Background: The tropomyosin receptor kinase B (TrkB) is a potential novel substrate of protein-tyrosine phosphatase 1B (PTP1B).
Results: PTP1B associates with and plays a modulatory role in BDNF-induced TrkB signaling.
Conclusion: PTP1B is a novel negative regulator of central BDNF/TrkB signaling.
Significance: This is the first evidence that PTP1B deficiency enhances central TrkB signaling and alters BDNF-induced thermogenesis in vivo.
Keywords: Brain-derived Neurotrophic Factor (BDNF), Hypothalamus, Metabolism, Phosphotyrosine Signaling, Receptor Tyrosine Kinase, Signal Transduction, Tyrosine-Protein Phosphatase (Tyrosine Phosphatase)
Abstract
Neuronal protein-tyrosine phosphatase 1B (PTP1B) deficiency in mice results in enhanced leptin signaling and protection from diet-induced obesity; however, whether additional signaling pathways in the brain contribute to the metabolic effects of PTP1B deficiency remains unclear. Here, we show that the tropomyosin receptor kinase B (TrkB) receptor is a direct PTP1B substrate and implicate PTP1B in the regulation of the central brain-derived neurotrophic factor (BDNF) signaling. PTP1B interacts with activated TrkB receptor in mouse brain and human SH-SY5Y neuroblastoma cells. PTP1B overexpression reduces TrkB phosphorylation and activation of downstream signaling pathways, whereas PTP1B inhibition augments TrkB signaling. Notably, brains of Ptpn1−/− mice exhibit enhanced TrkB phosphorylation, and Ptpn1−/− mice are hypersensitive to central BDNF-induced increase in core temperature. Taken together, our findings demonstrate that PTP1B is a novel physiological regulator of TrkB and that enhanced BDNF/TrkB signaling may contribute to the beneficial metabolic effects of PTP1B deficiency.
Introduction
Energy homeostasis is a complex process that involves numerous tightly regulated signaling pathways, and its dysregulation can lead to obesity (1). PTP1B2 is implicated in regulation of energy balance; single nucleotide polymorphisms within the human PTPN1 gene are associated with obesity and metabolic disorders (2), and deletion of PTP1B in mice (Ptpn1−/−) results in reduced adiposity and resistance to diet-induced obesity (3, 4).
PTP1B antagonizes the action of the adipocyte-secreted hormone leptin by directly dephosphorylating the leptin receptor-associated Janus kinase 2 (5–8). Leptin acts within the brain to regulate energy homeostasis by suppressing food intake and elevating energy expenditure, and the metabolic effects of central PTP1B deficiency have been attributed to improved leptin sensitivity (9–13). Mice with compound deletion of leptin and PTP1B (ob/ob:Ptpn1−/−) weigh less than obese ob/ob mice, suggesting possible leptin-independent metabolic effects of PTP1B deficiency (6). Indeed, PTP1B has other known protein substrates that are implicated in metabolic control, including the insulin receptor (14); yet the relative contribution of specific signaling pathways to the metabolic effects of PTP1B deficiency still remain to be determined (15).
Biochemical and structural studies have revealed a substrate recognition motif that, in addition to being present in known targets of PTP1B (7, 14, 16), is present in the receptor tyrosine kinase domain of the Trk family of proteins (17). Notably, the TrkB receptor and its ligand, BDNF, are key players in the regulation of energy homeostasis (18–20). The hypothalamus and the hindbrain are two major regions within the brain that are implicated in BDNF regulation of metabolism, although both BDNF and the TrkB receptor are broadly distributed throughout the central nervous system (21, 22). Intraparenchymal administration of BDNF into the ventromedial nucleus and paraventricular nucleus of the hypothalamus reduces food intake and increases energy expenditure (23–28). Similarly, delivery of BDNF directly into the dorsal vagal complex (29, 30) or nucleus tractus solitarius (31) of the hindbrain reduces food intake and increases energy expenditure.
Here, we show that PTP1B is a novel physiological regulator of BDNF/TrkB signaling in neurons. Mice lacking PTP1B are hypersensitive to central BDNF-induced increased core temperature, suggesting that alterations in this signaling pathway may be mediating some of the beneficial metabolic effects induced by disruption of PTP1B. These studies have important implications for the future use of PTP1B inhibitors in the treatment of obesity and metabolic disease.
EXPERIMENTAL PROCEDURES
Animal Care
Ptpn1−/− mice were generated and genotyped as described previously (9). Individually housed adult male Ptpn1−/− mice and their wild-type littermates (on a 129Sv/J × C57BL/6 background) were maintained in a temperature-controlled barrier facility on a 12:12 h light/dark cycle with ad libitum access to water and standard chow (Purina Rodent Chow 5001) or custom high-fat diet (Teklad TD93075; calories provided by protein (21.2%), fat (54.8%), and carbohydrate (24%)) for 12 weeks upon weaning (3 weeks of age) where indicated. All procedures were approved by University of Pennsylvania Institutional Animal Care and Use Committee.
Constructs
pGEX4T.1-hPTP1B and pMT2-hPTP1B constructs (and all parental vectors) were supplied by Dr. Benjamin Neel (Ontario Cancer Institute). pLNCX2-hTrkB was supplied by Dr. Garrett Brodeur (Children's Hospital of Philadelphia). The pGEX4T.1-hPTP1B-D181A/Y46F substrate trapping mutant of PTP1B, the pMT2-hPTP1B-C215S catalytically inactive mutant of PTP1B, and the TrkB kinase mutants pLNCX2-hTrkB-Y702F/Y706F/Y707F and Y706F/Y707F were generated using a standard site-directed mutagenesis kit (QuikChange II, Agilent Technologies).
Cell Culture
SH-SY5Y-TrkB cells stably expressing human TrkB were a generous gift of Dr. Garrett Brodeur (Children's Hospital of Philadelphia) and were maintained at 37 °C in 95%/5% O2/CO2 chamber in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Hyclone), 100 international units of penicillin and 100 μg/ml streptomycin (Invitrogen). Cells were cultured in the presence of 200 μg/ml geneticin (G418) (Cellgro) to maintain TrkB expression. Where indicated, cells were stimulated with human recombinant BDNF (R&D Systems) or vehicle (PBS for BDNF and dimethyl sulfoxide for PTP1B inhibitor).
Transient Transfection
For PTP1B overexpression studies, SH-SY5Y-TrkB cells were transiently transfected with eukaryotic expression constructs of PTP1B (WT PTP1B, C215S PTP1B, or parental pMT2 vector) using the polyethylenimine (PEI) method. Briefly, DNA was diluted in RPMI medium and mixed with PEI (Polysciences, Inc.) at 1:4 DNA:PEI for 15 min at room temperature. This mixture was directly added to the cells, which were then incubated at 37 °C in 95%/5% O2/CO2 chamber overnight. Experiments were performed ∼24 h after transfection.
Retroviral Transduction and Stable Cell Lines
SH-SY5Y parental cells were infected with retroviruses expressing tyrosine kinase-dead constructs of TrkB (Y702F/Y706F/Y707F or Y706F/Y707F in pLNCX2 retroviral vector) using the retroviral transduction method. Briefly, Plat-E packaging cells were transiently transfected using the PEI method. Retroviral supernatants were filter-sterilized, mixed with polybrene (5 μg/ml) (Sigma), and directly added to the SH-SY5Y-TrkB cells, which were incubated at 37 °C in a 95%/5% O2/CO2 chamber for 48 h. Approximately 24 h after transfection, the mixture was removed and replaced with fresh medium. Cells were allowed to recover for an additional 24 h, and pools of infected cells were selected with 600 μg/ml G418 (based on a G418 kill curve) for 2 weeks prior to experiments.
Recombinant Protein Production and Purification
BL21(DE3)pLysS competent cells (Stratagene) were transformed with bacterial expression constructs of PTP1B (pGEX4T.1-PTP1B-WT, pGEX4T.1-PTP1B-D181A/Y46F or parental pGEX4T.1 vector) according to the manufacturer's instructions. Recombinant protein production and purification was adapted from a previous protocol (32). Briefly, 0.1 mm isopropyl-1-thio-β-d-galactopyranoside was added to the bacterial cultures at an optical density of 0.6 to induce expression of recombinant proteins for 4 h at 37 °C. Bacterial pellets were lysed in ice-cold lysis buffer (10 mm Tris, pH 8.0, 150 mm sodium chloride, 1% Triton X-100, 10 mm dithiothreitol, and 1× bacterial protease inhibitor mixture (Roche Applied Science)) and sonicated on ice. Lysates were centrifuged, and the supernatants were incubated with glutathione-Sepharose beads (GE Healthcare) for 1 h at 4 °C. Collected beads were washed three times in lysis buffer at 4 °C. Recombinant proteins were eluted from the beads in six fractions with glutathione elution buffer (10 mm reduced glutathione in 50 mm Tris, pH 8.0, with 10 mm dithiothreitol and 1× bacterial protease inhibitors) at 4 °C. Supernatants containing the purified recombinant proteins were dialyzed into 1× TBS with 10 mm dithiothreitol and 10% glycerol using Slide-A-Lyzer® dialysis cassettes (10,000 molecular weight cut-off; Thermo Scientific). Enzymatic activity of purified PTP1B proteins was tested using a colorimetric phosphatase assay (BML-AK822, Enzo Life Sciences).
Substrate Trapping
For glutathione-S-transferase (GST) pulldown experiments, brain or SH-SY5Y-TrkB cell lysates were prepared in Nonidet P-40 buffer (150 mm sodium chloride, 20 mm Tris, pH 7.4, 5 mm EDTA, 10 mm sodium pyrophosphate, 1% Nonidet P-40, 50 mm sodium fluoride, 10 mm β-glycerophosphate) with protease inhibitor mixture (1:100 dilution; Roche Applied Science) with or without sodium orthovanadate (2 mm) (Sigma). Recombinant proteins were conjugated to glutathione-Sepharose beads (GE Healthcare) for 1 h at 4 °C. Lysed samples were incubated with bead-bound recombinant proteins for 4 h at 4 °C. Protein complexes were washed four times in lysis buffer at 4 °C and prepared for immunoblotting (as described below).
Immunoblotting
For BDNF signaling studies in the SH-SY5Y-TrkB cell line, lysates were prepared in radioimmune precipitation assay buffer (10 mm Tris-HCl, pH 7.4, 150 mm sodium chloride, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, 5 mm EDTA, 1 mm sodium fluoride) with protease inhibitor mixture (1:100 dilution), and sodium orthovanadate (2 mm). Protein concentrations were determined using a BCA Protein Assay (Thermo Scientific), and proteins were resolved by SDS-PAGE and transferred to PVDF membrane. Immunoblots were performed using the following antibodies: p-TrkB (Tyr-706) (sc-135645, Santa Cruz Biotechnology, Inc.), p-AKT (Ser-473) (193H12, Cell Signaling Technology), phospho-p44/42 MAPK (Thr-202/Tyr-204) (9101, Cell Signaling Technology), Trk (C-14) (sc-11, Santa Cruz Biotechnology, Inc.), AKT (9272, Cell Signaling Technology), p44/42 MAPK (137F5, Cell Signaling Technology), GST (2622, Cell Signaling Technology), homemade mouse PTP1B antibody (4), PTP1B (H-135), (sc-14021, Santa Cruz Biotechnology, Inc.), PTP1B (FG6, Oncogene Sciences), and SH-PTP2 (C-18) (sc-280, Santa Cruz Biotechnology, Inc). Proteins were visualized using enhanced chemiluminescence by HRP-linked mouse or rabbit secondary antibodies (GE Healthcare) and quantified by ImageJ software.
Neurite Outgrowth Assay
SH-SY5Y-TrkB cells were plated at 1 × 106 cells/per plate on poly-d-lysine (100 μg/ml) coated 60-mm plates (Millipore). Cells were pretreated with PTP1B inhibitor (compound II, 500 nm for 5 min (33)) or vehicle (dimethyl sulfoxide) prior to stimulation with BDNF (25 ng/ml) (R&D Systems) or vehicle (PBS). Cells were visualized prior to and 2, 4, 6, and 24 h following BDNF stimulation using a Nikon Eclipse TS100 microscope at 20× magnification and Nikon Coolpix S10 camera at 10× magnification. Primary neurite length was quantified using ImageJ software (NeuronJ plugin). Five neurites from a representative field were measured and averaged for each time point, and the experiment was repeated three separate times.
Central BDNF-induced Metabolic Effects
Individually housed, ad libitum fed, 4–5-month-old Ptpn1−/− male mice (n = 5) and their wild-type littermates (n = 10) were used in this study. Mice were injected intramuscularly with a mixture of ketamine (9 mg/kg), xylazine (0.27 mg/kg), and acepromazine (0.064 mg/kg) into the right hind limb. Following anesthesia, the mice had the head shaved and cleaned with antiseptic, and an incision was made starting from rostral to bregma and extending just beyond occipital notch. Mouthpiece and earbars of the digital stereotaxic apparatus (KOPF) were adjusted so that bregma and lambda were on the same dorsal-ventral coordinate. Using a high-speed dental drill (Fine Science Tools), a small hole was drilled 0.3 mm posterior to bregma and 0.9 mm lateral to the midsaggital suture. Chronic in-dwelling cannulae (C315GS-4-SPC, Plastics One) were lowered 1.5 mm ventral from dura. Cannulae were attached to the skull with glue adhesive and closed with an obturator. During the same surgery, miniature telemetric transponders (G2 E-mitters, Mini Mitter) were securely anchored to the abdominal muscle wall as described previously (31). Following surgery, mice were given approximately a week to fully recover. Correct cannula placement was tested through a single i.c.v. injection of neuropeptide Y (5 μg/ul) (Sigma). Only mice that showed >0.5 g food intake in the 1-h post-injection period were included in the study. Carrier-free human recombinant BDNF (0.05 μg/μl and 0.2 μg/ul) (R&D Systems) was prepared fresh in artificial cerebrospinal fluid (aCSF) (Harvard Apparatus). All injections were given at the onset of the dark cycle in a within-subjects design. Using a syringe pump (Harvard Apparatus PHD 4400 Hpsi programmable syringe pump), mice received a single 1-μl i.c.v. infusion of BDNF or aCSF at a rate of 1 μl/min. The injector tip was held in place for 30 s post-infusion. Chow intake was determined by weighing the food pellets (Purina Rodent Chow 5001) to the nearest 0.1 g before and 1, 3, and 6 h post-drug delivery. Body weight was measured to the nearest 0.1 g before and 24 h post-drug delivery. Core body temperature and locomotor activity were automatically recorded (VitalView Software) via minimitter every 5 min for 24 h following drug delivery. For substrate-trapping experiments, mice were injected with 0.5 μg of BDNF and euthanized 45 min later.
Statistical Analyses
Results are expressed as mean ± S.E. Graphs show quantified results from at least three separate experiments, and the blots are representative. Statistical analyses were performed using Prism (GraphPad) software. Comparisons between groups were made by unpaired two-tailed Student's t test, one-way analysis of variance, or two-way analysis of variance with Bonferroni's multiple comparison test used for post hoc comparisons, as appropriate. p values of < 0.05 were considered statistically significant.
RESULTS
PTP1B Overexpression Suppresses BDNF/TrkB Signaling
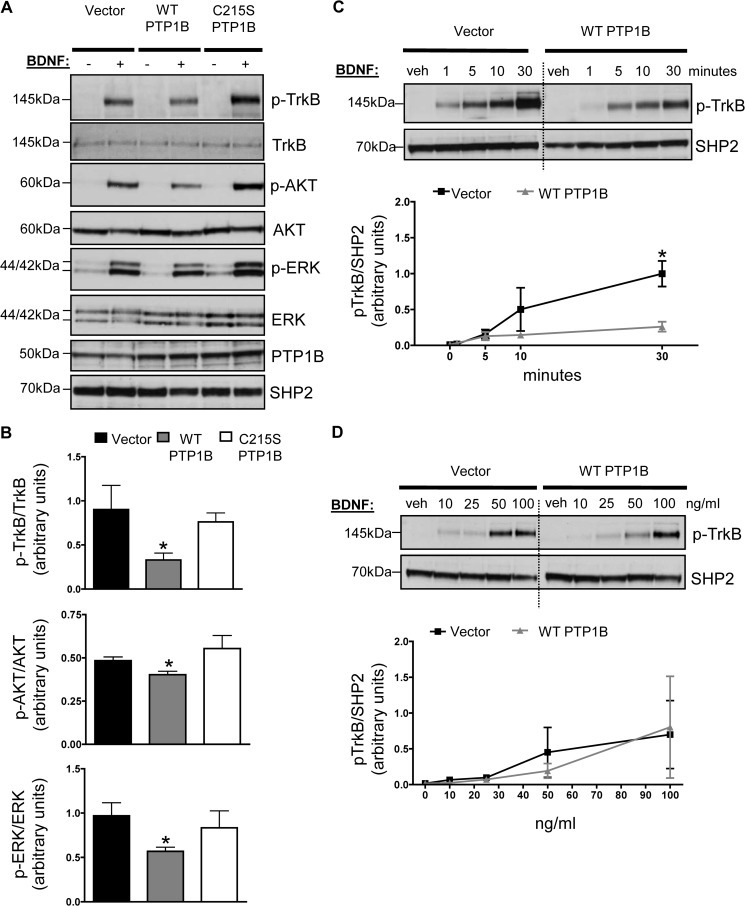
Given that the Trk family of receptors contains an ideal PTP1B target sequence and that the TrkB receptor ligand BDNF is an important central regulator of metabolism, we tested whether increasing levels of PTP1B antagonized BDNF/TrkB signaling. BDNF stimulation results in TrkB receptor dimerization and transphosphorylation of tyrosine residues, including Tyr702 and Tyr706/Tyr707 in the tyrosine kinase domain. This in turn triggers activation of different signaling cascades including PI3K/AKT, Ras stimulation of ERK, and phospholipase Cγ-1 activation (34). In neuronal SH-SY5Y cells stably expressing wild-type TrkB receptor (hereafter referred to as SH-SY5Y-TrkB), transient overexpression of wild-type PTP1B significantly suppressed BDNF-induced phosphorylation of the TrkB receptor on Tyr706 and activation of its downstream targets, AKT and ERK (Fig. 1, A and B). Expression of a catalytically inactive mutant (C215S) of PTP1B had no effect on TrkB, AKT, or ERK phosphorylation levels (Fig. 1, A and B), suggesting that the phosphatase activity of PTP1B is required for the effects of PTP1B on TrkB signaling. Expression of wild-type PTP1B suppressed peak BDNF-induced TrkB phosphorylation at 30 min (Fig. 1C), suggesting that PTP1B plays a negative regulatory role in BDNF-induced TrkB phosphorylation. Expression of wild-type PTP1B did not significantly alter the dose response to BDNF at the 5 min BDNF time point (Fig. 1D),
FIGURE 1.
A, SH-SY5Y-TrkB cells were transiently transfected with eukaryotic expression vectors of PTP1B (WT, C215S PTP1B, or parental pMT2 vector) using the PEI method. Cells were stimulated with BDNF (25 ng/ml) or vehicle (veh; PBS) for 5 min. Representative anti-phospho-TrkB, anti-phospho-AKT, anti-phospho-ERK, anti-TrkB, anti-AKT, anti-ERK, anti-human PTP1B, and anti-SHP2 immunoblots of total lysates are shown and quantified in B. Data are represented as mean ± S.E. (n = 3; *, p < 0.05 compared with empty vector group, Student's t test). C and D, lysates were immunoblotted with anti-phospho-TrkB and anti-SHP2. BDNF dose for each time point in C is 25 ng/ml. Time point for each dose in D is 5 min. Graphs show quantified results from at least three separate experiments, and representative blots are shown. Dashed lines show where the image from the same blot was cropped. Data are represented as mean ± S.E. (n = 3; *, p < 0.05 compared with empty vector group, two-way ANOVA with post hoc Bonferroni test).
PTP1B Inhibition or Genetic PTP1B Deficiency Enhances BDNF/TrkB Signaling
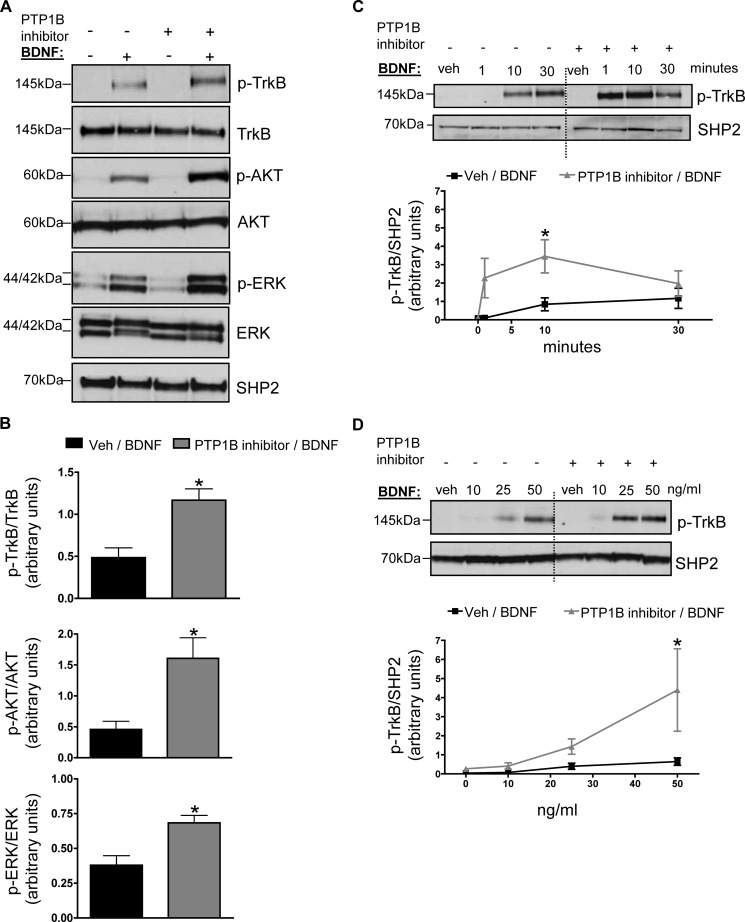
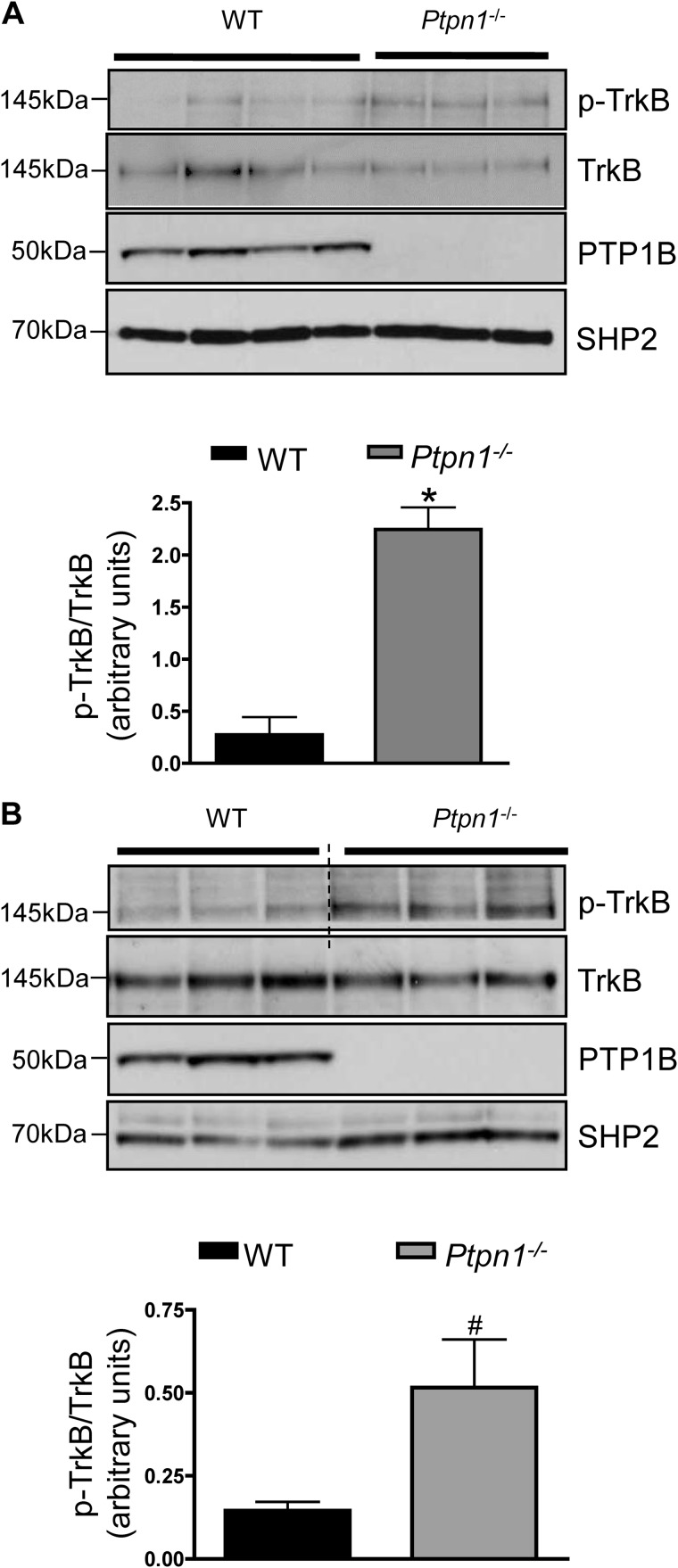
Inhibition of PTP1B activity sensitizes the leptin- and insulin-signaling pathways in vitro and in vivo (35–37). To investigate whether acute inhibition of PTP1B activity similarly enhances BDNF/TrkB signaling, SH-SY5Y-TrkB cells were pretreated with a cell permeable PTP1B-specific inhibitor (compound II, 100 nm for 5 min (33)) or vehicle prior to either vehicle or BDNF stimulation. PTP1B inhibition significantly increased BDNF-induced phosphorylation of the TrkB receptor on Tyr706 and enhanced activation of its downstream targets AKT and ERK (Fig. 2, A and B), showing that endogenous PTP1B acts as a physiological “brake” on TrkB signaling. PTP1B inhibitor pretreatment affected both the time course and the dose response of BDNF-induced TrkB phosphorylation (Fig. 2, C and D). Given our in vitro findings, we examined endogenous levels of phosphorylated TrkB in the hypothalamus of high-fat diet fed PTP1B-deficient mice compared with wild-type littermate controls. Male Ptpn1−/− mice fed a high-fat diet for 12 weeks showed increased levels of hypothalamic TrkB phosphorylation on Tyr706 compared with their wild-type littermates (Fig. 3A). No differences in p-TrkB or total TrkB protein were noted in hypothalamus of mice fed a chow diet (data not shown). TrkB receptors are highly expressed in the hippocampus (38, 39); notably, levels of p-TrkB were also elevated in this brain region of Ptpn1−/− mice compared with wild-type littermates (Fig. 3B).
FIGURE 2.
A, SH-SY5Y-TrkB cells were pretreated with cell permeable PTP1B-specific inhibitor (compound II (100 nm)) or vehicle (veh; dimethyl sulfoxide) for 5 min prior to stimulation with BDNF (25 ng/ml) or vehicle (PBS) for 5 min. Representative anti-phospho-TrkB, anti-phospho-AKT, anti-phospho-ERK, anti-TrkB, anti-AKT, anti-ERK, and anti-SHP2 immunoblots of total lysates are shown and quantified in B. Data are represented as mean ± S.E. (n = 3; *, p < 0.05 compared with control group, Student's t test). C and D, lysates were immunoblotted with anti-phosphoTrkB and anti-SHP2. BDNF dose for each time point in C is 25 ng/ml; time point for each dose in D is 5 min. Graphs show quantified results from at least three separate experiments and representative blots are shown. Dashed lines show where the image from the same blot was cropped. Data are represented as mean ± S.E. (n = 3; *, p < 0.05 compared with control group, two-way ANOVA with post hoc Bonferroni test).
FIGURE 3.
A, hypothalamic TrkB phosphorylation in 15-week-old male Ptpn1−/− mice and wild-type littermates after 12 weeks of high-fat diet feeding. Representative immunoblots of lysates using anti-phospho-TrkB, anti-TrkB, anti-mouse PTP1B, and anti-SHP2 antibodies are shown. Data are represented as mean ± S.E. (n = 4 for wild-type and n = 3 for Ptpn1−/−; *, p < 0.05 compared with wild-type group, Student's t test). B, hippocampal TrkB phosphorylation in 15-week-old, male Ptpn1−/− mice and wild-type littermates after 12 weeks of high-fat diet feeding. Dashed lines show where the image from the same blot was cropped. Representative immunoblots of lysates using anti-phosphoTrkB, anti-TrkB, anti-mouse PTP1B, and anti-SHP2 antibodies are shown. Data are represented as mean ± S.E. (n = 3 for each group; #, p = 0.067, Student's t test).
PTP1B Interacts with the TrkB Receptor
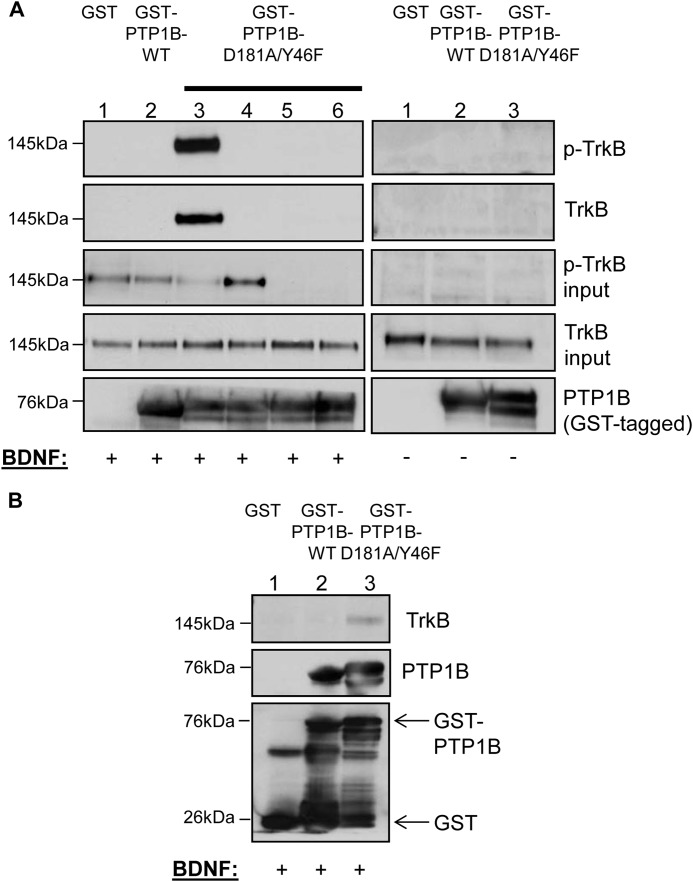
Although previous biochemical and in vitro peptide phosphorylation studies show a likely PTP1B substrate recognition motif in the receptor tyrosine kinase domain of TrkB (17), this interaction has not been confirmed in a physiologically relevant context. To test whether the TrkB receptor is indeed a bona fide PTP1B substrate, we utilized substrate trapping mutants of PTP1B that have reduced catalytic activity despite retained substrate binding ability (16). A robust substrate trapping mutant of PTP1B was generated by introducing two point mutations (D181A/Y46F) in the WPD and YRD loops surrounding the catalytic domain of the enzyme (40). As shown in Fig. 4A, GST-PTP1B-D181A/Y46F stably bound the tyrosine phosphorylated TrkB receptor when incubated with BDNF-stimulated SH-SY5Y-TrkB cell lysates in vitro (Fig. 4A, left panel, lane 3). PTP1B phosphatase activity is required for this association because the GST-PTP1B-D181A/Y46F interaction with TrkB was abolished in the presence of a competitive phosphatase inhibitor, sodium orthovanadate (Fig. 4A, left panel, lane 4). No binding of TrkB was detected to GST alone or GST-PTP1B-WT, showing that there is no nonspecific binding of TrkB to GST and that the phosphatase-substrate interaction is likely rapid and transient (Fig. 4A, left panel, lanes 1 and 2, respectively). To define the critical tyrosines mediating the PTP1B-TrkB interaction, two kinase-dead TrkB constructs with mutations corresponding to the tyrosine residues in the receptor tyrosine kinase domain (Y702F/Y706F/Y707F and Y706F/Y707F) were generated and stably expressed in SH-SY5Y parental cells that do not express endogenous TrkB receptors. The PTP1B-TrkB interaction was completely disrupted when GST-PTP1B-D181A/Y46F was incubated with TrkB mutant stable cell lysates (Fig. 4A, left panel, lanes 5 (Y702F/Y706F/Y707F) and 6 (Y706F/Y707F)), demonstrating that these tyrosine residues are necessary for this interaction. Binding of TrkB to GST-PTP1B-D181A/Y46F (or to GST alone or GST-PTP1B-WT) was not detected in the absence of BDNF stimulation, suggesting that this association is ligand-dependent and requires receptor phosphorylation (Fig. 4A, right panel, lanes 1–3). Overall, these data show that BDNF-induced tyrosine phosphorylation of the TrkB receptor activation site is necessary for PTP1B binding to TrkB. In addition to these in vitro findings, GST-PTP1B-D181A/Y46F stably associated with the activated TrkB receptor in brain lysates of mice injected i.c.v. with recombinant BDNF as shown by GST pulldown assay (Fig. 4B, lane 3). We did not detect association with GST-PTP1B-WT using this protocol (Fig. 4B, lane 2), again likely due to the rapid and transient nature of the phosphatase-substrate interaction. As a control, no binding was detected when using GST alone (Fig. 4B, lane 1).
FIGURE 4.
A, purified, active, recombinant GST fusion proteins (GST only control, WT, and D181A/Y46F PTP1B) (10 μg) were incubated with BDNF-stimulated (50 ng/ml, 5 min) (left panel, lanes 1–3, respectively) or unstimulated (right panel, lanes 1–3, respectively) SH-SY5Y-TrkB cell lysates (1. 5 mg) in a substrate-trapping (GST pulldown) experiment. Additionally, D181A/Y46F PTP1B (10 μg) was incubated with BDNF-stimulated (50 ng/ml, 5 min) SH-SY5Y-TrkB cell lysate (1.5 mg) in the presence of sodium orthovanadate (left panel, lane 4) and with BDNF-stimulated (50 ng/ml, 5 min) kinase-dead SH-SY5Y-TrkB cell lysates (Y702F/Y706F/Y707F TrkB and Y706F/Y707F TrkB (left panel, lanes 5 and 6, respectively)) (1.5 mg). Protein complexes were pulled down with glutathione beads and immunoblotted with anti-phospho-TrkB, anti-TrkB, and anti-human PTP1B as indicated. Lysates used as input were separately immunoblotted with anti-phospho-TrkB and anti-TrkB. B, purified, active, recombinant GST fusion proteins (GST-only control, WT and D181A/Y46F PTP1B) (10 μg) were incubated with BDNF-stimulated (0.5 μg, 45 min) mouse brain tissue lysate (3 mg) in a substrate-trapping (GST pulldown) experiment. Protein complexes were pulled down with glutathione beads and immunoblotted with anti-TrkB and anti-GST antibodies, as indicated.
Metabolic Effects of Acute Central BDNF Delivery in Ptpn1−/− Mice and Wild-type Mice
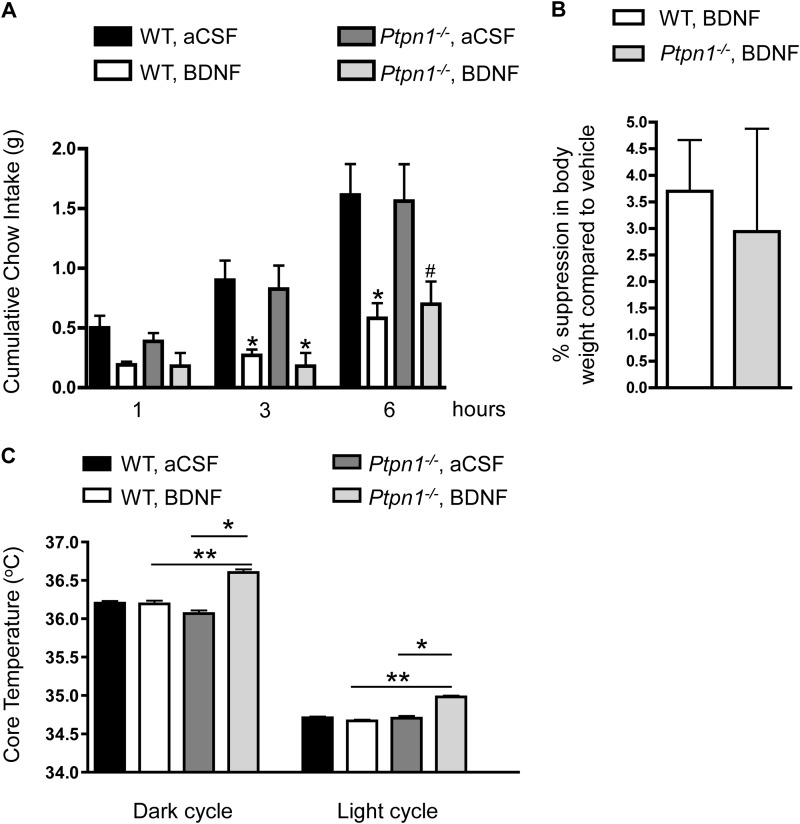
To determine the metabolic response of Ptpn1−/− mice to exogenous BDNF, male Ptpn1−/− mice and their wild-type littermates were given a single dose of BDNF or aCSF (vehicle) i.c.v. into the lateral ventricle just prior to the onset of the dark cycle. BDNF delivery into the brain has previously been shown to reduce food intake and suppress body weight in rodents (20). Both Ptpn1−/− mice and their wild-type littermates displayed significantly reduced cumulative food intake at 3 and 6 h after administration of BDNF compared with aCSF (Fig. 5A). Similarly, body weight was suppressed to a similar extent in both genotypes 24 h after BDNF administration (calculated as % body weight suppression compared with aCSF vehicle; Fig. 5B).
FIGURE 5.
A, cumulative chow intake (in grams) of 4–5-month-old Ptpn1−/− male mice and wild-type littermates at 1, 3, and 6 h following BDNF (0. 2 μg) or aCSF (vehicle) infusion into the lateral ventricle. Data are represented as mean ± S.E. (n = 10 for wild-type, n = 5 for Ptpn1−/− mice; *, p ≤ 0.05; #, p = 0.066 compared with vehicle treatment, one-way ANOVA). B, 24 h % body weight suppression (compared with vehicle treatment) of 4–5-month-old Ptpn1−/− male mice and wild-type littermates following BDNF (0.2 μg) or aCSF (vehicle) infusion into the lateral ventricle. Data are represented as mean ± S.E. (n = 10 for wild-type; n = 5 for Ptpn1−/− mice; *, p < 0.05 compared with wild-type controls, Student's t test). C, core temperature of 4–5-month-old Ptpn1−/− male mice and wild-type littermates following BDNF (0.05 μg) or aCSF (vehicle) infusion into the lateral ventricle. Data are represented as mean ± S.E. (n = 10 for wild-type; n = 5 for Ptpn1−/− mice; *, p ≤ 0.05 compared with vehicle treatment; **, p < 0.05 compared with wild-type controls, one-way ANOVA).
In addition to its effects on food intake, BDNF administration into the ventromedial nucleus and paraventricular nucleus of the hypothalamus as well as the nucleus tractus solitarius of the hindbrain increases energy expenditure through changes in spontaneous physical activity, resting metabolic rate, core temperature, and thermogenic gene expression (24, 26, 31). To test the hypothesis that PTP1B-deficient mice may display differential energy expenditure responses to exogenous BDNF, core body temperature and spontaneous physical activity were recorded in Ptpn1−/− and wild-type littermates in response to aCSF or a lower dose of BDNF that did not reduce food intake in either group (data not shown). Ptpn1−/− mice showed significantly elevated core temperature in response to BDNF in both the dark and light cycles compared with aCSF delivery, whereas this dose of BDNF did not induce elevated core temperature in wild-type controls (Fig. 5C). As expected, all groups display higher core temperatures during the dark cycle when mice are more active. A single dose of BDNF did not alter locomotor activity compared with vehicle in either genotype (data not shown). Results of these in vivo studies support a novel role for PTP1B in regulating BDNF/TrkB-induced signaling within the brain and suggest that enhanced central BDNF signaling may contribute to the increased energy expenditure noted in mouse models of PTP1B deficiency.
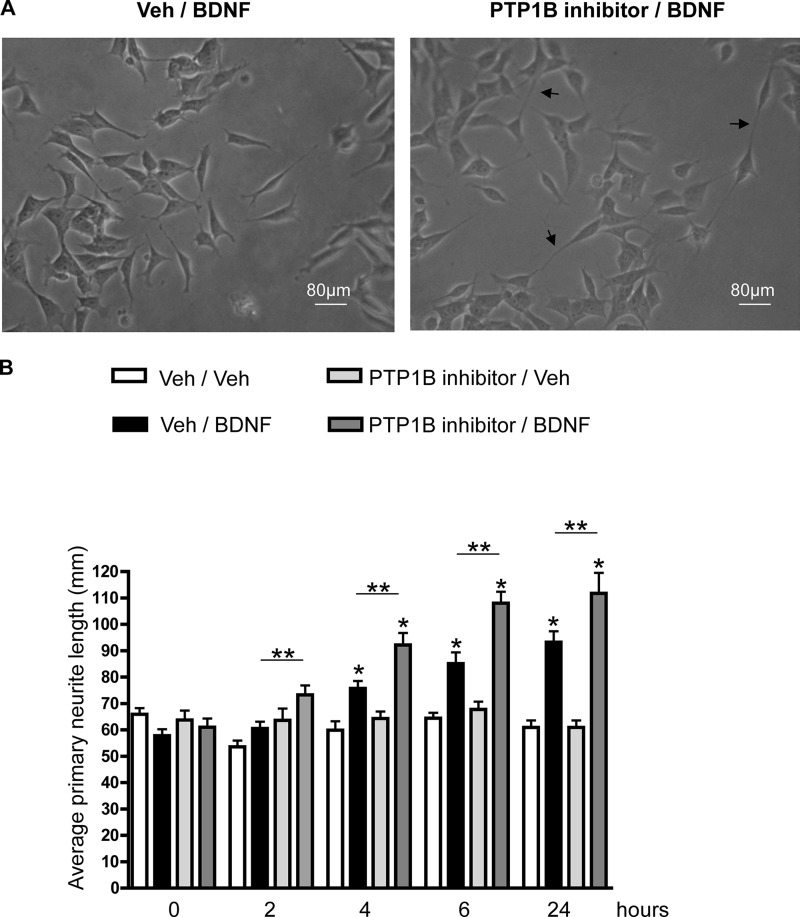
Acute Pretreatment with a PTP1B-specific Inhibitor Increases BDNF-induced Primary Neurite Outgrowth
BDNF is a neurotrophic factor and is crucial for many aspects of neuronal development, including neuronal survival, differentiation, and connectivity through axonal arborization and dendritic branching (34, 41). To determine whether PTP1B affects BDNF-induced neurite outgrowth, SH-SY5Y-TrkB cells were pretreated with vehicle or a cell permeable PTP1B-specific inhibitor (compound II, 500 nm for 5 min (33)) prior to BDNF or vehicle stimulation. Primary neurite outgrowth was assessed prior to and at 2, 4, 6, and 24 h following BDNF stimulation. PTP1B inhibitor pretreatment resulted in a significant increase in primary neurite length as early as 2 h post-BDNF and remained significantly higher throughout the experiment compared with cells without PTP1B inhibitor treatment (Fig. 6, A and B). These results demonstrate that PTP1B plays a role in regulating BDNF-induced neurite outgrowth in vitro and raises the intriguing possibility that central PTP1B deficiency could influence BDNF-mediated neural plasticity in the brain.
FIGURE 6.
A, representative image of SH-SY5Y-TrkB cells with vehicle (Veh)/BDNF treatment (left) or PTP1B inhibitor/BDNF treatment (right) at 6 h post-stimulation (t = 6). Arrows point at primary neurites (scale bar, 80 μm). B, SH-SY5Y-TrkB cells were pretreated with cell permeable, PTP1B-specific inhibitor (compound II (500 nm)) or vehicle (dimethyl sulfoxide) for 5 min prior to stimulation with BDNF (25 ng/ml) or vehicle (PBS). Primary neurite outgrowth was assessed prior to and 2, 4, 6, and 24 h following BDNF or vehicle stimulation. Average primary neurite length was quantified using ImageJ software (Neuron J plugin). Data are represented as mean ± S.E. (five neurites in a representative field were quantified and averaged for each time point, and the experiment was repeated three times; *, p < 0.05 compared with respective vehicle controls and **, p < 0.05 PTP1B inhibitor/BDNF treatment compared with vehicle/BDNF treatment, one-way ANOVA).
DISCUSSION
PTP1B is a known regulator of central metabolism, at least partially via negative regulation of leptin signaling. However, PTP1B is a ubiquitously expressed phosphatase with many substrate targets that are implicated in metabolic control and whether PTP1B regulates other pathways that are relevant to energy balance regulation is yet to be determined. Peptide studies have revealed a substrate recognition motif that, in addition to being present in known substrates of PTP1B, is present in the Trk family of receptors, suggesting a possible PTP1B-Trk receptor interaction. Using both a neuronal cell line and a genetic mouse model of PTP1B deficiency, we find that PTP1B is indeed a novel physiological regulator of central BDNF/TrkB signaling and BDNF-induced increase in core temperature.
In SH-SY5Y-TrkB cells, PTP1B overexpression impairs TrkB signaling. Furthermore, our data show that the interaction of PTP1B and TrkB requires PTP1B phosphatase activity and BDNF-induced TrkB tyrosine phosphorylation at the active site Tyr706/707. Notably, elevated PTP1B expression occurs in the obese state in vivo, and PTP1B expression is induced by a variety of factors, including diet, inflammation, and endoplasmic reticulum stress (2).
PTP1B is thought to play a complex role in interacting with and modulating the activity of its protein substrates (42). TrkB is a transmembrane receptor tyrosine kinase, whereas PTP1B is an intracellular phosphatase that is localized to cytoplasmic face of the endoplasmic reticulum (43). It is conceivable that the PTP1B-TrkB interaction and TrkB receptor dephosphorylation occur after ligand-induced receptor internalization, similar to epidermal growth factor and platelet-derived growth factor receptors (44). Alternatively, a protein complex may recruit PTP1B for TrkB receptor dephosphorylation, as is the case for insulin receptor signaling (45). Yet another possible mechanism is cleavage and subcellular relocalization of a truncated, active form of PTP1B into the cytosol (46). The precise biochemical mechanism by which PTP1B attenuates TrkB signaling needs further investigation. Interestingly, TrkB signaling is known to be modulated by other protein-tyrosine phosphatases (47–50), but how or whether PTP1B interacts with these proteins to regulate TrkB signaling remains unknown.
In SH-SY5Y-TrkB cells, PTP1B inhibition enhances TrkB signaling. It will be crucial to determine whether the observed in vitro effects of acute PTP1B inhibition on TrkB signaling are recapitulated in vivo through either PTP1B inhibitor administration into brain regions where TrkB is highly expressed or by chronic administration of BDNF in mouse models of central PTP1B deficiency.
The improved metabolic phenotype of whole body PTP1B-deficient mice is recapitulated in brain-specific PTP1B-deficient mice (13), implicating the central nervous system as the primary site of the metabolic effects of PTP1B. Within the central nervous system, the hypothalamus (12) and the hindbrain (11) have been identified as important sites of metabolic regulation by PTP1B. Notably, these same brain regions have been identified previously as major sites of BDNF and TrkB expression and implicated in mediating the metabolic effects of BDNF (20). Consistent with a role for PTP1B in regulating hypothalamic TrkB signaling, we find that high-fat diet fed Ptpn1−/− mice display increased TrkB active site phosphorylation. Given that high-fat diet feeding has been shown previously to impair TrkB phosphorylation in the brain (51, 52), enhanced central TrkB activation in the absence of PTP1B may play a role in mediating some of the beneficial metabolic effects in Ptpn1−/− mice. A previous study from our laboratory (12) has emphasized the importance of intact hypothalamic leptin signaling for the metabolic effects of PTP1B; however, incomplete recapitulation of the metabolic phenotype of brain-specific PTP1B deficiency with hypothalamus-specific PTP1B deficiency suggests the importance of extra-hypothalamic sites such as the hindbrain for the metabolic effects of PTP1B. In fact, sensitivity to hindbrain/nucleus tractus solitarius BDNF may underlie the enhanced thermogenic response to fourth i.c.v. BDNF (31). It remains to be determined whether PTP1B regulates BDNF/TrkB signaling in the hindbrain.
BDNF is a key neurotrophic factor implicated in neural connectivity specifically through enhancing axonal arborization and neurite outgrowth (41). PTP1B has previously been shown to enhance neurite extension in PC12 cells (53) and to influence plasticity in hippocampal neurons (54). Present data show that pretreatment with PTP1B inhibitor increases BDNF-induced neurite outgrowth in SH-SY5Y-TrkB cells. Although the molecular mechanism behind this effect is yet unknown, one possibility is that hyperphosphorylation of the TrkB receptor due to PTP1B inhibition results in receptor internalization followed by trafficking through signaling endosomes and consequent delivery to specific parts of the neurons to induce neurite outgrowth (55). In the context of metabolic control, this interesting finding leads us to speculate that disruption of PTP1B may lead to altered developmental wiring and/or plasticity-induced rewiring of brain regions implicated in metabolic control due to augmented BDNF/TrkB signaling. Given that leptin also has trophic effects in the hypothalamus (56), sensitization to BDNF and leptin in the absence of PTP1B may act synergistically to affect these processes, which may contribute to the overall improved metabolic phenotype of Ptpn1−/− mice.
Exogenous BDNF administration into the brain reduces energy intake and increases energy expenditure, leading to overall body weight loss in rodents (23–27, 29, 31, 57). Our data indicate that acute BDNF administration into the lateral ventricle reduces food intake and body weight in Ptpn1−/− mice and wild-type littermates to a similar extent. Although there are no differential energy intake effects between genotypes with our dose and injection paradigm, Ptpn1−/− mice are more sensitive to BDNF-induced increase in core temperature compared with wild-type littermates. Previous studies on the energy intake and expenditure effects of BDNF were conducted using rats and employing acute or chronic BDNF administration to the ventricles or targeted to specific nuclei of the hypothalamus or the hindbrain (23–27, 29, 31). To our knowledge, this is the first study examining the metabolic effects of acute BDNF administration into the lateral ventricle of mice. Intraparenchymal BDNF injections into different brain regions of interest will further elucidate the key brain regions that are responsible for metabolic effects observed in Ptpn1−/− mice. Notably, BDNF expression is induced by leptin through melanocortin signaling in both the hypothalamus (58) and the hindbrain (30); thus, the observed effects might not completely be leptin-independent and will require further investigation.
Taken together, our results suggest a previously unidentified role of PTP1B in central BDNF/TrkB signaling. These intriguing results bring us one step closer to understanding the role of BDNF/TrkB pathway in energy balance regulation, as well as the mechanism of action of PTP1B in exerting its metabolic effects. Importantly, these results open up new avenues for understanding PTP1B regulation of Trk signaling in the context of neurodevelopment and neuronal plasticity in addition to metabolism.
This work was supported by National Institutes of Health Grants R01DK082417 (to K. K. B.), R01DK021397 (to H. J. G.), K01DK097147 (to S. E. K.), and R01CA69202 (to Z. Y. Z.) and University of Pennsylvania Diabetes Endocrinology Research Center Grant 5P30DK019525.
- PTP1B
- protein-tyrosine phosphatase 1B
- PEI
- polyethylenimine
- aCSF
- artificial cerebrospinal fluid
- i.c.v.
- intracerebroventricular
- ANOVA
- analysis of variance.
REFERENCES
- 1. Flier J. S. (2004) Obesity wars: molecular progress confronts an expanding epidemic. Cell 116, 337–350 [DOI] [PubMed] [Google Scholar]
- 2. Tsou R. C., Bence K. K. (2012) The genetics of PTPN1 and obesity: insights from mouse models of tissue-specific PTP1B deficiency. J. Obes. 2012, 926857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elchebly M., Payette P., Michaliszyn E., Cromlish W., Collins S., Loy A. L., Normandin D., Cheng A., Himms-Hagen J., Chan C. C., Ramachandran C., Gresser M. J., Tremblay M. L., Kennedy B. P. (1999) Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283, 1544–1548 [DOI] [PubMed] [Google Scholar]
- 4. Klaman L. D., Boss O., Peroni O. D., Kim J. K., Martino J. L., Zabolotny J. M., Moghal N., Lubkin M., Kim Y. B., Sharpe A. H., Stricker-Krongrad A., Shulman G. I., Neel B. G., Kahn B. B. (2000) Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 20, 5479–5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zabolotny J. M., Bence-Hanulec K. K., Stricker-Krongrad A., Haj F., Wang Y., Minokoshi Y., Kim Y. B., Elmquist J. K., Tartaglia L. A., Kahn B. B., Neel B. G. (2002) PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2, 489–495 [DOI] [PubMed] [Google Scholar]
- 6. Cheng A., Uetani N., Simoncic P. D., Chaubey V. P., Lee-Loy A., McGlade C. J., Kennedy B. P., Tremblay M. L. (2002) Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell 2, 497–503 [DOI] [PubMed] [Google Scholar]
- 7. Myers M. P., Andersen J. N., Cheng A., Tremblay M. L., Horvath C. M., Parisien J. P., Salmeen A., Barford D., Tonks N. K. (2001) TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 276, 47771–47774 [DOI] [PubMed] [Google Scholar]
- 8. Kaszubska W., Falls H. D., Schaefer V. G., Haasch D., Frost L., Hessler P., Kroeger P. E., White D. W., Jirousek M. R., Trevillyan J. M. (2002) Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol. Cell. Endocrinol. 195, 109–118 [DOI] [PubMed] [Google Scholar]
- 9. Tsou R. C., Zimmer D. J., De Jonghe B. C., Bence K. K. (2012) Deficiency of PTP1B in leptin receptor-expressing neurons leads to decreased body weight and adiposity in mice. Endocrinology 153, 4227–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Banno R., Zimmer D., De Jonghe B. C., Atienza M., Rak K., Yang W., Bence K. K. (2010) PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J. Clin. Invest. 120, 720–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Jonghe B. C., Hayes M. R., Zimmer D. J., Kanoski S. E., Grill H. J., Bence K. K. (2012) Food intake reductions and increases in energetic responses by hindbrain leptin and melanotan II are enhanced in mice with POMC-specific PTP1B deficiency. Am. J. Physiol. Endocrinol. Metab. 303, E644–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsou R. C., Rak K. S., Zimmer D. J., Bence K. K. (2014) Improved metabolic phenotype of hypothalamic PTP1B-deficiency is dependent upon the leptin receptor. Mol. Metab. 3, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bence K. K., Delibegovic M., Xue B., Gorgun C. Z., Hotamisligil G. S., Neel B. G., Kahn B. B. (2006) Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat. Med. 12, 917–924 [DOI] [PubMed] [Google Scholar]
- 14. Kenner K. A., Anyanwu E., Olefsky J. M., Kusari J. (1996) Protein-tyrosine phosphatase 1B is a negative regulator of insulin- and insulin-like growth factor-I-stimulated signaling. J. Biol. Chem. 271, 19810–19816 [DOI] [PubMed] [Google Scholar]
- 15. Tiganis T., Bennett A. M. (2007) Protein tyrosine phosphatase function: the substrate perspective. Biochem. J. 402, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flint A. J., Tiganis T., Barford D., Tonks N. K. (1997) Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. U.S.A. 94, 1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Espanel X., Huguenin-Reggiani M., Hooft van Huijsduijnen R. (2002) The SPOT technique as a tool for studying protein tyrosine phosphatase substrate specificities. Protein Sci. 11, 2326–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vanevski F., Xu B. (2013) Molecular and neural bases underlying roles of BDNF in the control of body weight. Front. Neurosci. 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cordeira J., Rios M. (2011) Weighing in the role of BDNF in the central control of eating behavior. Mol. Neurobiol. 44, 441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rios M. (2013) BDNF and the central control of feeding: accidental bystander or essential player? Trends Neurosci. 36, 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yan Q., Radeke M. J., Matheson C. R., Talvenheimo J., Welcher A. A., Feinstein S. C. (1997) Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J. Comp. Neurol. 378, 135–157 [PubMed] [Google Scholar]
- 22. Ohira K., Hayashi M. (2009) A new aspect of the TrkB signaling pathway in neural plasticity. Curr. Neuropharmacol. 7, 276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang C., Bomberg E., Billington C., Levine A., Kotz C. M. (2007) Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1003–1012 [DOI] [PubMed] [Google Scholar]
- 24. Wang C., Bomberg E., Billington C., Levine A., Kotz C. M. (2007) Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R992–1002 [DOI] [PubMed] [Google Scholar]
- 25. Wang C., Bomberg E., Levine A., Billington C., Kotz C. M. (2007) Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1037–1045 [DOI] [PubMed] [Google Scholar]
- 26. Wang C., Bomberg E., Billington C. J., Levine A. S., Kotz C. M. (2010) Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain Res. 1336, 66–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang C., Godar R. J., Billington C. J., Kotz C. M. (2010) Chronic administration of brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reverses obesity induced by high-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1320–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Godar R., Dai Y., Bainter H., Billington C., Kotz C. M., Wang C. F. (2011) Reduction of high-fat diet-induced obesity after chronic administration of brain-derived neurotrophic factor in the hypothalamic ventromedial nucleus. Neuroscience 194, 36–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bariohay B., Lebrun B., Moyse E., Jean A. (2005) Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology 146, 5612–5620 [DOI] [PubMed] [Google Scholar]
- 30. Bariohay B., Roux J., Tardivel C., Trouslard J., Jean A., Lebrun B. (2009) Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology 150, 2646–2653 [DOI] [PubMed] [Google Scholar]
- 31. Spaeth A. M., Kanoski S. E., Hayes M. R., Grill H. J. (2012) TrkB receptor signaling in the nucleus tractus solitarius mediates the food intake-suppressive effects of hindbrain BDNF and leptin. Am. J. Physiol. Endocrinol. Metab. 302, E1252–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blanchetot C., Chagnon M., Dubé N., Hallé M., Tremblay M. L. (2005) Substrate-trapping techniques in the identification of cellular PTP targets. Methods 35, 44–53 [DOI] [PubMed] [Google Scholar]
- 33. Xie L., Lee S. Y., Andersen J. N., Waters S., Shen K., Guo X. L., Moller N. P., Olefsky J. M., Lawrence D. S., Zhang Z. Y. (2003) Cellular effects of small molecule PTP1B inhibitors on insulin signaling. Biochemistry 42, 12792–12804 [DOI] [PubMed] [Google Scholar]
- 34. Reichardt L. F. (2006) Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1545–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ma Y. M., Tao R. Y., Liu Q., Li J., Tian J. Y., Zhang X. L., Xiao Z. Y., Ye F. (2011) PTP1B inhibitor improves both insulin resistance and lipid abnormalities in vivo and in vitro. Molecular and cellular biochemistry 357, 65–72 [DOI] [PubMed] [Google Scholar]
- 36. Morrison C. D., White C. L., Wang Z., Lee S. Y., Lawrence D. S., Cefalu W. T., Zhang Z. Y., Gettys T. W. (2007) Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology 148, 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zinker B. A., Rondinone C. M., Trevillyan J. M., Gum R. J., Clampit J. E., Waring J. F., Xie N., Wilcox D., Jacobson P., Frost L., Kroeger P. E., Reilly R. M., Koterski S., Opgenorth T. J., Ulrich R. G., Crosby S., Butler M., Murray S. F., McKay R. A., Bhanot S., Monia B. P., Jirousek M. R. (2002) PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc. Natl. Acad. Sci. U.S.A. 99, 11357–11362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klein R., Martin-Zanca D., Barbacid M., Parada L. F. (1990) Expression of the tyrosine kinase receptor gene trkB is confined to the murine embryonic and adult nervous system. Development 109, 845–850 [DOI] [PubMed] [Google Scholar]
- 39. Webster M. J., Herman M. M., Kleinman J. E., Shannon Weickert C. (2006) BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr. Patterns 6, 941–951 [DOI] [PubMed] [Google Scholar]
- 40. Boubekeur S., Boute N., Pagesy P., Zilberfarb V., Christeff N., Issad T. (2011) A new highly efficient substrate-trapping mutant of protein tyrosine phosphatase 1B (PTP1B) reveals full autoactivation of the insulin receptor precursor. J. Biol. Chem. 286, 19373–19380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jeanneteau F., Deinhardt K., Miyoshi G., Bennett A. M., Chao M. V. (2010) The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat. Neurosci. 13, 1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stuible M., Tremblay M. L. (2010) In control at the ER: PTP1B and the down-regulation of RTKs by dephosphorylation and endocytosis. Trends Cell Biol. 20, 672–679 [DOI] [PubMed] [Google Scholar]
- 43. Frangioni J. V., Beahm P. H., Shifrin V., Jost C. A., Neel B. G. (1992) The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell 68, 545–560 [DOI] [PubMed] [Google Scholar]
- 44. Haj F. G., Verveer P. J., Squire A., Neel B. G., Bastiaens P. I. (2002) Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science 295, 1708–1711 [DOI] [PubMed] [Google Scholar]
- 45. Wu C. L., Buszard B., Teng C. H., Chen W. L., Warr C. G., Tiganis T., Meng T. C. (2011) Dock/Nck facilitates PTP61F/PTP1B regulation of insulin signalling. Biochem. J. 439, 151–159 [DOI] [PubMed] [Google Scholar]
- 46. Frangioni J. V., Oda A., Smith M., Salzman E. W., Neel B. G. (1993) Calpain-catalyzed cleavage and subcellular relocation of protein phosphotyrosine phosphatase 1B (PTP-1B) in human platelets. EMBO J. 12, 4843–4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rusanescu G., Yang W., Bai A., Neel B. G., Feig L. A. (2005) Tyrosine phosphatase SHP-2 is a mediator of activity-dependent neuronal excitotoxicity. EMBO J. 24, 305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang T., Massa S. M., Longo F. M. (2006) LAR protein tyrosine phosphatase receptor associates with TrkB and modulates neurotrophic signaling pathways. J. Neurobiol. 66, 1420–1436 [DOI] [PubMed] [Google Scholar]
- 49. Ambjørn M., Dubreuil V., Miozzo F., Nigon F., Møller B., Issazadeh-Navikas S., Berg J., Lees M., Sap J. (2013) A loss-of-function screen for phosphatases that regulate neurite outgrowth identifies PTPN12 as a negative regulator of TrkB tyrosine phosphorylation. PLoS One 8, e65371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gatto G., Dudanova I., Suetterlin P., Davies A. M., Drescher U., Bixby J. L., Klein R. (2013) Protein tyrosine phosphatase receptor type O inhibits trigeminal axon growth and branching by repressing TrkB and Ret signaling. J. Neurosci. 33, 5399–5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sharma S., Zhuang Y., Gomez-Pinilla F. (2012) High-fat diet transition reduces brain DHA levels associated with altered brain plasticity and behaviour. Sci. Rep. 2, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Woo J., Shin K. O., Park S. Y., Jang K. S., Kang S. (2013) Effects of exercise and diet change on cognition function and synaptic plasticity in high fat diet induced obese rats. Lipids Health Dis. 12, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pathre P., Arregui C., Wampler T., Kue I., Leung T. C., Lilien J., Balsamo J. (2001) PTP1B regulates neurite extension mediated by cell-cell and cell-matrix adhesion molecules. J. Neurosci. Res. 63, 143–150 [DOI] [PubMed] [Google Scholar]
- 54. Fuentes F., Zimmer D., Atienza M., Schottenfeld J., Penkala I., Bale T., Bence K. K., Arregui C. O. (2012) Protein tyrosine phosphatase PTP1B is involved in hippocampal synapse formation and learning. PLoS One 7, e41536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lazo O. M., Gonzalez A., Ascaño M., Kuruvilla R., Couve A., Bronfman F. C. (2013) BDNF regulates Rab11-mediated recycling endosome dynamics to induce dendritic branching. J. Neurosci. 33, 6112–6122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bouret S. G., Gorski J. N., Patterson C. M., Chen S., Levin B. E., Simerly R. B. (2008) Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab. 7, 179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pelleymounter M. A., Cullen M. J., Wellman C. L. (1995) Characteristics of BDNF-induced weight loss. Exp. Neurol. 131, 229–238 [DOI] [PubMed] [Google Scholar]
- 58. Xu B., Goulding E. H., Zang K., Cepoi D., Cone R. D., Jones K. R., Tecott L. H., Reichardt L. F. (2003) Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 6, 736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]