white matter/Layer VI of the cortex. Application of D(−)-amino-5-phosphono-valeric acid (DAPV), a NMDAR selective antagonist, blocked this long-latency response thus establishing its dependence upon NMDAR activity. One possible role for this long-latency activity is to ensure that delayed afferent stimuli could still reach threshold and stabilize activity-dependent connections critical for functional network development (Luhmann et al.1990).
The developing brain displays periods of plasticity, during which modulation can affect the structure and connectivity of the mature brain. One classical model of critical period plasticity is the visual cortex. Studies show that the GABAergic inhibitory system modulates onset of the critical period in the visual cortex. Enhancing GABAergic inhibition in a rodent model by infusing the newly opened eye with benzodiazepines, a GABAA receptor allosteric modulator, results in an earlier onset critical period. Conversely, deletion of GAD65 shifts the onset of critical period later. In fact, application of the benzodiazepine diazepam was sufficient to fully induce plasticity in a monocular deprivation model even in GAD65 knockout mice (Iwai et al. 2003). Interestingly, reduction of intracortical inhibition can partially reactivate ocular dominance plasticity in the adult mouse cortex (Harauzov et al. 2010).
GABAergic inhibition was also shown to affect the structural development of visual columns. Infusion of diazepam in the cat visual cortex resulted in wider than usual columns, whereas infusion of an inverse agonist (methyl-6,7-dimethoxy-4-ethyl-beta-carboline-3-carboxylate) DMCM led to the development of narrower than usual columns (Hensch and Stryker 2004).
6. GABA in the white matter
Neurons located in the adult cortical white matter are termed interstitial neurons, which can be largely classified into GABAergic and glutamatergic subtypes, though the exact proportions are unknown and a potential area for future research (Suarez-Sola 2009; Judaš et al. 2010). Generally, interstitial neurons of the white matter are accepted to be the remnants of a subplate population of neurons (Chun and Shatz 1989). Studies in cat and rodent models show that those neurons which remain in the subplate either undergo cell death or become adult interstitial neurons, as the subplate develops into adult white matter. Therefore, the interstitial neurons of the adult neocortex are likely the oldest neurons in the cerebral cortex, due to their origin in the subplate (Chun and Shatz 1989). A recent study has proposed that there are least two distinct and topographically separate interstitial neuron populations in the human cortex, one arising from fetal subplate neurons and manifesting as superficial interstitial neurons in adult white matter, and the other arising from the subventricular/intermediate zones in the fetus and developing into deep interstitial neurons of the adult periventricular and central white matter (Judaš et al.2010). Notably, this second population is absent in non-primates. In general, interstitial neuronal development and presence in white matter differs significantly between humans and non-humans. This poses a significant problem given that most information on this population of neurons is derived from studies in non-human models, and suggests a critical area of further investigation.
As previously stated, interstitial neurons of the white matter can be classified as GABAergic but specific information on their proportion, development, and migration is poorly elucidated. As studies on the origin of cortical GABAergic interneurons have revealed, GABAergic neurons of the cortex arise from the subplate or intermediate/subventricular zone and migrate through the white matter into the overlying cortex (Letinic et al. 2002; Xu et al. 2011). In human periventricular and central white matter, this migration occurs mostly over the second half of gestation, peaks at term, and decreases dramatically after birth. At least 20% of GABAergic neurons in the white matter was found to migrate towards the cortex during the second half of gestation. A population of non-migrating GABAergic neurons that remained in the developing white matter and subplate may become fixed in the subplate (which eventually becomes adult white matter) and white matter, or undergo programmed loss postnatally (Xu et al. 2011). Evidence in a feline model shows that that cortical interstitial neurons of the mature cat cortex originated in and distributed throughout the cortex from an early subplate population. Furthermore, a similar decrease in density was seen postnatally that was likely due to cell death, as suggested by a parallel increase in small, atrophic neurons (Chun and Shatz 1989). Postnatally, a population of GABAergic interneuron precursors are seen in the dorsal white matter area of the cortex. These GAD65-GFP positive cells later migrate into the overlaying anterior cingulate cortex, a process that was seen up to P21 and suggested that these GABAergic precursors could be a reservoir for postnatally migrating interneurons (Riccio et al. 2012). In the adult white matter, a population of large, aspiny NADPH-d neurons that express GABA was found to localize in the superficial white matter of non-human primates (Yan et al. 1996). In the spine, long GABAergic fibers can be seen in the ventral white matter as early as E12.5 (Allain et al. 2004).
7. GABA and oligodendrocytes
Oligodendroglial cells are the cells that give rise to myelin in the CNS and thus a major component of CNS white matter. The subpallium which gives rise to GABAergic neurons also generates oligodendrocytes that migrate in a similarly tangential path to the cortex (Marin and Rubenstein 2001). Therefore, it is possible that oligodendrocytes and GABAergic neurons share further characteristics due to their development in a similar milieu. Interactions between GABAergic neurons and oligodendrocyte precursor cells (OPCs) have been shown to occur in the hippocampus, with GABA acting at OPC GABAA receptors to increase intracellular Ca2+ levels (Lin and Bergles 2004). Developing oligodendrocytes express GABAA receptors, and GABA acting at these receptors has been shown to depolarize the developing cell (Káradóttir and Attwell 2007).
Activity at GABAA receptors has also been shown to exhibit a protective effect on oligodendrocytes. This effect is dependent upon and modulated by the activity of NKCC1, which maintains a high intracellular Cl− concentration. The protective effect of GABAA activity in oligodendrocyte survival may be due to a GABAA receptor mediated rise in intracellular Ca2+ levels (Wang et al. 2003). Activation of GABAA receptors with 30 µM of the GABAA agonist muscimol significantly reduced oligodendrocyte mortality in the absence of growth factors, and this neuroprotective affect required NKCC1 activation. Blockage of NKCC1 activity with 10 µM of the NKCC1 antagonist bumetanide negated any protective effect. There is evidence that oligodendrocytes also express GABAB receptors and activation of these receptors increases the proliferation and migration of oligodendroglial precursor cells, via a negatively coupled adenlyl cyclase (AC) signaling pathway. Application of the GABAB receptor agonist baclofan significantly reduced cyclic adenosine monophosphate (cAMP) and AC and led to an increase in migration (Luyt et al. 2007).
Oligodendrocytes arise from the white matter glial precursor cell O-2A, as do type-1 astrocytes. Optic nerve O-2A cells in culture have been shown to express GABA, though its synthesis does not appear to involve GAD (Barres et al. 1990). It remains unclear precisely what the effect of this GABA synthesis may be. O-2A cells are not the sole oligodendryocyte precursor to have connections to the GABAergic system. Nerve/glial antigen 2 expressing oligodendrocyte precursor (NG2) cells have been shown to receive direct synaptic input from axons (Lin and Bergles, 2004). Some of these synapses are GABAergic, as NG2 cells also have been shown to express GABAA receptor, although it is not known precisely what effect activity at these synpases might have. There is some evidence that exogenously applied GABA can induce an intracellular rise in Ca2+ concentrations (Mangin and Gallo 2011). NG2 cells are the main source of oligodendrocytes as well as the main proliferating cells in white and gray matter of the postnatal adult brain. They undergo proliferation in response to trauma, infection and demyelination. NG2 cells have been shown to form neuron-NG2 synapses during the process of spontaneous remylination following demylination, a response that often contributes to the the formation of a glial scar (Mangin and Gallo 2011).
II. GABA and GABAergic neurons in immature brain injury
1. GABA and its role in white matter injury: anoxia-ischemia and periventricular leukomalacia
Several white matter lesions may proceed through mechanisms during development that involve the GABAergic system, including anoxic-ischemic injury, periventricular leukomalacia (PVL), and schizophrenia. Release of GABA in the white matter has been shown to have a neuroprotective effect following anoxic injury, as measured by post-anoxic compound action potential (CAP) recovery in the rat optic nerve (Fern et al. 1995). Selective GABAA receptor antagonists such as bicuculline and agonists such as muscimol did not influence CAP recovery. In contrast, the GABAB receptor specific antagonist phaclofen completely abolished the neuroprotective effect. Furthermore, perfusion of nerve tissue with pertussis toxin (PTX), an irreversible G-protein inhibitor, significantly reduced CAP recovery, as did perfusion of nerve tissue with the PKC-inhibitor staurosporine. Taken together, these findings implicate a GABAB/G protein/PKC dependent protective pathway that is activated following anoxia. Notably, this protective pathway is in place even under control conditions, thus demonstrating that endogenous GABA is sufficient for this protective pathway. The authors suggest that this second messenger system could target the Na+/Ca2+ exchanger, as downregulation of this membrane transport protein could produce a protective effect consistent with the data (Fern et al. 1995). Interestingly, there have been findings that increased GABA synthesis produces a neuroprotective effect on GABAergic neurons in the striatum (Li et al.2010). While these results were observed in GABAergic neurons of the grey matter, it raises the question whether presynaptic GABA synthesis may similarly be elevated following white matter injury events.
Comparison of telencephalon sections of human neonates (25–32 weeks of gestation) with white matter lesions (WMLs) and without WMLs revealed differential loss of GABAergic neurons and oligodendrocytes in the white matter and subplate. Sections from neonates with white matter lesions showed a fourfold decrease in density of GAD67 cells, as well as a significant decrease in GABAergic receptor expression in the subplate and cortex as compared to controls. While a loss of GABA receptor expression in the subplate and increased receptor expression in the cortex is expected with migration of GABAergic cells from the subplate to the cortex, this decrease of expression in both subplate and cortex indicates a significant loss of GABA receptor expression in infants with WMLs (Robinson et al. 2006). Since this period of time corresponds to a window of increased migration of late-born GABAergic neurons through the white matter, it is likely that these findings are due to injury to this population of migrating neurons. Infants who suffer periventricular leukomalacia (PVL), the most common WML responsible for cognitive deficits of preterm infants, often exhibit a sequelae of cognitive defects that may arise from abnormal cortical development as a result of damage to these migrating GABAergic neurons (Robinson et al. 2006).
GABA and endogenously produced adenosine can interact to produce a neuroprotective effect during white matter anoxia in the optic nerve. The mechanism also appears to be PKC-mediated, as administration of staurosporine abolished the observed neuroprotection. Interaction between GABA and adenosine appears to be synergistic at low concentrations, whereas high concentrations of one in the presence of the other removed any protective effect (Fern et al. 1994). In PVL, there is evidence that a population of migrating doublecortin expressing (DCX+) cells may travel to necrotic foci and contribute to neuronal regeneration/repair. A subpopulation of these DCX+ cells was shown to express GAD 65/67, which is consistent with the idea that migratory DCX+ cells differentiate into the GABAergic phenotype (Haynes et al.2011).
In the human brain, granular subcortical (i.e. subplate and white matter) neurons were shown to be 50–80% less in density in white matter lesion slices than in controls. While the study did not specifically determine which neurotransmitter the granular subcortical neurons expressed, it is known that granular neurons in the subventricular zone/ventricular zone, white matter, and subplate express GAD65/67. Thus, there is a likelihood that the lost granular subcortical neurons were GABAergic in nature (Kinney et al. 2012).
In PVL and other chronic white matter hypoxia-ischemia pathologies, astrogliosis and microgliosis, both signifiers of CNS cell damage, can be seen. Also seen is hypomyelination, although interestingly, this is not accompanied by a significant decrease in oligodendrocytelineage (OL) cell density. One possible explanation for this retention of OL cell density is the possibility that oligodendrocyte progenitor cells, including NG2 cells, proliferate following injury and thus mask any loss of oligodendrocytes. The authors suggest that the hypomyelination may be due to inadequate repair of necrotic foci, improper maturation of OL progenitors, an inability of mature OL to produce myelin, and/or lesion to the axon that arrests proper signaling for myelin production (Billiards et al. 2008). Another study supports the suggestion that a mechanism for the observed hypomyelination in PVL involves abnormal maturation of OL cells. It was shown that in a neonatal rat model, late oligodendrocyte precursors (pre-OLS) displayed arrested maturation, as well as failure to differentiate and produce myelin following chronic perinatal white matter injury (Segovia et al. 2008).
2. GABA and Schizophrenia
A substantial body of evidence points to developmental abnormalities as a crucial part of the etiology of schizophrenia. In particular, studies show a link between dramatic abnormalities in the white matter and this disease (Suarez-Sola 2009). A significant general increase in the density of superficial interstitial white matter neurons (IWMNs), a population of neurons that contains many of GABAergic phenotype, was seen in human brain tissue of schizophrenics (Anderson et al.1996; Eastwood and Harrison 2003; Yang et al. 2011). Intriguingly, an increase in GABAergic interstitial white matter neurons (IWMNS) found in the dorsolateral prefrontal cortex white matter was found to be accompanied by a deficit of GABAergic interneuron markers in the overlying gray matter (Yang et al. 2011). A later study of GABAergic IWMNS in the orbital frontal cortex of schizophrenics similarly showed a GABAergic deficit in the overlying gray matter (Joshi et al. 2012). These findings suggest that GABAergic interneuron decreases in the cortical grey matter are mirrored by increased density of neurons deeper in the white matter (Yang et al.2011; Joshi et al. 2012; Connor et al. 2012). One possible explanation for this finding is that the migration of interneurons from white matter to the cortex may be abnormal among schizophrenic patients (Yang et al. 2011). In support of the neurodevelopmental hypothesis for deficits seen in the schizophrenic brain, findings suggest that the second trimester in humans is a time period when insults that lead to abnormalities seen in schizophrenia are most likely to arise (Eastwood and Harrison 2003). Interestingly, the latter half of gestation (28–38 weeks) is also the time period during which GAD67 cell density increases the most in human subplate and white matter, indicating a window of maximal migration. It is possible that this period of high GABAergic neuron migration may be a period of vulnerability that leads to schizophrenic cortical deficits. This view is supported by the distribution of upregulation in GABAA receptor binding in the prefrontal cortex of schizophrenics, with receptor binding most markedly apparent in cortical layer II, the last lamina to appear during corticogenesis (Benes et al. 1996). However, other potential explanations for the increase in IWMNS—such as in response to changes in the adult CNS—cannot be ruled out (Conner et al. 2012).
Another observation in schizophrenic brains that may connect to GABA is a decrease of reelin mRNA per cell. Reelin, a key developmental signaling molecule, is an extracellular matrix protein shown to be secreted by GABAergic interneurons in the telencephalon and plays a critical role in neuronal migration, proper morphology development, and synaptogenesis (Pesold et al. 1998; Rice and Curran 2001). In patients with schizophrenia, the gene transcript for reelin, RELN, has been shown to be significantly decreased in inhibitory white matter neurons (Eastwood and Harrison 2003). Reelin is also found in GABAergic neurons of the adult rat cortex and hippocampus (Pesold et al. 1998). Studies using a heterozygous reeler mouse (HRM) which displays roughly half the reelin expression of controls exhibits similar behaviors and structural deficits to those found in schizophrenic patients. Furthermore, schizophrenic patients show a decrease in GABAergic Purkinje neurons in the cerebellum, as well as a decrease in reelin mRNA expression in cerebellar granular cells. This decrease in Purkinje neurons is similarly found in HRM. Taken together, this seems to indicate that reelin modulates the expression of GABAergic Purkinje neurons and is dysregulated in schizophrenia (Maloku et al. 2010).
Significant gray matter abnormalities are also seen in schizophrenia. Several studies have shown a decrease in GAD67 expression in the frontal cortex and a few give evidence of a similar decrease in GAD65 expression (Akbarian and Huang 2006). Both GAD67 and GAD65 are key enzymes involved in the synthesis of GABA. Studies have also shown a decrease in GABA release thus pointing to downregulation in presynaptic GABAergic function in schizophrenia (Costa et al.2001). Furthermore, reuptake of GABA via GABA transporter 1 (GAT1) has been shown to be downregulated in the schizophrenic brain, suggesting decreased GABA reuptake. The authors also provided evidence that this finding is supported by schizophrenia-like behaviors displayed in by GAT1 knockout mice (Yu et al. 2013).
The developmental shift from GAD25, a gene transcript related to GABA signaling, to GAD67 is important for proper GABA synthesis. Evidence exists that a significantly increased GAD25/GAD67 ratio is seen in the hippocampus of patients with schizophrenia, suggesting improper maturation of the GABAergic system (Hyde et al. 2011). It has also been suggested that there may be a link between the downregulation of GAD67 expression and the previously mentioned downregulation of reelin (Costa et al. 2001). The authors propose that decreased reelin expression may decrease the overall number of GABAergic axon terminals by decreasing the overall number of dendritic spines, and in this way explain the downregulation of GAD67 (Costa et al. 2001).
The chloride-cation transporters NKCC1 and KCC2 have also been implicated in the pathology of schizophrenia. A general NKCC1/KCC2 expression imbalance has been described in patients with schizophrenia. A decreased ratio of NKCC1/KCC2 expression indicates the proper switch has occurred from expression of the early-acting NKCC1 transporter to expression of the later-acting KCC2 transporter, a hallmark of the mature brain. However, in schizophrenia, the NKCC1/KCC2 ratio is seen to be significantly increased. Findings that both a NKCC1/KCC2 ratio increase and a GAD25/GAD65 ratio increase were significantly predicted by the GAD1 genotype suggests that the mechanism underlying these observed abnormalities is genetic (Hyde et al. 2011). Furthermore, the GAD1 phenotype is associated with expression of a specific KCC2 transcript, AK098371, that is seen to be lower in schizophrenic patients than controls. Another KCC2 transcript, EXON6B, was shown to be significantly increased in schizophrenic patients. Together, this suggests that different transcripts of KCC2 may play a role in the abnormalities seen with the GABAergic system in schizophrenia (Tao et al. 2012).
III. GABA and GABAergic neurons in mature brain injury
1. GABAergic system in the mature brain
Networks of the adult cortex are generally built from excitatory projection neurons with long processes that are then modulated by local inhibitory interneurons expressing GABA receptors (Ben-Ari 2004; Druga 2009). GABAergic interneurons display a remarkable level of chemical, morphological, and connection heterogeneity, suggesting a wide array of specific functions for these neurons. Approximately 20–30% of cortical neurons in the mature brain are inhibitory interneurons (Druga 2009). Nearly all GABAergic interneurons can be classified into three broad groups based upon expression of the calcium-binding protein parvalbumin (PV), the neuropeptide somatostatin (SST), and the serotonin receptor 5HT3aR. Each group contains further types of interneurons with different morphology, properties, and function (for detailed review, see Rudy et al. 2011).
2. Transient ischemia in the mature brain
Transient global ischemia, also termed “mini-stroke,” can induce selective neuronal death. In a rodent model, large aspiny cholinergic neurons (LA) and most GABAergic interneurons of the striatum survive transient ischemia whereas medium spiny neurons (MS) eventually die. In fact, inhibitory synaptic transmission in LA neurons is enhanced. Application of the selective GABAA receptor agonist muscimol 24 hours following ischemia was shown to increase inhibitory transmission and paired-pulse data suggested that the increased inhibitory transmission was due to increased presynaptic release. Taken together, these findings suggest that increased inhibitory transmission in LA neurons is mediated by presynaptic GABAA receptors, although the exact presynaptic location of these receptors is not known (Li et al. 2009). A later study by the same authors found that expression of GAD65 in the striatum is increased significantly following ischemia, strongly suggesting increased GABA synthesis by the remaining MS neurons. The increase in GAD65-expressing neurons was also shown to arise from tonically active interneurons expressing GAD67 that undergo a genetic shift to express GAD65 following ischemia (Li et al. 2010). The overall neuroprotective mechanism may be one in which increased inhibition on LA neurons counterbalances excitotoxicity, a broadly accepted major cause of postischemic neuronal cell death (Lipton 1999).
3. Stroke and GABA
Stroke is a leading cause of both death and disability. Increasingly, evidence shows that the GABAergic system may play a role in the neuronal affects of stroke as well as in the potential for plasticity and repair. Tonic GABAA receptor activity is involved in long-term potentiation (LTP), functional organization of neuronal circuits, and plasticity in the brain. Whole-cell voltage-clamp recordings of mouse brain slices post-infarct show that tonic inhibition is increased in the peri-infarct zone (Clarkson et al. 2010). This tonic inhibition has been shown to be mediated by extrasynaptic GABAA receptors (Lee and Maguire 2014). Indeed, application of L655,708, an inverse agonist specific to the benzodiazepine binding site on extrasynaptic GABAA receptors, resulted in early and sustained motor function recovery after stroke. Genetic knockdown of GABAA receptors containing α5 and δ subunits, found primarily in extrasynaptic GABAA receptors, resulted in a similar increase functional recovery. These findings suggest chronic tonic inhibition mediated by extrasynaptic GABAA receptors following stroke impairs the plasticity required for functional recovery (Clarkson et al. 2010). A specific mechanism suggested may be a dysfunction of GABA reuptake from extracellular fluid, as tonic inhibition is mediated by the degree of this reuptake via GABA transporter proteins (GATs). Indeed, a reduced level of GAT 3/4 function was seen in the peri-infarct zone (Clarkson et al. 2010).
4. Epilepsy, Traumatic Brain Injury (TBI) and Post-Traumatic Epilepsy (PTE)
Epileptoform activity is strongly affected by GABAA receptor mediated inhibition. Slight suppression of inhibition induced by low concentrations (≤ 0.5µM) of the GABAA receptor antagonist bicuculline under conditions of focal stimulation was sufficient for horizontal spread of neural activity mimicking partial epilepsy (Chagnac-Amital and Connors 1989).
Traumatic brain injury (TBI) often results in epileptic-related changes to the brain and may eventually lead to long-term reorganization of neural circuits. Post-traumatic epilepsy (PTE), a consequence of TBI, shows certain pathophysiologies involving the GABAergic system. Evidence exists for a loss in inhibitory interneurons following experimental TBI in animal models (Hunt et al. 2013). In humans, postmortem analysis of the dentate gyrus shows substantial loss of hilar interneurons populations in those with PTE. These losses are sometimes accompanied by decreased synaptic inhibition of granule cells (Hunt et al. 2013). Interestingly, findings in a rodent model of TBI appear to show an increase in GABAA receptor mediated tonic inhibition following injury. The authors suggest that tonic inhibition may be a novel mechanism by which TBI contributes to functional impairment, notably loss of memory and cognitive function (Mtchedlishvili et al. 2010).
Another potential area of GABAergic influence is the inflammatory response following TBI. Inflammatory cytokines are known to modulate GABAA receptor mediated responses and glial overexpression of certain cytokines lead to spontaneous seizures and decrease in GABAergic cells. Chemokines may also increase the release of neurotransmitters including GABA, which may contribute to imbalance in network excitability and epileptogenesis after TBI (Hunt et al. 2013). In previous studies, interleukin-1 (IL-1) was shown to enhance postsynaptic GABAA receptor function (Miller et al. 1991). Given the important role inhibitory interneurons play in managing the balance of excitation and inhibition across cortical networks, and the relatively little that is currently known about which interneuron populations are affected by mechanical trauma, it is suggested that more research be conducted to better elucidate the effects of TBI on the inhibitory system.
SUMMARY
GABA, the main inhibitory transmitter in the adult brain, has a multitude of different functions during development and influences the migration, proliferation, and proper morphological development of neurons, as well as the timing of critical periods and potentially primes the earliest neuronal networks. These effects of GABA are through depolarizing actions of GABAA receptors. Furthermore, development-dependent regulation of the cation-chloride transporters NKCC1 and KCC2 controls Cl− homeostasis and dictates the “GABA shift” in the CNS. The GABAergic system encompasses cortical interneurons as well as white matter interneurons and connections to oligodendroglial cells. The latter two have been implicated in diseases of the white matter, including perinatal white matter injuries and schizophrenia. The specific roles of GABAergic neurons in the pathogenesis of these diseases are not well understood. Studies suggest the most dramatic white matter pathology in schizophrenia links its pathology to a diminishment of the GABAergic system at both the physiological and genetic levels. The GABAergic system is also robustly involved in the pathology of several brain injuries in the mature brain. Increased inhibitory activity at GABAA receptors appears to have a neuroprotective role in transient ischemia whereas tonic inhibitory activity at extrasynaptic GABAA receptors may play a detrimental role in stroke pathology. GABAA receptor mediated inhibition provides a strong restraint on epileptoform activity, a common consequence of traumatic brain injury. Taken together, a better understanding of the GABAergic system in the developing brain as well as under pathophysiological conditions is warranted.
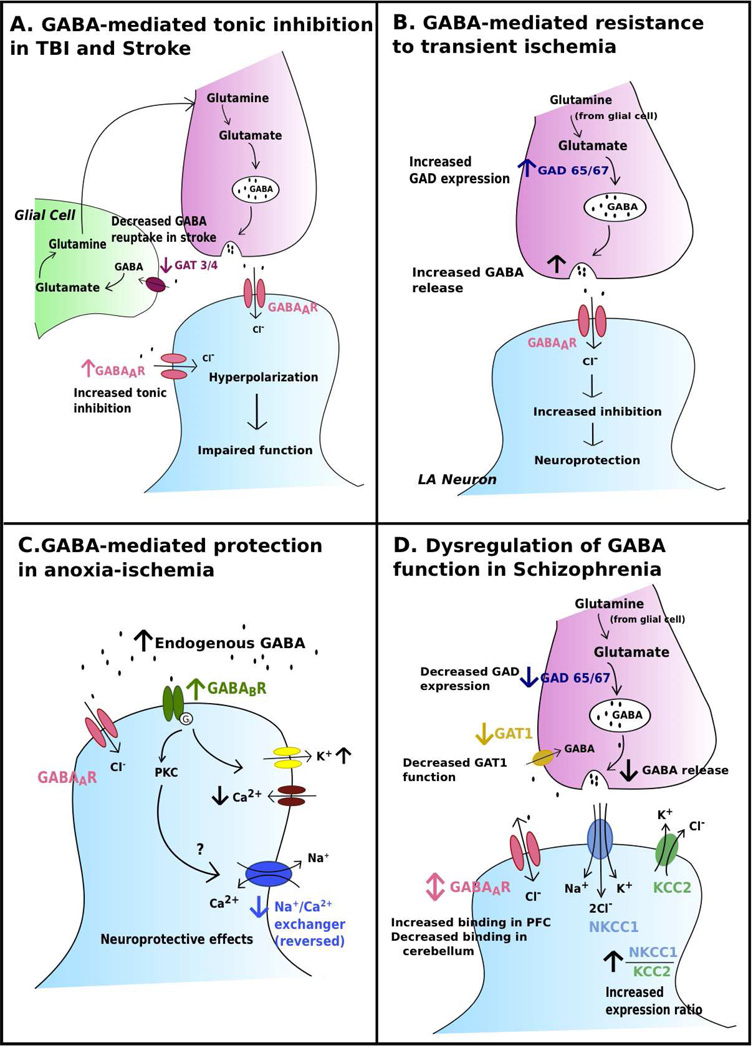
Figure 1. GABA receptors in disease.
a. Traumatic Brain Injury (TBI) and Stroke pathologies appear to involve increased tonic inhibition mediated by extrasynaptic GABAA receptors (Clarkson et al. 2010; Mtchedlishvili et al. 2010). Increased tonic inhibition in TBI may contribute to subsequent functional impairment (Mtchedlishvili et al. 2010). Increased tonic inhibition impairs functional recovery following stroke; this increase may be a result of reduced function of GABA transporter 3/4 (GAT 3/4) mediated GABA reuptake (Clarkson et al. 2010). b. Increased GABA release enhances neuroprotective inhibitory transmission in large aspiny (LA) neurons (Li et al. 2009). This effect is most likely mediated by presynaptic GABAA receptors, however the exact location of these receptors is not known. GAD expression increased following ischemia, which strongly suggests increased GABA synthesis.
C. Endogenous GABA acts at GABAB receptors to mediate neuroprotective effects following anoxia-ischemia in white matter (Fern et al. 1995). A possible target of the PKC second messenger system recruited is the Na+ /Ca2+ exchanger, which is reversed under anoxic conditions. Downregulation of the Na+ /Ca2+ exchanger may lead to neuroprotective effects (Fern et al. 1995). GABAB receptors also act upon K+ and Ca2+ channels (see text).
D. Gray matter abnormalities seen in schizophrenia include a decrease in GAD expression and decrease in presynaptic GABA release (Costa et al. 2001; Akbarian and Huang 2006). Reuptake of GABA via GABA transporter 1 (GAT1) is decreased (Yu et al. 2013). The ratio of NKCC1/KCC2 expression is increased in patients with schizophrenia, suggesting abnormal maturation, as normal maturation is accompanied by increased KCC2 expression and decreased NKCC1 expression (Hyde et al. 2011).
ACKNOWLEDGMENT
This work was supported in part by NIH grant R01NS38118, R01NS075995 (D. Sun).
REFERENCES
- Akbarian Schahram, Huang Hsien-Sung. Molecular and Cellular Mechanisms of Altered GAD1/GAD67 Expression in Schizophrenia and Related Disorders. Brain Research Reviews. 2006 Sep;52(no. 2):293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Allain Anne-Emilie, Baїri Alexia, Meyrand Pierre, Branchereau Pascal. Ontogenic Changes of the GABAergic System in the Embryonic Mouse Spinal Cord. Brain Research. 2004 Mar;1000(no. 1–2):134–147. doi: 10.1016/j.brainres.2003.11.071. [DOI] [PubMed] [Google Scholar]
- Anderson Stewart A, Volk David W, Lewis David A. Increased Density of Microtubule Associated Protein 2-Immunoreactive Neurons in the Prefrontal White Matter of Schizophrenic Subjects. Schizophrenia Research. 1996 May;19(no. 2–3):111–119. doi: 10.1016/0920-9964(96)88521-5. [DOI] [PubMed] [Google Scholar]
- Anderson SA. Interneuron Migration from Basal Forebrain to Neocortex: Dependence on Dlx Genes. Science. 1997 Oct 17;278(no. 5337):474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson Stewart A, Kaznowski Christine E, Horn Carrie, Rubenstein John LR, McConnell Susan K. Distinct Origins of Neocortical Projection Neurons and Interneurons In Vivo. Cerebral Cortex. 2002 Jul 1;12(no. 7):702–709. doi: 10.1093/cercor/12.7.702. [DOI] [PubMed] [Google Scholar]
- Antonopoulos J, Pappas IS, Parnavelas JG. Activation of the GABAA Receptor Inhibits the Proliferative Effects of bFGF in Cortical Progenitor Cells. The European Journal of Neuroscience. 1997 Feb;9(no. 2):291–298. doi: 10.1111/j.1460-9568.1997.tb01399.x. [DOI] [PubMed] [Google Scholar]
- Barres BA, Koroshetz WJ, Swartz KJ, Chun LL, Corey DP. Ion Channel Expression by White Matter Glia: The O-2A Glial Progenitor Cell. Neuron. 1990 Apr;4(no. 4):507–524. doi: 10.1016/0896-6273(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of Γ-Aminobutyric AcidA Receptors: Classification on the Basis of Subunit Structure and Receptor Function. Pharmacological Reviews. 1998 Jun 1;50(no. 2):291–314. [PubMed] [Google Scholar]
- Behar Toby N, Schaffner Anne E, Scott Catherine A, O’Connell Casey, Barker Jeffery L. Differential Response of Cortical Plate and Ventricular Zone Cells to GABA as a Migration Stimulus. The Journal of Neuroscience. 1998 Aug 15;18(no. 16):6378–6387. doi: 10.1523/JNEUROSCI.18-16-06378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Tseeb V, Raggozzino D, Khazipov R, Gaiarsa JL. Gamma-Aminobutyric Acid (GABA): A Fast Excitatory Transmitter Which May Regulate the Development of Hippocampal Neurones in Early Postnatal Life. Progress in Brain Research. 1994;102:261–273. doi: 10.1016/S0079-6123(08)60545-2. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Yehezkel, Khalilov Ilgam, Represa Alfonso, Gozlan Henri. Interneurons Set the Tune of Developing Networks. Trends in Neurosciences. 2004 Jul 1;27(no. 7):422–427. doi: 10.1016/j.tins.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Marie A, Khan Y. Up-Regulation of GABAA Receptor Binding on Neurons of the Prefrontal Cortex in Schizophrenic Subjects. Neuroscience. 1996 Nov 1;75(no. 4):1021–1031. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- Billiards Saraid S, Haynes Robin L, Folkerth Rebecca D, Borenstein Natalia S, Trachtenberg Felicia L, Rowitch David H, Ligon Keith L, Volpe Joseph J, Kinney Hannah C. Myelin Abnormalities without Oligodendrocyte Loss in Periventricular Leukomalacia. Brain Pathology (Zurich, Switzerland) 2008 Apr;18(no. 2):153–163. doi: 10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov Yuri A, Benavidez Jillian M, Black Mendy, Leiter Courtney R, Osterndorff-Kahanek Elizabeth, Johnson David, Borghese Cecilia M, et al. GABAA Receptors Containingγ1 Subunits Contribute to In Vivo Effects of Ethanol in Mice. PLoS ONE. 2014 Jan 16;9(no. 1) doi: 10.1371/journal.pone.0085525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boue-Grabot EM, L Roudbaraki, Tramu Bascles, G, Bloch B, Garret M. Expression of GABA Receptor Rho Subunits in Rat Brain. Journal of Neurochemistry. 1998 Mar;70(no. 3):899–907. doi: 10.1046/j.1471-4159.1998.70030899.x. [DOI] [PubMed] [Google Scholar]
- Cancedda Laura, Fiumelli Hubert, Chen Karen, Poo Mu-ming. Excitatory GABA Action Is Essential for Morphological Maturation of Cortical Neurons In Vivo. The Journal of Neuroscience. 2007 May 9;27(no. 19):5224–5235. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Connors BW. Horizontal Spread of Synchronized Activity in Neocortex and Its Control by GABA-Mediated Inhibition. Journal of Neurophysiology. 1989 Apr;61(no. 4):747–758. doi: 10.1152/jn.1989.61.4.747. [DOI] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, van den Pol AN. GABA Receptors Precede Glutamate Receptors in Hypothalamic Development; Differential Regulation by Astrocytes. Journal of Neurophysiology. 1995 Oct;74(no. 4):1473–1484. doi: 10.1152/jn.1995.74.4.1473. [DOI] [PubMed] [Google Scholar]
- Chun Jerold JM, Shatz Carla J. Interstitial Cells of the Adult Neocortical White Matter Are the Remnant of the Early Generated Subplate Neuron Population. Journal of Comparative Neurology. 1989;282(no. 4):555–569. doi: 10.1002/cne.902820407. [DOI] [PubMed] [Google Scholar]
- Clarkson Andrew N, Huang Ben S, MacIsaac Sarah E, Mody Istvan, Thomas Carmichael S. Reducing Excessive GABAergic Tonic Inhibition Promotes Post-Stroke Functional Recovery. Nature. 2010 Nov 11;468(no. 7321):305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor Caroline M, Crawford Benjamin C, Akbarian Schahram. White Matter Neuron Alterations in Schizophrenia and Related Disorders. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience. 2011 May;29(no. 3):325–334. doi: 10.1016/j.ijdevneu.2010.07.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Erminio, Davis John, Grayson Dennis R, Guidotti Alessandro, Pappas George D, Pesold Christine. Dendritic Spine Hypoplasticity and Downregulation of Reelin and GABAergic Tone in Schizophrenia Vulnerability. Neurobiology of Disease. 2001 Oct;8(no. 5):723–742. doi: 10.1006/nbdi.2001.0436. [DOI] [PubMed] [Google Scholar]
- Couve Andrés, Moss Stephen J, Pangalos Menelas N. GABAB Receptors: A New Paradigm in G Protein Signaling. Molecular and Cellular Neuroscience. 2000 Oct;16(no. 4):296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- Del Rio JA, Soriano E, Ferrer I. Development of GABA-Immunoreactivity in the Neocortex of the Mouse. The Journal of Comparative Neurology. 1992 Dec 22;326(no. 4):501–526. doi: 10.1002/cne.903260403. [DOI] [PubMed] [Google Scholar]
- Druga R. Neocortical Inhibitory System. Folia Biologica. 2009;55(no. 6):201–217. [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial White Matter Neurons Express Less Reelin and Are Abnormally Distributed in Schizophrenia: Towards an Integration of Molecular and Morphologic Aspects of the Neurodevelopmental Hypothesis. Molecular Psychiatry. 2003;8(no. 9):821–831. doi: 10.1038/sj.mp.4001399. [DOI] [PubMed] [Google Scholar]
- Fern Robert, Waxman Stephen G, Ransom BRUCER. Modulation of Anoxic Injury in CNS White Matter by Adenosine and Interaction between Adenosine and GABA. Journal of Neurophysiology. 1994;72(no. 6):2609–2616. doi: 10.1152/jn.1994.72.6.2609. [DOI] [PubMed] [Google Scholar]
- Fern R, Waxman SG, Ransom BR. Endogenous GABA Attenuates CNS White Matter Dysfunction Following Anoxia. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1995 Jan;15(no. 1 Pt 2):699–708. doi: 10.1523/JNEUROSCI.15-01-00699.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauzov Alexey, Spolidoro Maria, DiCristo Graziella, De Pasquale Roberto, Cancedda Laura, Pizzorusso Tommaso, Viegi Alessandro, Berardi Nicoletta, Maffei Lamberto. Reducing Intracortical Inhibition in the Adult Visual Cortex Promotes Ocular Dominance Plasticity. The Journal of Neuroscience. 2010 Jan 6;30(no. 1):361–371. doi: 10.1523/JNEUROSCI.2233-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama Nobuya, Shibuya Izumi, Tanaka Keiko, Kabashima Narutoshi, Ueta Yoichi, Yamashita Hiroshi. Inhibition of N- and P/Q-Type Calcium Channels by Postsynaptic GABAB Receptor Activation in Rat Supraoptic Neurones. The Journal of Physiology. 1998 Jun 1;509(no. 2):371–383. doi: 10.1111/j.1469-7793.1998.371bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydar Tarik F, Wang Feng, Schwartz Michael L, Rakic Pasko. Differential Modulation of Proliferation in the Neocortical Ventricular and Subventricular Zones. The Journal of Neuroscience. 2000 Aug 1;20(no. 15):5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes Robin L, Xu Gang, Folkerth Rebecca D, Trachtenberg Felicia L, Volpe Joseph J, Kinney Hannah C. Potential Neuronal Repair in Cerebral White Matter Injury in the Human Neonate. Pediatric Research. 2011 Jan;69(no. 1):62–67. doi: 10.1203/PDR.0b013e3181ff3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennou Sonia, Khalilov Ilgam, Diabira Diabé, Ben-Ari Yehezkel, Gozlan Henri. Early Sequential Formation of Functional GABAA and Glutamatergic Synapses on CA1 Interneurons of the Rat Foetal Hippocampus. European Journal of Neuroscience. 2002;16(no. 2):197–208. doi: 10.1046/j.1460-9568.2002.02073.x. [DOI] [PubMed] [Google Scholar]
- Hensch Takao K, Stryker Michael P. Columnar Architecture Sculpted by GABA Circuits in Developing Cat Visual Cortex. Science (New York, N.Y.) 2004 Mar 12;303(no. 5664):1678–1681. doi: 10.1126/science.1091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner Christian A, Stein Valentin, Hermans-Borgmeyer Irm, Meyer Torsten, Ballanyi Klaus, Jentsch Thomas J. Disruption of KCC2 Reveals an Essential Role of K-Cl Cotransport Already in Early Synaptic Inhibition. Neuron. 2001 May;30(no. 2):515–524. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Hunt Robert F, Boychuk Jeffery A, Smith Bret N. Neural Circuit Mechanisms of Post-Traumatic Epilepsy. Frontiers in Cellular Neuroscience. 2013 Jun 18;7 doi: 10.3389/fncel.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde Thomas M, Lipska Barbara K, Ali Towhid, Mathew Shiny V, Law Amanda J, Metitiri Ochuko E, Straub Richard E, et al. Expression of GABA Signaling Molecules KCC2, NKCC1, and GAD1 in Cortical Development and Schizophrenia. The Journal of Neuroscience. 2011 Jul 27;31(no. 30):11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Youichi, Fagiolini Michela, Obata Kunihiko, Hensch Takao K. Rapid Critical Period Induction by Tonic Inhibition in Visual Cortex. The Journal of Neuroscience. 2003 Jul 30;23(no. 17):6695–6702. doi: 10.1523/JNEUROSCI.23-17-06695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi Dipesh, Fung Samantha J, Rothwell Alice, Weickert Cynthia Shannon. Higher Gamma-Aminobutyric Acid Neuron Density in the White Matter of Orbital Frontal Cortex in Schizophrenia. Biological Psychiatry. 2012 Nov;72(no. 9):725–733. doi: 10.1016/j.biopsych.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Jovanovic Jasmina N, Thomson Alex M. Development of Cortical GABAergic Innervation. Frontiers in Cellular Neuroscience. 2011 Jul 14;5 doi: 10.3389/fncel.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judaš Miloš, Sedmak Goran, Pletikos Mihovil, Jovanov-Milošević Nataša. Populations of Subplate and Interstitial Neurons in Fetal and Adult Human Telencephalon. Journal of Anatomy. 2010 Oct;217(no. 4):381–399. doi: 10.1111/j.1469-7580.2010.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir R, Attwell D. Neurotransmitter Receptors in the Life and Death of Oligodendrocytes. Neuroscience. 2007 Apr 14;145(no. 4):1426–1438. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasyanov Alexander M, Safiulina Victoria F, Voronin Leon L, Cherubini Enrico. GABA-Mediated Giant Depolarizing Potentials as Coincidence Detectors for Enhancing Synaptic Efficacy in the Developing Hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2004 Mar 16;101(no. 11):3967–3972. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilb Werner. Development of the GABAergic System from Birth to Adolescence. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2012 Dec;18(no. 6):613–630. doi: 10.1177/1073858411422114. [DOI] [PubMed] [Google Scholar]
- Kinney Hannah C, Haynes Robin L, Xu Gang, Andiman Sarah E, Folkerth Rebecca D, Sleeper Lynn A, Volpe Joseph J. Neuron Deficit in the White Matter and Subplate in Periventricular Leukomalacia. Annals of Neurology. 2012 Mar;71(no. 3):397–406. doi: 10.1002/ana.22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koós Tibor, Tepper James M. Inhibitory Control of Neostriatal Projection Neurons by GABAergic Interneurons. Nature Neuroscience. 1999 May;2(no. 5):467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Lee Vallent, Maguire Jamie. The Impact of Tonic GABAA Receptor-Mediated Inhibition on Neuronal Excitability Varies across Brain Region and Cell Type. Frontiers in Neural Circuits. 2014 Feb 3;8 doi: 10.3389/fncir.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic Kresimir, Zoncu Roberto, Rakic Pasko. Origin of GABAergic Neurons in the Human Neocortex. Nature. 2002 Jun 6;417(no. 6889):645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Leto Ketty, Bartolini Alice, Yanagawa Yukio, Obata Kunihiko, Magrassi Lorenzo, Schilling Karl, Rossi Ferdinando. Laminar Fate and Phenotype Specification of Cerebellar GABAergic Interneurons. The Journal of Neuroscience. 2009 May 27;29(no. 21):7079–7091. doi: 10.1523/JNEUROSCI.0957-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Yan, Lei Zhigang, Xu Zao C. Enhancement of Inhibitory Synaptic Transmission in Large Aspiny Neurons after Transient Cerebral Ischemia. Neuroscience. 2009 Mar 17;159(no. 2):670–681. doi: 10.1016/j.neuroscience.2008.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Yan, Blanco Glenn Dave, Lei Zhigang, Xu Zao Cheng. Increased GAD Expression in the Striatum after Transient Cerebral Ischemia. Molecular and Cellular Neurosciences. 2010 Dec;45(no. 4):370–377. doi: 10.1016/j.mcn.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Shih-chun, Bergles Dwight E. Synaptic Signaling between GABAergic Interneurons and Oligodendrocyte Precursor Cells in the Hippocampus. Nature Neuroscience. 2004 Jan;7(no. 1):24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic Cell Death in Brain Neurons. Physiological Reviews. 1999 Oct;79(no. 4):1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- López-Bendito Guillermina, Luján Rafael, Shigemoto Ryuichi, Ganter Paul, Paulsen Ole, Molnár Zoltán. Blockade of GABAB Receptors Alters the Tangential Migration of Cortical Neurons. Cerebral Cortex. 2003 Sep 1;13(no. 9):932–942. doi: 10.1093/cercor/13.9.932. [DOI] [PubMed] [Google Scholar]
- LoTurco Joseph J, Owens David F, Heath Mark J. S, Davis Marion B. E, Kriegstein Arnold R. GABA and Glutamate Depolarize Cortical Progenitor Cells and Inhibit DNA Synthesis. Neuron. 1995 Dec;15(no. 6):1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Luhmann Heiko J, Prince David A. Control of NMDA Receptor-Mediated Activity by GABAergic Mechanisms in Mature and Developing Rat Neocortex. Developmental Brain Research. 1990 Jul 1;54(no. 2):287–290. doi: 10.1016/0165-3806(90)90152-o. [DOI] [PubMed] [Google Scholar]
- Luján R, Shigemoto R, López-Bendito G. Glutamate and GABA Receptor Signalling in the Developing Brain. Neuroscience. 2005;130(no. 3):567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Luyt Karen, Slade Timothy P, Dorward Jienchi J, Durant Claire F, Wu Yue, Shigemoto Ryuichi, Mundell Stuart J, Váradi Anikó, Molnár Elek. Developing Oligodendrocytes Express Functional GABAB Receptors That Stimulate Cell Proliferation and Migration. Journal of Neurochemistry. 2007;100(no. 3):822–840. doi: 10.1111/j.1471-4159.2006.04255.x. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. Gabaa Receptor Channels. Annual Review of Neuroscience. 1994;17(no. 1):569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Maloku Ekrem, Covelo Ignacio R, Hanbauer Ingeborg, Guidotti Alessandro, Kadriu Bashkim, Hu Qiaoyan, Davis John M, Costa Erminio. Lower Number of Cerebellar Purkinje Neurons in Psychosis Is Associated with Reduced Reelin Expression. Proceedings of the National Academy of Sciences of the United States of America. 2010 Mar 2;107(no. 9):4407–4411. doi: 10.1073/pnas.0914483107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin Jean-Marie, Gallo Vittorio. The Curious Case of NG2 Cells: Transient Trend or Game Changer? ASN NEURO. 2011 Mar 10;3(no. 1):37–49. doi: 10.1042/AN20110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín Oscar, Rubenstein John LR. A Long, Remarkable Journey: Tangential Migration in the Telencephalon. Nature Reviews Neuroscience. 2001 Nov;2(no. 11):780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Markram Henry, Toledo-Rodriguez Maria, Wang Yun, Gupta Anirudh, Silberberg Gilad, Wu Caizhi. Interneurons of the Neocortical Inhibitory System. Nature Reviews Neuroscience. 2004 Oct;5(no. 10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Miller LG, Galpern WR, Dunlap K, Dinarello CA, Turner TJ. Interleukin-1 Augments Gamma-Aminobutyric acidA Receptor Function in Brain. Molecular Pharmacology. 1991 Feb;39(no. 2):105–108. [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. A Physiological Role for GABAB Receptors and the Effects of Baclofen in the Mammalian Central Nervous System. Progress in Neurobiology. 1995 Jul;46(no. 4):423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Zakaria, Lepsveridze Eka, Xu Hong, Kharlamov Elena A, Lu Bo, Kelly Kevin M. Neurobiology of Disease. no. 3. Vol. 38. Frontiers in Brain Stimulation; 2010. Jun, Increase of GABAA Receptor-Mediated Tonic Inhibition in Dentate Granule Cells after Traumatic Brain Injury; pp. 464–475. [DOI] [PubMed] [Google Scholar]
- Obrietan Karl, van den Pol Anthony N. GABAB Receptor-Mediated Inhibition of GABAA Receptor Calcium Elevations in Developing Hypothalamic Neurons. Journal of Neurophysiology. 1998 Mar 1;79(no. 3):1360–1370. doi: 10.1152/jn.1998.79.3.1360. [DOI] [PubMed] [Google Scholar]
- Owens David F, Kriegstein Arnold R. Is There More to Gaba than Synaptic Inhibition? Nature Reviews Neuroscience. 2002 Sep;3(no. 9):715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, Caruncho HJ. Reelin Is Preferentially Expressed in Neurons Synthesizingγ-Aminobutyric Acid in Cortex and Hippocampus of Adult Rats. Proceedings of the National Academy of Sciences of the United States of America. 1998 Mar 17;95(no. 6):3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Zdravko, Dujmović Ana, Kostović Ivica, Esclapez Monique. Distinct Origin of GABA-Ergic Neurons in Forebrain of Man, Nonhuman Primates and Lower Mammals. Collegium Antropologicum. 2008 May 13;32(no. 1) Supplement 1:9–17. [PubMed] [Google Scholar]
- Petanjek Zdravko, Berger Brigitte, Esclapez Monique. Origins of Cortical GABAergic Neurons in the Cynomolgus Monkey. Cerebral Cortex (New York, NY) 2009 Feb;19(no. 2):249–262. doi: 10.1093/cercor/bhn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer Carsten K, Stein Valentin, Keating Damien J, Maier Hannes, Rinke Ilka, Rudhard York, Hentschke Moritz, Rune Gabriele M, Jentsch Thomas J, Hübner Christian A. NKCC1-Dependent GABAergic Excitation Drives Synaptic Network Maturation during Early Hippocampal Development. The Journal of Neuroscience. 2009 Mar 18;29(no. 11):3419–3430. doi: 10.1523/JNEUROSCI.1377-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio O, Murthy S, Szabo G, Vutskits L, Kiss JZ, Vitalis T, Lebrand C, Dayer AG. New Pool of Cortical Interneuron Precursors in the Early Postnatal Dorsal White Matter. Cerebral Cortex (New York, N.Y.: 1991) 2012 Jan;22(no. 1):86–98. doi: 10.1093/cercor/bhr086. [DOI] [PubMed] [Google Scholar]
- Rice Dennis S, Curran Tom. Role of the Reelin Signaling Pathway in Central Nervous System Development. Annual Review of Neuroscience. 2001;24(no. 1):1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- Rivera Claudio, Voipio Juha, Payne John A, Ruusuvuori Eva, Lahtinen Hannele, Lamsa Karri, Pirvola Ulla, Saarma Mart, Kaila Kai. The K+/Cl|[minus]| Co-Transporter KCC2 Renders GABA Hyperpolarizing during Neuronal Maturation. Nature. 1999 Jan 21;397(no. 6716):251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Robinson Shenandoah, Li Qing, DeChant Anne, Cohen Mark L. Neonatal Loss of Γ–aminobutyric Acid Pathway Expression after Human Perinatal Brain Injury. Journal of Neurosurgery. 2006;104(no. 6) Suppl:396. doi: 10.3171/ped.2006.104.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy Bernardo, Fishell Gordon, Lee SooHyun, Hjerling-Leffler Jens. Three Groups of Interneurons Account for Nearly 100% of Neocortical GABAergic Neurons. Developmental Neurobiology. 2011 Jan 1;71(no. 1):45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia Kristen N, McClure Melissa, Moravec Matthew, Ling Luo Ning, Wan Ying, Gong Xi, Riddle Art, et al. ARRESTED OLIGODENDROCYTE LINEAGE MATURATION IN CHRONIC PERINATAL WHITE MATTER INJURY. Annals of Neurology. 2008 Apr;63(no. 4):520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strata Fabrizio, Atzori Marco, Molnar Margherita, Ugolini Gabriele, Tempia Filippo, Cherubini Enrico. A Pacemaker Current in Dye-Coupled Hilar Interneurons Contributes to the Generation of Giant GABAergic Potentials in Developing Hippocampus. The Journal of Neuroscience. 1997 Feb 15;17(no. 4):1435–1446. doi: 10.1523/JNEUROSCI.17-04-01435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Sola M Luisa. Neurons in the White Matter of the Adult Human Neocortex. Frontiers in Neuroanatomy. 2009;3 doi: 10.3389/neuro.05.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Dandan, Murali Sangita G. Na+-K+-2Cl−Cotransporter in Immature Cortical Neurons: A Role in Intracellular Cl−Regulation. Journal of Neurophysiology. 1999 Apr 1;81(no. 4):1939–1948. doi: 10.1152/jn.1999.81.4.1939. [DOI] [PubMed] [Google Scholar]
- Takesian Anne E, Hensch Takao K. Chapter 1 - Balancing Plasticity/Stability Across Brain Development. In: Mor Nahum, Van Vleet Thomas M, Merzenich Michael M., editors. Progress in Brain Research. Volume 207. Elsevier: Changing Brains Applying Brain Plasticity to Advance and Recover Human Ability; 2013. pp. 3–34. http://www.sciencedirect.com/science/article/pii/B9780444633279000011. [Google Scholar]
- Tan Seong-Seng, Kalloniatis Michael, Sturm Karin, Tam Patrick PL, Reese Benjamin E, Faulkner-Jones Beverly. Separate Progenitors for Radial and Tangential Cell Dispersion during Development of the Cerebral Neocortex. Neuron. 1998;1998;21(no. 2):295–304. doi: 10.1016/s0896-6273(00)80539-5. [DOI] [PubMed] [Google Scholar]
- Tao Ran, Li Chao, Newburn Erin N, Ye Tianzhang, Lipska Barbara K, Herman Mary M, Weinberger Daniel R, Kleinman Joel E, Hyde Thomas M. Transcript-Specific Associations of SLC12A5 (KCC2) in Human Prefrontal Cortex with Development, Schizophrenia, and Affective Disorders. The Journal of Neuroscience. 2012 Apr 11;32(no. 15):5216–5222. doi: 10.1523/JNEUROSCI.4626-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Pol Anthony N, Gao Xiao-Bing, Patrylo Peter R, Ghosh Prabhat K, Obrietan Karl. Glutamate Inhibits GABA Excitatory Activity in Developing Neurons. The Journal of Neuroscience. 1998;18(no. 24):10749–10761. doi: 10.1523/JNEUROSCI.18-24-10749.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR. Defining the Role of GABA in Cortical Development. The Journal of Physiology. 2009 Jan 19;587(no. 9):1873–1879. doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Hao, Yan Yiping, Kintner Douglas B, Lytle Christian, Sun Dandan. GABA-Mediated Trophic Effect on Oligodendrocytes Requires Na-K-2Cl Cotransport Activity. Journal of Neurophysiology. 2003 Aug 1;90(no. 2):1257–1265. doi: 10.1152/jn.01174.2002. [DOI] [PubMed] [Google Scholar]
- Williams Jeffery R, Sharp James W, Kumari Vijaya G, Wilson Martin, Payne John A. The Neuron-Specific K-Cl Cotransporter, KCC2 ANTIBODY DEVELOPMENT AND INITIAL CHARACTERIZATION OF THE PROTEIN. Journal of Biological Chemistry. 1999 Apr 30;274(no. 18):12656–12664. doi: 10.1074/jbc.274.18.12656. [DOI] [PubMed] [Google Scholar]
- Xu Gang, Broadbelt Kevin G, Haynes Robin L, Folkerth Rebecca D, Borenstein Natalia S, Belliveau Richard A, Trachtenberg Felicia L, Volpe Joseph J, Kinney Hannah C. Late Development of the GABAergic System in the Human Cerebral Cortex and White Matter. Journal of Neuropathology and Experimental Neurology. 2011 Oct;70(no. 10):841–858. doi: 10.1097/NEN.0b013e31822f471c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Junko, Okabe Akihito, Toyoda Hiroki, Kilb Werner, Luhmann Heiko J, Fukuda Atsuo. Cl− Uptake Promoting Depolarizing GABA Actions in Immature Rat Neocortical Neurones Is Mediated by NKCC1. The Journal of Physiology. 2004 Jun 15;557(no. 3):829–841. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX, Jen LS, Garey LJ. NADPH-Diaphorase-Positive Neurons in Primate Cerebral Cortex Colocalize with GABA and Calcium-Binding Proteins. Cerebral Cortex. 1996 May 1;6(no. 3):524–529. doi: 10.1093/cercor/6.3.524. [DOI] [PubMed] [Google Scholar]
- Yang Yang, Fung Samantha J, Rothwell Alice, Tianmei Si, Weickert Cynthia Shannon. Increased Interstitial White Matter Neuron Density in the DLPFC of People with Schizophrenia. Biological Psychiatry. 2011 Jan 1;69(no. 1):63–70. doi: 10.1016/j.biopsych.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Zhe, Fang Qi, Xiao Xian, Wang Yi-Zhi, Cai You-Qing, Cao Hui, Hu Gang, et al. GABA Transporter-1 Deficiency Confers Schizophrenia-Like Behavioral Phenotypes. PLoS ONE. 2013 Jul 29;8(no. 7) doi: 10.1371/journal.pone.0069883. [DOI] [PMC free article] [PubMed] [Google Scholar]