Abstract
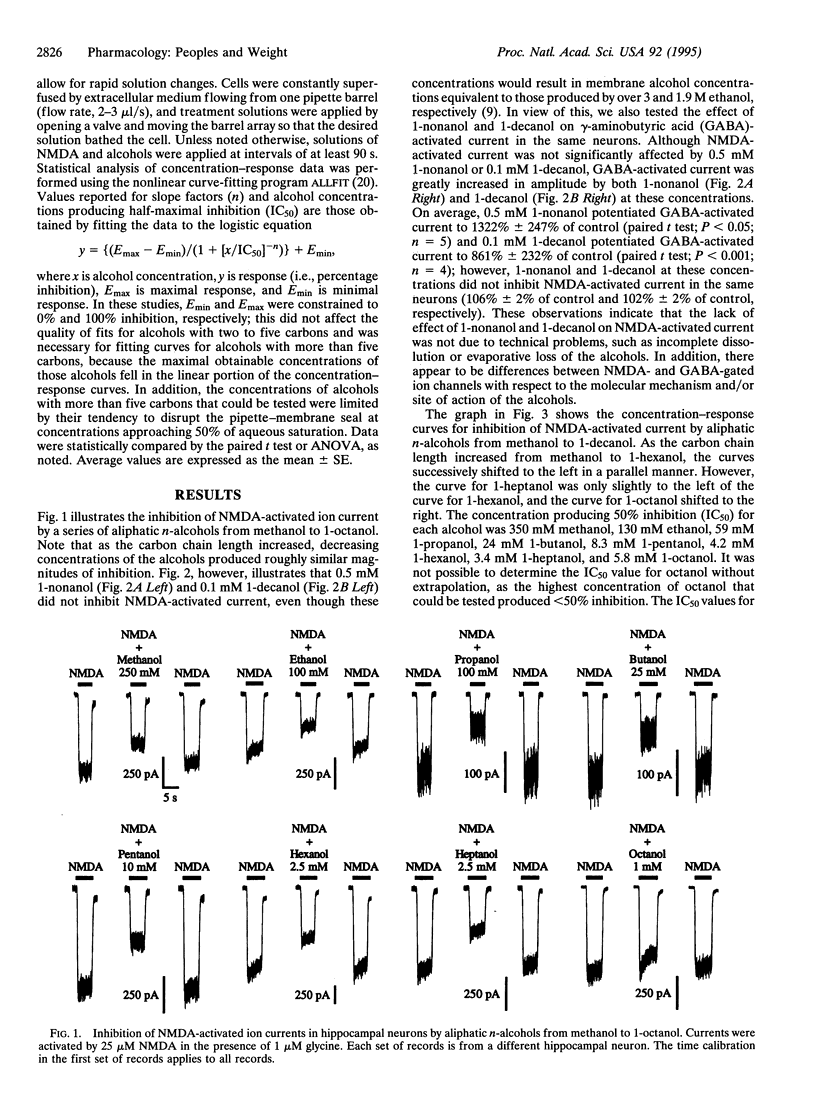
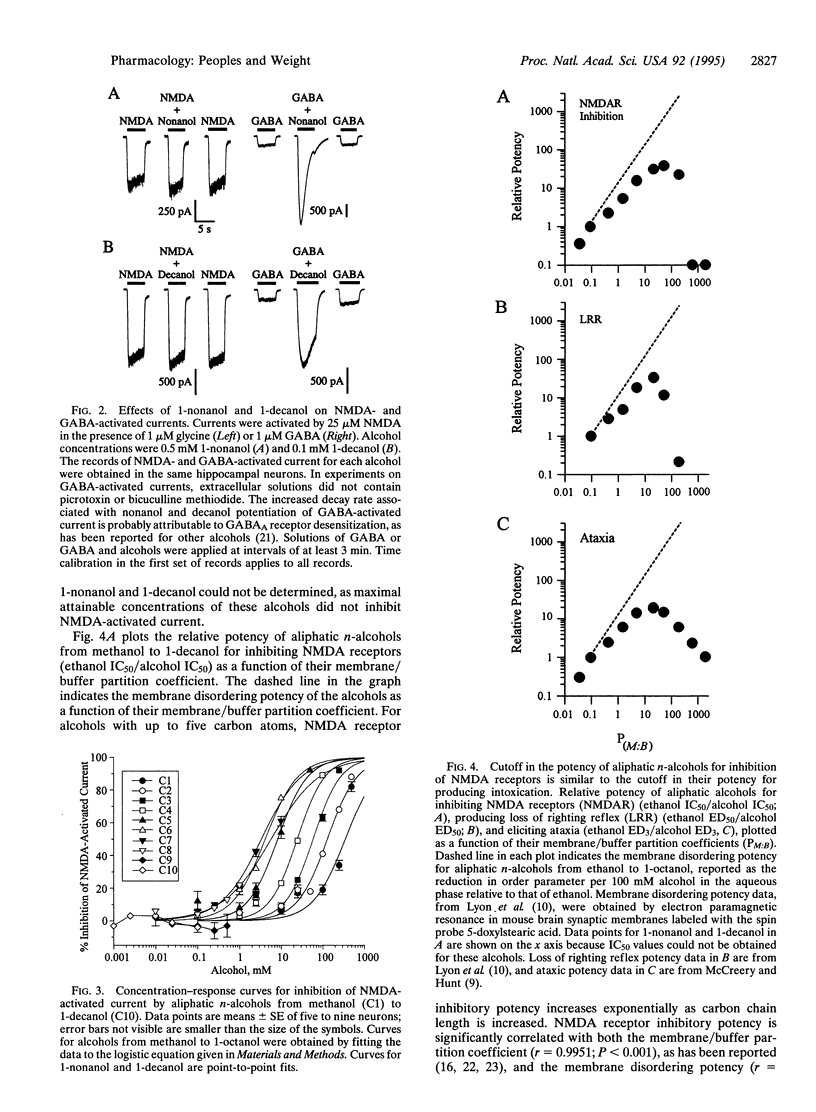
As the number of carbon atoms in an aliphatic n-alcohol is increased from one to five, intoxicating potency, lipid solubility, and membrane lipid disordering potency all increase in a similar exponential manner. However, the potency of aliphatic n-alcohols for producing intoxication reaches a maximum at six to eight carbon atoms and then decreases. The molecular basis of this "cutoff" effect is not understood, as it is not correlated with either the lipid solubility or the membrane disordering potency of the alcohols, which continue to increase exponentially. Since it has been suggested that inhibition of N-methyl-D-aspartate (NMDA) receptors by alcohols may play a role in alcohol intoxication, we investigated whether a series of aliphatic n-alcohols would exhibit a cutoff in potency for inhibition of NMDA receptors. We found that although potency for inhibition of NMDA receptors increased exponentially for alcohols with one to five carbon atoms, potency for inhibition of NMDA receptors reached a maximum at six to eight carbon atoms and then abruptly disappeared. This cutoff for alcohol inhibition of NMDA receptors is consistent with an interaction of the alcohols with a hydrophobic pocket on the receptor protein. In addition, the similarity of the cutoffs for alcohol inhibition of NMDA receptors and alcohol intoxication suggests that the cutoff for NMDA receptor inhibition may contribute to the cutoff for alcohol intoxication, which is consistent with an important role of NMDA receptors in alcohol intoxication.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Fink K., Göthert M. Inhibition of N-methyl-D-aspartate-induced noradrenaline release by alcohols is related to their hydrophobicity. Eur J Pharmacol. 1990 Nov 27;191(2):225–229. doi: 10.1016/0014-2999(90)94152-n. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Mapping of general anaesthetic target sites provides a molecular basis for cutoff effects. Nature. 1985 Jul 25;316(6026):349–351. doi: 10.1038/316349a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Partitioning of long-chain alcohols into lipid bilayers: implications for mechanisms of general anesthesia. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5116–5120. doi: 10.1073/pnas.83.14.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. B. The effects of drugs on membrane fluidity. Annu Rev Pharmacol Toxicol. 1984;24:43–64. doi: 10.1146/annurev.pa.24.040184.000355. [DOI] [PubMed] [Google Scholar]
- Gonzales R. A., Westbrook S. L., Bridges L. T. Alcohol-induced inhibition of N-methyl-D-aspartate-evoked release of [3H]norepinephrine from brain is related to lipophilicity. Neuropharmacology. 1991 May;30(5):441–446. doi: 10.1016/0028-3908(91)90004-u. [DOI] [PubMed] [Google Scholar]
- Huidobro-Toro J. P., Bleck V., Allan A. M., Harris R. A. Neurochemical actions of anesthetic drugs on the gamma-aminobutyric acid receptor-chloride channel complex. J Pharmacol Exp Ther. 1987 Sep;242(3):963–969. [PubMed] [Google Scholar]
- Hunt W. A. Neuroscience research: how has it contributed to our understanding of alcohol abuse and alcoholism? A review. Alcohol Clin Exp Res. 1993 Oct;17(5):1055–1065. doi: 10.1111/j.1530-0277.1993.tb05664.x. [DOI] [PubMed] [Google Scholar]
- Li C., Peoples R. W., Weight F. F. Alcohol action on a neuronal membrane receptor: evidence for a direct interaction with the receptor protein. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8200–8204. doi: 10.1073/pnas.91.17.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Dilger J. P., Vidal A. M. Effects of alcohols and volatile anesthetics on the activation of nicotinic acetylcholine receptor channels. Mol Pharmacol. 1994 Jun;45(6):1235–1241. [PubMed] [Google Scholar]
- Lovinger D. M., White G., Weight F. F. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989 Mar 31;243(4899):1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lyon R. C., McComb J. A., Schreurs J., Goldstein D. B. A relationship between alcohol intoxication and the disordering of brain membranes by a series of short-chain alcohols. J Pharmacol Exp Ther. 1981 Sep;218(3):669–675. [PubMed] [Google Scholar]
- McCreery M. J., Hunt W. A. Physico-chemical correlates of alcohol intoxication. Neuropharmacology. 1978 Jul;17(7):451–461. doi: 10.1016/0028-3908(78)90050-3. [DOI] [PubMed] [Google Scholar]
- Miller K. W., Firestone L. L., Alifimoff J. K., Streicher P. Nonanesthetic alcohols dissolve in synaptic membranes without perturbing their lipids. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1084–1087. doi: 10.1073/pnas.86.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nakahiro M., Arakawa O., Narahashi T. Modulation of gamma-aminobutyric acid receptor-channel complex by alcohols. J Pharmacol Exp Ther. 1991 Oct;259(1):235–240. [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Weight F. F. Cellular and molecular physiology of alcohol actions in the nervous system. Int Rev Neurobiol. 1992;33:289–348. doi: 10.1016/s0074-7742(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Willetts J., Balster R. L., Leander J. D. The behavioral pharmacology of NMDA receptor antagonists. Trends Pharmacol Sci. 1990 Oct;11(10):423–428. doi: 10.1016/0165-6147(90)90150-7. [DOI] [PubMed] [Google Scholar]


