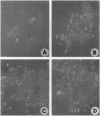
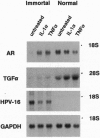
Abstract
Infection with multiple sexually transmitted agents has been associated with inflammation of the cervix and an increased risk of cervical cancer in women infected with human papillomaviruses (HPVs). Two proinflammatory cytokines, interleukin 1 alpha (IL-1 alpha) and tumor necrosis factor alpha (TNF-alpha), inhibited proliferation of normal epithelial cells cultured from human cervix. In contrast, both cytokines significantly stimulated proliferation of cervical cell lines (5 of 7) immortalized by transfection with HPV-16 or -18 DNAs or lines derived from cervical carcinomas (7 of 11). Stimulation was dose dependent from 0.01 to 1.0 nM and was blocked by specific inhibitors, such as the IL-1 receptor antagonist or the TNF type 1 or 2 soluble receptors. Growth stimulation by IL-1 alpha or TNF-alpha was accompanied by a 6- to 10-fold increase in RNA encoding amphiregulin, an epidermal growth factor (EGF) receptor ligand. Recombinant human amphiregulin (0.1 nM) was as effective as IL-1 alpha or TNF-alpha in promoting proliferation. Monoclonal antibodies that blocked signal transduction by the EGF receptor or that neutralized amphiregulin activity prevented mitogenic stimulation by IL-1 alpha or TNF-alpha. These studies indicate that IL-1 alpha and TNF-alpha stimulate proliferation of immortal and malignant cervical epithelial cells by an EGF receptor-dependent pathway requiring autocrine stimulation by amphiregulin. Furthermore, they suggest that chronic inflammation and release of proinflammatory cytokines might provide a selective growth advantage for abnormal cervical cells in vivo.
Full text
PDF
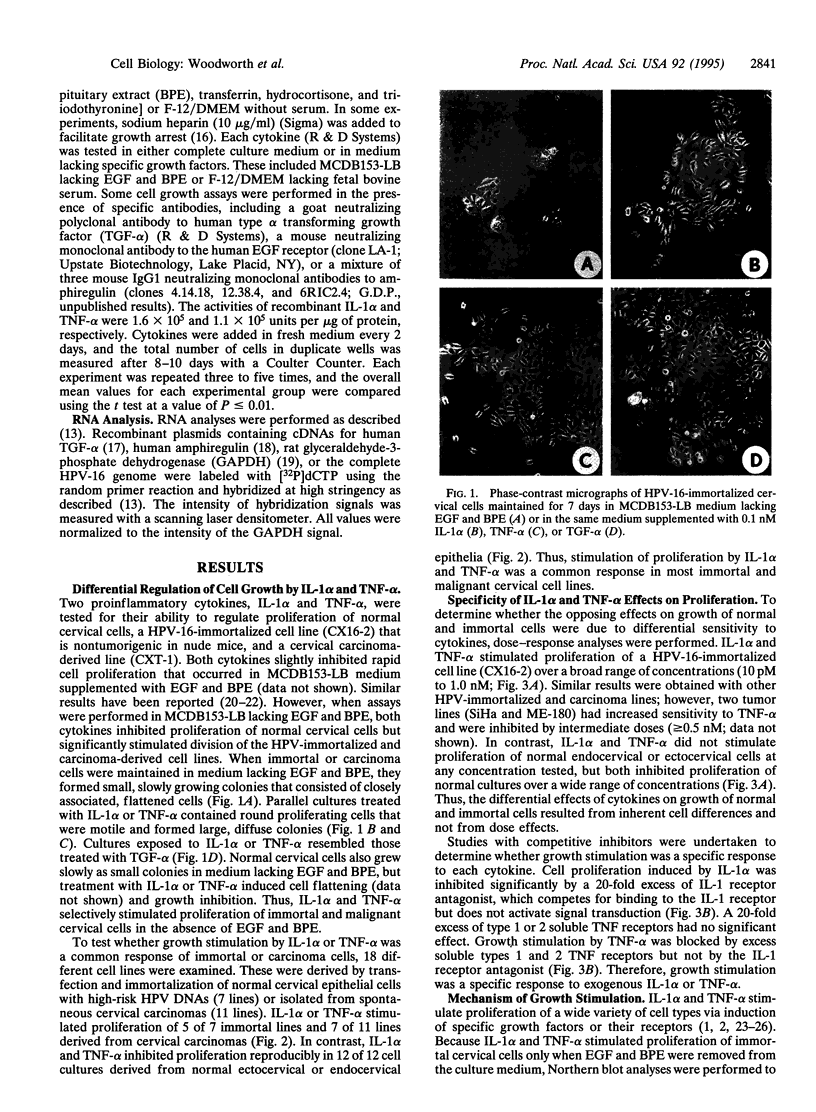
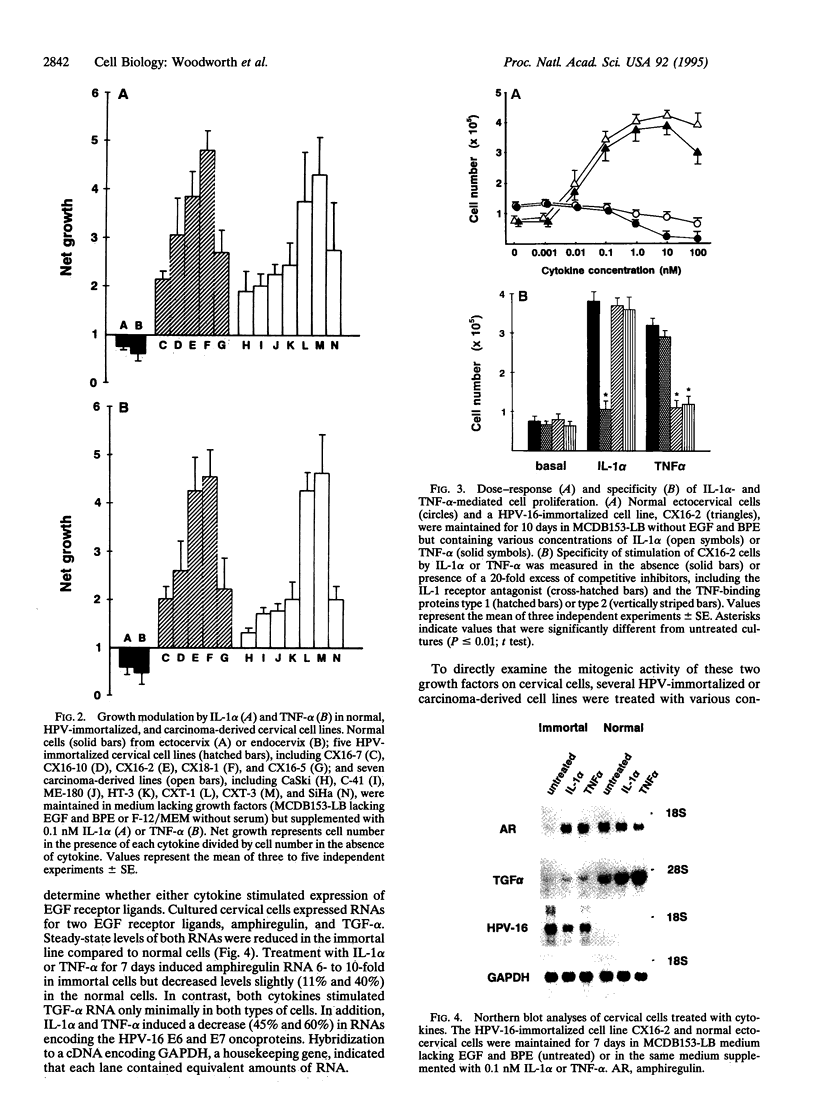

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. N., Mitra R. S., Griffiths C. E., Dixit V. M., Nickoloff B. J. Keratinocytes as initiators of inflammation. Lancet. 1991 Jan 26;337(8735):211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- Ciardiello F., Kim N., Saeki T., Dono R., Persico M. G., Plowman G. D., Garrigues J., Radke S., Todaro G. J., Salomon D. S. Differential expression of epidermal growth factor-related proteins in human colorectal tumors. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7792–7796. doi: 10.1073/pnas.88.17.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. W., Mattox P. A., Keeble W. W., Pittelkow M. R., Plowman G. D., Shoyab M., Adelman J. P., Shipley G. D. A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol Cell Biol. 1991 May;11(5):2547–2557. doi: 10.1128/mcb.11.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992 Dec 15;52(24):6735–6740. [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan 14;328(2):106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Ito R., Kitadai Y., Kyo E., Yokozaki H., Yasui W., Yamashita U., Nikai H., Tahara E. Interleukin 1 alpha acts as an autocrine growth stimulator for human gastric carcinoma cells. Cancer Res. 1993 Sep 1;53(17):4102–4106. [PubMed] [Google Scholar]
- Johnson G. R., Saeki T., Gordon A. W., Shoyab M., Salomon D. S., Stromberg K. Autocrine action of amphiregulin in a colon carcinoma cell line and immunocytochemical localization of amphiregulin in human colon. J Cell Biol. 1992 Aug;118(3):741–751. doi: 10.1083/jcb.118.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K., Yamamoto M., Kameyama S., Kawamata H., Rademaker A., Oyasu R. Enhancement of rat urinary bladder tumorigenesis by lipopolysaccharide-induced inflammation. Cancer Res. 1993 Nov 1;53(21):5172–5175. [PubMed] [Google Scholar]
- Koutsky L. A., Holmes K. K., Critchlow C. W., Stevens C. E., Paavonen J., Beckmann A. M., DeRouen T. A., Galloway D. A., Vernon D., Kiviat N. B. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992 Oct 29;327(18):1272–1278. doi: 10.1056/NEJM199210293271804. [DOI] [PubMed] [Google Scholar]
- LeJeune S., Leek R., Horak E., Plowman G., Greenall M., Harris A. L. Amphiregulin, epidermal growth factor receptor, and estrogen receptor expression in human primary breast cancer. Cancer Res. 1993 Aug 1;53(15):3597–3602. [PubMed] [Google Scholar]
- Lee S. W., Morhenn V. B., Ilnicka M., Eugui E. M., Allison A. C. Autocrine stimulation of interleukin-1 alpha and transforming growth factor alpha production in human keratinocytes and its antagonism by glucocorticoids. J Invest Dermatol. 1991 Jul;97(1):106–110. doi: 10.1111/1523-1747.ep12478503. [DOI] [PubMed] [Google Scholar]
- Lewis G. D., Aggarwal B. B., Eessalu T. E., Sugarman B. J., Shepard H. M. Modulation of the growth of transformed cells by human tumor necrosis factor-alpha and interferon-gamma. Cancer Res. 1987 Oct 15;47(20):5382–5385. [PubMed] [Google Scholar]
- Lowy D. R., Kirnbauer R., Schiller J. T. Genital human papillomavirus infection. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2436–2440. doi: 10.1073/pnas.91.7.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malejczyk J., Malejczyk M., Köck A., Urbanski A., Majewski S., Hunzelmann N., Jablonska S., Orth G., Luger T. A. Autocrine growth limitation of human papillomavirus type 16-harboring keratinocytes by constitutively released tumor necrosis factor-alpha. J Immunol. 1992 Oct 15;149(8):2702–2708. [PubMed] [Google Scholar]
- Normanno N., Selvam M. P., Qi C. F., Saeki T., Johnson G., Kim N., Ciardiello F., Shoyab M., Plowman G., Brandt R. Amphiregulin as an autocrine growth factor for c-Ha-ras- and c-erbB-2-transformed human mammary epithelial cells. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2790–2794. doi: 10.1073/pnas.91.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombella V. J., Yamashiro D. J., Maxfield F. R., Decker S. J., Vilcek J. Tumor necrosis factor increases the number of epidermal growth factor receptors on human fibroblasts. J Biol Chem. 1987 Feb 15;262(5):1950–1954. [PubMed] [Google Scholar]
- Parkin D. M., Lärä E., Muir C. S. Estimates of the worldwide frequency of sixteen major cancers in 1980. Int J Cancer. 1988 Feb 15;41(2):184–197. doi: 10.1002/ijc.2910410205. [DOI] [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S., Bikle D. D., Eessalu T. E., Aggarwal B. B., Elias P. M. Binding and biological effects of tumor necrosis factor alpha on cultured human neonatal foreskin keratinocytes. J Clin Invest. 1989 Mar;83(3):816–821. doi: 10.1172/JCI113963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirisi L., Yasumoto S., Feller M., Doniger J., DiPaolo J. A. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987 Apr;61(4):1061–1066. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman G. D., Green J. M., McDonald V. L., Neubauer M. G., Disteche C. M., Todaro G. J., Shoyab M. The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Mol Cell Biol. 1990 May;10(5):1969–1981. doi: 10.1128/mcb.10.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J. W. Lymphohematopoietic cytokines in the female reproductive tract. Curr Opin Immunol. 1991 Oct;3(5):772–777. doi: 10.1016/0952-7915(91)90112-e. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Lindquist P. B., Bringman T. S., Goeddel D. V., Derynck R. Expression in rat fibroblasts of a human transforming growth factor-alpha cDNA results in transformation. Cell. 1986 Jul 18;46(2):301–309. doi: 10.1016/0092-8674(86)90747-6. [DOI] [PubMed] [Google Scholar]
- Schmauz R., Okong P., de Villiers E. M., Dennin R., Brade L., Lwanga S. K., Owor R. Multiple infections in cases of cervical cancer from a high-incidence area in tropical Africa. Int J Cancer. 1989 May 15;43(5):805–809. doi: 10.1002/ijc.2910430511. [DOI] [PubMed] [Google Scholar]
- Schmiegel W., Roeder C., Schmielau J., Rodeck U., Kalthoff H. Tumor necrosis factor alpha induces the expression of transforming growth factor alpha and the epidermal growth factor receptor in human pancreatic cancer cells. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):863–867. doi: 10.1073/pnas.90.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasawa H., Tomita Y., Sekiya S., Takamizawa H., Simizu B. Integration and transcription of human papillomavirus type 16 and 18 sequences in cell lines derived from cervical carcinomas. J Gen Virol. 1987 Feb;68(Pt 2):583–591. doi: 10.1099/0022-1317-68-2-583. [DOI] [PubMed] [Google Scholar]
- Shoyab M., McDonald V. L., Bradley J. G., Todaro G. J. Amphiregulin: a bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6528–6532. doi: 10.1073/pnas.85.17.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- Villa L. L., Vieira K. B., Pei X. F., Schlegel R. Differential effect of tumor necrosis factor on proliferation of primary human keratinocytes and cell lines containing human papillomavirus types 16 and 18. Mol Carcinog. 1992;6(1):5–9. doi: 10.1002/mc.2940060103. [DOI] [PubMed] [Google Scholar]
- Woodworth C. D., Bowden P. E., Doniger J., Pirisi L., Barnes W., Lancaster W. D., DiPaolo J. A. Characterization of normal human exocervical epithelial cells immortalized in vitro by papillomavirus types 16 and 18 DNA. Cancer Res. 1988 Aug 15;48(16):4620–4628. [PubMed] [Google Scholar]
- Woodworth C. D., Simpson S. Comparative lymphokine secretion by cultured normal human cervical keratinocytes, papillomavirus-immortalized, and carcinoma cell lines. Am J Pathol. 1993 May;142(5):1544–1555. [PMC free article] [PubMed] [Google Scholar]
- Wu S., Boyer C. M., Whitaker R. S., Berchuck A., Wiener J. R., Weinberg J. B., Bast R. C., Jr Tumor necrosis factor alpha as an autocrine and paracrine growth factor for ovarian cancer: monokine induction of tumor cell proliferation and tumor necrosis factor alpha expression. Cancer Res. 1993 Apr 15;53(8):1939–1944. [PubMed] [Google Scholar]