Abstract
Living in an oxygen-rich environment is dangerous for a cell. Reactive oxygen species can damage DNA, RNA, protein and lipids. The MutT protein in Escherichia coli removes 8-oxo-deoxyguanosine triphosphate (8-oxo-dGTP) and 8-oxo-guanosine triphosphate (8-oxo-GTP) from the nucleotide pools precluding incorporation into DNA and RNA. While 8-oxo-dGTP incorporation into DNA is mutagenic, it is not clear if 8-oxo-GTP incorporation into RNA can have phenotypic consequences for the cell. We use a bistable epigenetic switch sensitive to transcription errors in the Escherichia coli lacI transcript to monitor transient RNA errors. We do not observe any increase in epigenetic switching in mutT cells. We revisit the original observation of partial phenotypic suppression of a lacZamber allele in a mutT background that was attributed to RNA errors. We find that Lac+ revertants can completely account for the increase in β-galactosidase levels in mutT lacZamber cultures, without invoking participation of transient transcription errors. Moreover, we observe a fluctuation type of distribution of β-galactosidase appearance in a growing culture, consistent with Lac+ DNA revertant events. We conclude that the absence of MutT produces a DNA mutator but does not equally create an RNA mutator.
INTRODUCTION
Errors in information transfer from DNA to RNA to protein are inevitable. Transcription errors occur at a rate of ∼10−5 per residue in Escherichia coli (1,2), over 10 000× higher than errors in DNA synthesis. Errors in DNA synthesis can produce heritable change in phenotype due to alteration of protein function. Studies that have focused on the mechanisms of DNA replication and repair in E. coli have provided a major framework for understanding the fidelity of genetic transmission from cell to cell and have revealed a series of fidelity mechanisms responsible for maintaining DNA integrity (3). Transcription errors, although transient in nature, can also have phenotypic consequences for the cell, including transient (4) and heritable phenotypic change (5,6). Unlike DNA fidelity, the mechanisms ensuring transcription fidelity in vivo are not well characterized due to the difficulty of isolating such transient errors in mRNA (7). We have harnessed the classical bistable switch in the lac operon, a memory-module, to capture and monitor the consequences of transient transcription errors in living E. coli cells, providing an appropriate tool to study proteins involved in modulating RNA fidelity (5,6).
The lac operon comprises an autocatalytic positive feedback loop allowing a heritable all-or-none epigenetic switch at a maintenance concentration of inducer [that concentration of inducer which does not activate transcription of the operon but allows an already induced cell to remain induced (5,8–9)]. The lac repressor is rare (∼5 tetramers per cell) (10). A transient depletion of repressor within a cell will lead to a transient derepression of the operon, producing a burst of lacY permease gene expression (11). At the maintenance concentration of the nonmetabolizable inducer thio-methylgalactoside (TMG), this burst of permease will trigger an autocatalytic positive feedback response, so that the new induced state will be heritably maintained through cell division in a clonal cell population (5,6).
Using single-cell analysis, we have previously shown that the frequency of epigenetic switching from the OFF expression state to the ON expression state of the lac operon is increased when the fidelity of RNA transcription is decreased due to error-prone RNA polymerases, error-prone transcription sequences or to the absence of auxiliary RNA fidelity factors GreA and GreB (functional analogues of eukaryotic TFIIS) (5,6). In addition, the 1000-fold difference between the genetic mutation frequency (lacI+ to lacI−) and the epigenetic switch frequency observed in the wild-type strain (5) demonstrates that this epigenetic switch system is not affected by mutation, even the increased mutagenesis seen in mutator strains (5).
Treffers was the first to discover a mutator strain of bacteria (12). His mutT allele facilitated a unique unidirectional mutational signature, A:T → C:G transversions (13). It was later shown that MutT protein can hydrolyze an oxidized dGTP nucleotide, 8-oxo-deoxyguanosine triphosphate (8-oxo-dGTP), back to the monophosphate form 8-oxo-dGMP (14). 8-oxo-dGTP is a potent mutagenic nucleotide that is readily incorporated into DNA opposite template C or A (14), with a preference for template A (15,16). Therefore, by cleaning the 8-oxo-dGTP pool, MutT reduces spontaneous transversion mutations ∼1000-fold (12–13,17). In addition, MutT can also act on 8-oxo-guanosine triphosphate (8-oxo-GTP) and convert it to 8-oxo-GMP averting mutagenic nucleotide incorporation into RNA (18). mutT mutants are known to exhibit phenotypes independent of DNA mutational effects, which have been attributed to a decrease in transcription fidelity (18–20). The persistence of oxidized ribonucleotides in the available nucleotide pool and the subsequent incorporation of 8-oxo-GTP into mRNA, causing T to G transversions in the nascent transcript, was assumed to be the mechanism for RNA errors and these phenotypes. It was suggested that the absence of MutT can increase the readthrough of a stop codon mutation through 8-oxo-GTP incorporation generating a 30-fold increase in functional protein levels in mutT cultures compared to wild-type cultures (18). Such RNA infidelity in mutT strains may account for the accumulation of misfolded proteins (19) and the observed cytotoxicity of aminoglycoside antibiotics (20).
To investigate the role of MutT on transcription fidelity, we have used our bistable lac assay that is sensitive to transcription errors and find no increase in epigenetic switch frequency in the absence of MutT function; we also show that the phenotype previously attributed to transcription errors [partial phenotypic suppression or leakiness (18)] is principally due to mutagenic events in the DNA of the cells and not due to the incorporation of oxidized GTP into mRNA. In light of our results we suggest that other observed mutT phenotypes, such as protein mistranslation or antibiotic sensitivity, that have been attributed to 8-oxo-GTP require other explanations than simply 8-oxo-GTP misincorporation into mRNA.
MATERIALS AND METHODS
Bacterial strains
All strains used in this study are derived from the wild-type sequenced E. coli MG1655 strain or strain CC101 (gift of Susan Rosenberg, Baylor College of Medicine, USA) and are found in Table 1. Manipulation of the MG1655 and CC101 genomes was accomplished by standard methodologies (21,22). The dnaE941 allele (gift of Roel Schaaper, National Institute of Environmental Health Sciences, USA) and the ΔmutT and ΔmutY deletion mutations (Keio Collection, Keio University, Japan) (23) were moved into CC101 by P1 transduction; the lacIq allele (Coli Genetic Stock Center, Yale, USA) was moved into CH1118 by P1 transduction. The kanamycin resistance cassettes were removed by a pCP20 flippase reaction.
Table 1. Bacterial strains.
| Strain | Genotype | Reference |
|---|---|---|
| CH30 | MG1655: λ−, rph-1 | laboratory stock |
| CH458 | MG1655 lacZYA::gfp-catR | (5) |
| CH5201 | ΔlacI::cmR lacZYA::gfpFRT (CH2163 with pKD46 lost) | (6) |
| CH1071 | NCM514 lacIp-4000 (lacIq) zah-2224::catR λ−, rph-1 | CGSC 8249 |
| CH1118 | MG1655 lacZYA::gfpFRT | (5) |
| CH1143 | MG1655 lacIqzah-2224::catR lacZYA::gfpFRT | CH1118 x P1 (CH1071) |
| BW25113 | F−, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ−, rph-1, Δ(rhaD-rhaB)568, hsdR514 | (23) |
| CH584 | BW25113 mutY::knR | JW2928 (23) |
| CH336 | BW25113 mutT::knR | JW0097 (23) |
| CH4198 | NR10778: ara-9, fhuA1, lacY1 or lacZ4, tsx-3, supE44, galK2, λ−, hisG4(oc), rfbD1?, trp-3(Oc), rpsL8 or rpsL9 malA1 (λr), metE46, mtl-1, thi-1, dnaE941, zae502::Tn10, lacZ118(oc) | (37) |
| CH353 | MG1655 mutT::knR | CH30 x P1 (CH336) |
| CH605 | MG1655 mutY::knR | CH30 x P1 (CH584) |
| CH586 | CC101: F` lacI−Z− proA+B+; ara Δ(gpt-lac)5 | (17) via S.M.Rosenberg |
| CH2956 | F` lacI−Z+ proA+B+; ara Δ(gpt-lac)5 | lacZ+ revertant |
| CH952 | CH586 mutT::knR | CH586 x P1 (CH353) |
| CH4404 | CH586 ΔmutTFRT | CH952 flipped |
| CH4406 | CH586 ΔmutTFRT mutY::knR | CH4404 x P1 (CH605) |
| CH4408 | CH586 ΔmutTFRT ΔmutYFRT | CH4406 flipped |
| CH4412 | CH586 ΔmutTFRT ΔmutYFRT dnaE941 zae502::Tn10 | CH4408 x P1 (CH4198) |
| CH4416 | CH586 ΔmutTFRT dnaE941 zae502::Tn10 | CH4404 x P1 (CH4198) |
| CH458 | MG1655 lacZYA::gfp-cmR | (5) |
| CH505 | MG1655 lacZYA::gfp-cmR mutT::knR | CH458 x P1 (CH353) |
Growth conditions and media
To determine the level of Lac+ revertants for each strain, a bacterial culture grown in LB media, was diluted and ∼200 cells were seeded to new tubes containing fresh LB media and shaken at 37°C overnight. The cultures were then washed 3× in minimal A salts (21) and then aliquots were plated onto minimal A lactose plates for Lac+ revertant determination and onto minimal A glucose plates for viability. Mutation frequency was determined by dividing Lac+ revertants by the number of viable cells in the culture (17,21). To determine the lacI+ to lacI− genetic mutation frequency, a bacterial culture grown in minimal A succinate media, was diluted and ∼200 cells were seeded to new tubes containing fresh minimal A succinate media and shaken at 37°C overnight. The cultures were then washed 3× in minimal A salts and we selected for colony forming ability on agar plates containing phenyl-β-D-galactoside (Pgal; 75 μg/ml) as the sole carbon source, and onto minimal A glucose plates for viability. Only cells constitutively expressing β-galactosidase (lacI− and lacOc mutants) can form colonies on Pgal plates. Mutation frequency was determined by dividing the number of lacI− mutants by the number of viable cells in the culture.
To demonstrate hysteresis and bistability in lac operon expression in single cells, a bacterial culture grown in minimal A salts plus MgSO4 (1 mM) with succinate (0.2%), was diluted 1:5 in fresh medium with (ON culture) or without 1-mM TMG (OFF culture) and shaken at 37°C for 7 h. After this induction period, the two cultures were individually diluted and ∼200 cells were seeded to new tubes containing fresh medium that contained varying amounts of TMG, and shaken at 37°C for 42 h. Flow cytometry was used to determine the percentage of cells that were induced for lac operon expression (ON cells), as previously described (6).
To determine epigenetic switch frequencies, a bacterial culture grown in minimal succinate media, was diluted and ∼200 cells were seeded to new tubes containing fresh medium, with a maintenance level of 6-μM TMG, and shaken at 37°C for 42 h, as previously described (6), and subjected to flow cytometry.
A reconstruction test was performed to determine the dynamic range of the β-galactosidase assay. Overnight LB cultures of CH586 (lacZ−) and a Lac+ revertant strain (CH2956) were used; the CH2956 culture was 10-fold serially diluted into CH586 to make 1 ml in total cell volume, in duplicate, and with these reconstructed Z+:Z− populations β-galactosidase assays were performed. The cell titers of the initial cultures were determined by diluting and plating onto LB plates; the number of Lac+ revertants in the initial CH586 culture was determined by plating onto minimal lactose plates, and diluting and plating onto minimal glucose plates for cell titer.
Single-molecule mRNA fluorescent in situ hybridization (smFISH)
The smFISH protocol has been described in detail (24,25). Fluorescently labeled oligonucleotide probe sequences designed against the lacI transcript (purchased from Biosearch Technologies, USA) are described in Supplementary Table S1. Bacterial strains for smFISH analysis were grown in minimal A salts with succinate (0.2%) and thiamine at 37°C. The estimation of mRNA number in the cell relies on quantifying localized fluorescence and is not achieved by counting discrete spots. The number of bound probes is measured on the basis of the total fluorescence intensity (photon flux) of the spots, without requiring that individual mRNAs appear as separate spots. By performing a calibration step, the total intensity of spots in the cell can then be converted to the number of target mRNAs (25).
Flow cytometry
To determine the percentage of cells that were induced for lac operon expression (ON cells), 10 μl of culture was diluted into 300 μl filtered minimal A salts plus MgSO4 (1 mM) and subjected to flow cytometry analysis with GFP fluorescence measured in a BD FACSCanto II Flow Cytometer (Becton, Dickinson and Company, USA) with Diva acquisition software (Becton Dickinson) and FloJo analysis software (Tree Star, Inc. USA). To monitor fluorescent cells in a culture we used a narrow gating for forward and side scattering so that the most represented cell population was evaluated. For each independent culture 10 000 cells were interrogated, as previously described (6).
β-galactosidase levels monitored over time by an automated microplate reader
Overnight LB cultures of each strain were diluted and ∼200 cells were seeded into fresh LB media containing 0.5 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactoside (Xgal) in a microtiter dish (200 μl media per well) and grown at 37°C with shaking in a BioTek Synergy™ 2 multi-detection automated microplate reader. Readings were recorded every hour for 46 h (OD600 to monitor cell growth; OD615 to monitor Xgal cleavage). OD600 readings from the LB control wells (cells but no Xgal) were subtracted from OD615 readings from wells containing the same strain with Xgal to normalize readings to account for cell growth.
β-galactosidase assay
Cells were grown in LB media and β-galactosidase levels were determined by the method of Miller (21). A Z buffer ortho-nitrophenyl-β-D-galactopyranoside (ONPG) control β-galactosidase assay (no cells) was included due to the prolonged time of some of the reactions with very low enzyme levels.
RESULTS AND DISCUSSION
Stochastic switching in the lac bistable gene network in ΔmutT cells
Although it is clear that incorporation of 8-oxo-dGTP into DNA can have mutagenic consequences for the cell, with heritable phenotypic consequences, it remains unknown if incorporation of 8-oxo-GTP into RNA can also have heritable phenotypic consequences for the cell (26). To study the effect of mutT on heritable phenotypic change, we used the bistable lac switch assay in E. coli. This bistable switch assay is sensitive to transcription errors: we previously observed that a 5-fold decrease in transcription fidelity due to an RNA polymerase mutation [measured both in vitro and in vivo (27)] leads to a 4- to 6-fold increase in epigenetic switching frequency in our bistable lac assay (5). Moreover, the number of lacI mRNA per cell is very low making this system susceptible to transcription errors. Based on equilibrium dialysis against radioactive isopropyl-thio-galactoside it was estimated that there are about five lac repressor tetramers per cell (10). It was therefore suggested that the lacI gene has an inefficient promoter and that only one or two mRNA molecules are synthesized per lacI gene per generation (28). We directly measured the number of lacI mRNA molecules in single cells using single-molecule mRNA fluorescent in situ hybridization (smFISH) (24,25) to quantify mRNA statistics in three E. coli strains: an entire deletion of the lacI gene (CH5201), the wild-type lacI promoter (CH458) and the lacI up-promoter Iq (CH1143; Figure 1). Fully 86% of wild-type lacI cells do not exhibit any lacI mRNA, 11% of cells have one lacI mRNA and 3% have two or more lacI mRNA per cell at any given time (2081 cells monitored); see Figure 1. No lacI mRNA molecules were observed in cells that have the entire lacI gene deleted. A mutant that makes about 10× more lac repressor, lacIq, has been previously isolated (28) which carries an up-mutation at the −35 position of the lacI promoter (29). smFISH analysis showed that the lacIq strain exhibited an average of 3.05 lacI mRNA per cell (2638 cells monitored; Figure 1). This is the first demonstration of wild-type lacI mRNA statistics and is in excellent agreement with the low lac repressor numbers indirectly determined during the initial isolation of the lac repressor almost 50 years ago (10). Thus, these results suggest that wild-type lac repressor production is subject to large fluctuations in protein number, due to rare stochastic transcription events (30), making the bistable switch system sensitive to transcription errors during lacI mRNA production (31).
Figure 1.
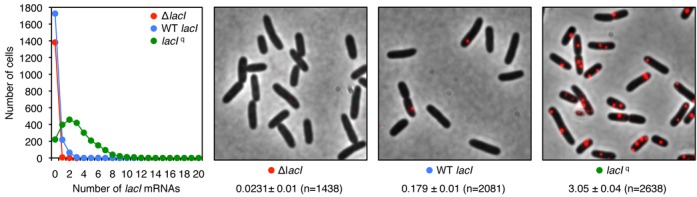
Single-molecule FISH (smFISH) to characterize lacI mRNA copy number statistics. Typical images of smFISH-labeled cells in different strain backgrounds (ΔlacI, CH5201; wild-type lacI, CH458; lacIq, CH1143). An overlay of the phase contrast (grayscale) and smFISH probes targeting the lacI gene (red) is shown. n, number of cells monitored. Mean and standard error values for absolute lacI mRNA numbers per cell are shown for each strain. The histogram shows the distribution of lacI mRNA molecules per cell observed within each strain population monitored.
To determine the proportion of cells that switch to the ON state for lac operon expression, we used a lacZYA::gfp construct expressing β-galactosidase, galactoside permease and green fluorescent protein (5). During growth of OFF cells in a maintenance concentration of TMG (6-μM TMG; see Figure 2A and B), if a cell suffers a stochastic event leading to derepression of the lac operon, this transient derepression will trigger permease synthesis and activation of the autocatalytic positive-feedback loop, resulting in green fluorescent cells (5,6). As a result, the OFF state will transition to the ON state and be heritably maintained in the following generations, mimicking lacI mutation in this system (Figure 2A). To determine the epigenetic switch frequency, we measured the number of green cells within the resulting cultures by flow cytometry (Figure 2C). We calculate the epigenetic switch frequency as number of ON cells over the total number of cells interrogated, following the convention used in determining lacI− mutation frequencies in a population (21). The observed ON switch frequency is therefore dependent on both the number of switch events that have occurred and the number of generations after a discrete switch event has occurred, as in a classical fluctuation test (see ‘Materials and Methods’ section).
Figure 2.
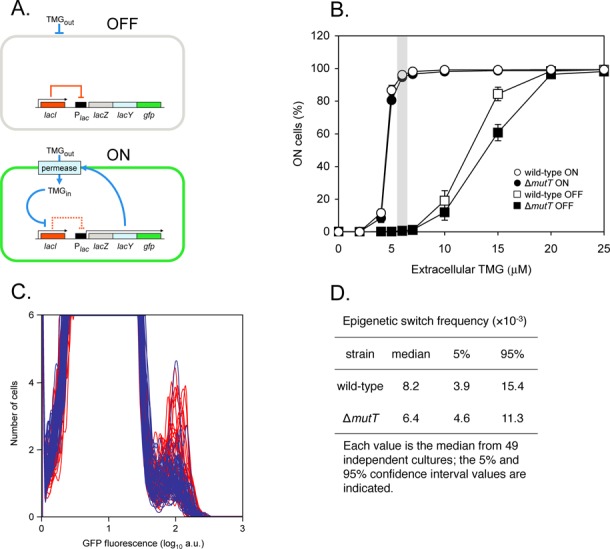
Stochastic switching in the lac bistable gene network. (A) Under maintenance conditions, the lac operon is OFF when the lac repressor is bound to the lac operator (indicated by the solid red line) and the inducer thio-methylgalactoside (TMG) remains extracellular; stochastic events that lead to a transient derepression of the lac operon will result in a burst of lac operon functions and the appearance of permease will initiate an autocatalytic positive-feedback response (indicated by solid blue lines), which will heritably maintain the ON state (TMG induces an allosteric transition in lac repressor, indicated by the dashed red line, so that it no longer binds to the lac operator), and the cell will exhibit green fluorescence. (B) Wild-type (CH458) and ΔmutT (CH505) cells that were originally ON or OFF were sub-cultured and grown in media containing various concentrations of TMG. Each value is the average ±SD from four independent cultures. The shaded area highlights the maintenance concentration of 6-μM TMG for these strains. (C) OFF wild-type cells (red histograms) and OFF ΔmutT cells (blue histograms) were diluted and grown in media containing 6-μM TMG. After 42 h growth, flow cytometry was performed to determine the frequency of epigenetically ON cells in 49 independent cultures of each strain; the ΔmutT histograms are superimposed over the wild-type histograms (104 cells interrogated for each histogram). (D) The ΔmutT epigenetic-switch frequency is not significantly increased over the wild-type value (Mann–Whitney Rank Sum Test, P = 0.13).
When we measured the epigenetic switch frequency in our bistable switch assay in a ΔmutT strain (CH505), we did not observe any increase in epigenetic switch frequency compared to wild-type cells (Mann–Whitney Rank Sum Test, P = 0.13) (Figure 2D). Both wild-type (CH458) and mutT strains exhibit bistability and similar hysteresis patterns, and the maintenance concentration of 6-μM TMG is the same for both strains (Figure 2B). As expected, we observed a 130-fold induction in lacI+ to lacI− forward mutation frequency in the mutT strain over the mutT+ strain (3.1 × 10−4 ± 4.1 × 10−4 SD for the mutT strain, three independent experiments; 2.4 × 10−6 ± 1.5 × 10−6 SD for the wild-type strain, nine independent experiments), which is similar to mutation induction found in other studies of mutT mutagenesis in the lacI system (32).
Our system directly compares the frequency of permanent and transient errors in information transfer that lead to the same phenotype (lac operon ON) in the same system (5). While the genetic mutation frequency is increased in ΔmutT cells, there is no observed increase in the epigenetic switch frequency compared to mutT+ cells. This result is intriguing because the susceptible sites of mutT-mediated mutation in the lacI gene should be equally susceptible to mutT-mediated RNA error in lacI mRNA generating non-functional repressor. Indeed, throughout the lacI gene there are 23 sites where 8-oxo-dGTP/8-oxo-GTP incorporated opposite A on the transcribed strand would give a non-functional lac repressor, whereas there are 14 sites where 8-oxo-dGTP incorporated opposite A on the non-transcribed strand would give a non-functional lac repressor (32–34). Therefore, the lacI transcript is a robust target to monitor the phenotypic consequences of 8-oxo-GTP incorporation opposite A residues during transcription.
Reducing A:T → C:G DNA mutation in a ΔmutT strain
The putative role for MutT in transcription fidelity was first observed using a lacZ amber mutant strain specific to mutT-mediated mutation (18). We note that study used the F′ from CC101 (carrying the lacZamber mutation: F′ lacI−Z− proA+B+) in a ara Δ(gpt-lac)5 thi trpE9777 background; we use the original CC101 strain (F′ lacI−Z− proA+B+; ara Δ(gpt-lac)5; Table 1) and our results should be directly comparable (both strains that carry the CC101 F′ will be referred to as CC101). E. coli strain CC101 (CH586) measures reversion from Lac− to Lac+ specifically via the A:T → C:G transversion at codon 461 of the lacZ gene; at this site the wild-type GAG Glu codon is now a TAG amber nonsense mutation (17). At the DNA level, in a mutT strain such A:T → C:G transversions are specifically produced through 8-oxo-dGTP incorporation opposite the initial A of the amber nonsense codon on the transcribed DNA strand during replication (Supplementary Figure S1). Similarly, at the RNA level, partial phenotypic suppression (leakiness) may occur through 8-oxo-GTP incorporation opposite the same A of the amber nonsense codon on the transcribed DNA strand during mRNA transcription (Supplementary Figure S1). As a result of 8-oxo-GTP incorporation, the 3′ATC5′ nonsense codon will be transcribed as 5′G*AG3′ (G* indicates 8-oxoG) in the nascent transcript and translated as a Glu residue by tRNA with a 3′CUU5′ anticodon sequence (35), providing functional β-galactosidase. Therefore, both permanent DNA mutations and transient mRNA misincorporations at this nucleotide position may contribute to the β-galactosidase levels observed in mutT cultures.
We sought to diminish the mutational burden of the absence of MutT function in the CC101 strain to allow the phenotypic consequences of 8-oxo-GTP incorporation into RNA to be enhanced. It has been shown that the absence of MutY function can decrease A:T → C:G mutations in a CC101 ΔmutT strain, i.e. MutY function is mutagenic in the absence of MutT function in E. coli (16,36). MutY is an adenine glycosylase that removes the A from A:G (and A:G*) mismatches with no apparent strand specificity: MutY does not distinguish between an A that is incorrectly incorporated opposite a template G (or G*) during DNA replication, nor a template A paired with a misincorporated G (or G*) (see Supplementary Figure S2). Vidmar and Cupples (36) observed a 71% decrease in A:T → C:G mutants in a CC101 mutT mutY strain compared to a CC101 mutT strain; we also find a similar 70% decrease in A:T → C:G mutants in an independently created CC101 mutT mutY strain (CH4406) compared to a mutT strain (CH4404) (Table 2).
Table 2. Reducing A:T → C:G mutation in a ΔmutT strain.
| Strain | Lac+ revertants per 108 cells | Reduction in ΔmutT consequences |
|---|---|---|
| CC101 mutT+ | 0.2 ± 0.2 | |
| CC101 ΔmutT | 1184 ± 313 | |
| CC101 ΔmutT dnaE941 | 476 ± 233 | 60% |
| CC101 ΔmutT ΔmutY | 352 ± 27 | 70% |
| CC101 ΔmutT ΔmutY dnaE941 | 153 ± 20 | 87% |
Frequencies are means (±SD) for eight independent cultures per strain.
To further decrease the mutational burden of the absence of MutT function in the CC101 strain, we replaced the wild-type dnaE gene with the dnaE941 anti-mutator allele. The dnaE941 allele encodes a DNA polymerase that was isolated as a suppressor of the high mutability of a mutT mutator strain (37). The dnaE941 allele in a mutT strain decreased the level of mutations to rifampicin resistance by 60% compared to a dnaE+ mutT strain (37). We also observe a 60% decrease in A:T → C:G events in the CC101 mutT dnaE941 strain (CH4416) compared to the CC101 mutT strain (Table 2). Although the mechanism by which the dnaE941 anti-mutator increases the fidelity of DNA replication is not known, it has been suggested to be due to an increase in polymerase base selectivity or an increase in exonucleolytic proofreading ability (37).
Moreover, when we combine the dnaE941 antimutator allele with an absence of MutY function we observe an 87% decrease in A:T → C:G events (strain CC101 mutT mutY dnaE941 (CH4412) versus strain CC101 mutT; Table 2). Therefore, the phenotypic consequences of transient errors in RNA transcription can now be assessed with less of the confounding influence of permanent Lac+ mutants in the bacterial culture. We emphasize that the levels of 8-oxo-dGTP and 8-oxo-GTP will be unchanged in all the mutT strains, but the DNA mutational consequences of those same levels of oxidized nucleotide pools will be different (diminished) in the mutT strains that carry the additional mutated or altered mutY and dnaE alleles. We note that cultures of the CC101 ΔmutT ΔmutY dnaE941 strain still generate over 500-fold more Lac+ mutants than the wild-type CC101 strain (Table 2).
Lac+ revertants can account for increased β-galactosidase levels in ΔmutT cultures
To concurrently study the genotypic and phenotypic effect of the absence of MutT function, we measured mutation frequency and β-galactosidase activity from the same culture in wild-type CC101, ΔmutT and the different strain backgrounds that exhibit reduced mutational load for mutT-mediated mutagenesis. Figure 3 shows the Lac+ reversion mutation frequencies and β-galactosidase enzyme activities for 10 independent CC101 and ΔmutT compromised cultures in the form of heat maps, with each panel representing an independent culture; the corresponding panels in the two maps represent the two methods of analysis undertaken on the same culture. When the mutational jackpot (row 2, column 3) is omitted from analysis, the same trend is observed as found in Table 2: the mutational consequences of the ΔmutT allele is decreased by 61% in a CC101 ΔmutT ΔmutY strain, and 86% in a CC101 ΔmutT ΔmutY dnaE941 strain in this independent experiment (Supplementary Table S2). Again, omitting the jackpot culture, we observe a 5.3-, 3.8-, 2.7-fold increase in β-galactosidase enzyme levels for the ΔmutT, ΔmutT ΔmutY and ΔmutT ΔmutY dnaE941 strains, respectively, over that observed in the wild-type CC101 strain (Supplementary Table S2). We observe that cultures of the ΔmutT ΔmutY dnaE941 strain generate over 500-fold more Lac+ mutants than the wild-type CC101 strain, but exhibit only a 2.7-fold increase in β-galactosidase enzyme level in the culture.
Figure 3.
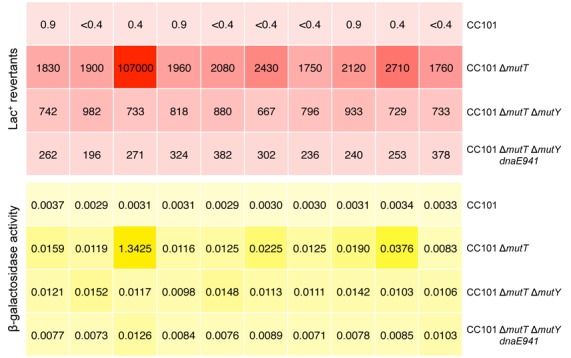
Lac+ mutation frequencies and β-galactosidase enzyme levels in CC101 and ΔmutT compromised cultures. The upper red heat map describes Lac+ revertants per 108 cells with each panel representing an independent culture (with actual revertant numbers presented); the lower yellow heat map describes β-galactosidase enzyme levels with each panel representing the same independent culture found in the corresponding position in the red heat map (actual activities in Miller units are presented). Each row depicts independent cultures of the same strain.
Our result is in contrast to that of Taddei et al. (18) not only in the fold increase in β-galactosidase levels, they observed a 30-fold increase in β-galactosidase levels ( leakiness ) in CC101 ΔmutT cultures compared to CC101 cultures, but also in the interpretation of the fold increase in β-galactosidase levels observed in ΔmutT cultures. The increased CC101 ΔmutT leakiness in that study was attributed to errors in transcription, with 8-oxo-GTP being incorporated into the nascent transcript across from the template A of the 3′ATC5′ nonsense mutation (Supplementary Figure S1); the possibility that this increased β-galactosidase enzyme level in ΔmutT cultures was due to Lac+ revertant mutations arising in the growing population, i.e. 8-oxo-dGTP being incorporated opposite the template A during DNA replication, was discounted (18). The following formula was previously used to consider the contribution of Lac+ revertants to the β-galactosidase activity of the population, M(pop), measured in Miller units: M(pop) = M(lac+)f(lac+) + M(lac−)f(lac−) where M(lac+) and M(lac−) are β-galactosidase activities of Lac+ and Lac− cells, and f(lac+) and f(lac−) their respective frequencies (18). Therefore, for an overnight CC101 culture: the frequency of Lac− is close to 1 and the frequency of A:T → C:G Lac+ revertants is ∼10−5 in the ΔmutT context (however, this value will fluctuate due to the stochastic nature of mutation appearance during growth of the bacterial culture). The wild-type level of β-galactosidase activity in a fully induced Lac+ CC101 culture is ∼3800 Miller units (38); we observe the same enzyme levels in fully induced lacZ+ cultures (Figure 4; data not shown). It was therefore argued (18) that the increase in β-galactosidase concentration Taddei et al. observed in a mutT strain was not due to reversion mutations [M(pop) = 0.38; f(lac+) = 10−5; M(lac+) = 38 000], because each revertant would need to produce 10-fold more β-galactosidase Miller units than the value obtained for a fully induced wild-type lacZ gene.
Figure 4.
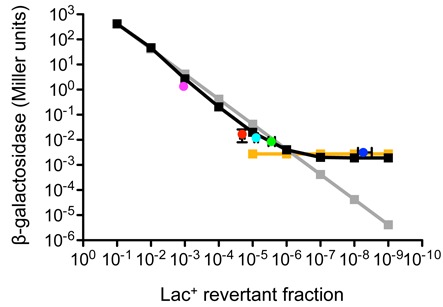
A reconstruction test of the contribution of Lac+ revertants within a Lac− population monitored by β-galactosidase assay. Black squares represent 10-fold serial dilutions of Z+ into Z− cells and then β-galactosidase assays were performed. Yellow squares represent the Z buffer ONPG (no cells) control assayed at the same time as the 1:105 to 1:109 (Z+:Z−) dilution time points. Gray squares represent the theoretical decrease in β-galactosidase units if the initial time point (1:10 dilution) β-galactosidase level is maintained throughout dilution in the reconstruction experiment. CC101 (dark blue circle), CC101 ΔmutT jackpot (purple circle), CC101 ΔmutT (red circle), CC101 ΔmutT ΔmutY (light blue circle), CC101 ΔmutT ΔmutY dnaE941 (green circle) values are from Figure 3 and Table 3; mean ± SD for 9 (red) or 10 (light blue, green, dark blue) cultures (the purple jackpot is a singular culture).
Here, we used the same formula and found that the β-galactosidase activities in the CC101 ΔmutT background, and in all the backgrounds that reduce the mutational burden of the absence of MutT function, can be readily explained solely by the generation of Lac+ DNA revertant bacteria that arise during the growth of the cultures. All the revertant Lac+ β-galactosidase levels observed in the ΔmutT strain, and the ΔmutT derivatives, approach the β-galactosidase levels made by a fully induced wild-type lacZ gene, or are less than the β-galactosidase levels made in a fully induced wild-type lacZ gene, including the ΔmutT mutation jackpot culture down to the ΔmutT ΔmutY dnaE941 background, which is a ∼400-fold difference in Lac+ mutation frequency (Table 3). In the analysis of Taddei et al., bacterial cultures containing more than 10−5 Lac+ revertants were said to be discarded from their analysis (18); here, we do not discard any results but retain them for their information content. By performing both mutation assays and β-galactosidase assays for each culture, which in total forms a robust fluctuation distribution, we show that Lac+ revertants can completely account for the increase in β-galactosidase levels in all these cultures even though the reversion frequencies from CC101 ΔmutT cultures to CC101 ΔmutT ΔmutY dnaE941 cultures vary from 10−3 to 10−6 (Figure 3; Table 3).
Table 3. Contribution of DNA errors to the observed population β-galactosidase levels.
| Strain | M(pop) | = | M(lac+) | f(lac+) | + | M(lac−) | f(lac−) |
|---|---|---|---|---|---|---|---|
| Wild-type Lac+ | 3800 | 3800 | 1 | ND | ∼10−6 | ||
| CC101 ΔmutT | 0.0169 | 805 | 2.1 × 10−5 | ND | 1 | ||
| CC101 ΔmutT jackpot | 1.3425 | 1220 | 1.1 × 10−3 | ND | 1 | ||
| CC101 ΔmutT ΔmutY | 0.0121 | 1513 | 8 × 10−6 | ND | 1 | ||
| CC101 ΔmutT ΔmutY dnaE941 | 0.00862 | 3079 | 2.8 × 10−6 | ND | 1 |
When we plot these results (Figure 3; Table 3) against the reconstruction experiment we generated the situation becomes clear (Figure 4). A LacZ+ revertant culture (CH2956) was 10-fold serially diluted into a CC101 LacZ− culture (the initial LB titers of the two cultures were the same, 4.4 × 109 cells per ml; the number of Lac+ revertants in the initial CC101 culture was 0.2 per 108 cells, and therefore this low Lac+ number renders any contribution to the total β-galactosidase units in the reconstruction test negligible) and β-galactosidase assays were performed. As the number of Lac+ revertants falls, the β-galactosidase units in the reconstruction test falls in parallel until the level of 1 Z+: 106 Z− is achieved; after that point, any further dilution in Lac+ numbers does not affect the observed β-galactosidase units level which levels out at the same β-galactosidase unit level as the Z buffer ONPG control. The numbers of Lac+ revertants, and the corresponding β-galactosidase units, for CC101, ΔmutT and all other the mutT-compromised strains, including the ΔmutT jackpot culture, all fall on, or close to, the reconstruction line (see Figure 4). It is clear that the dynamic range of the β-galactosidase assay has been exceeded when the CC101 strain is monitored: in theory, the CC101 strain should exhibit a value more than 100-fold less than that observed (the CC101 level is simply the level of the Z buffer ONPG control); in practice, the β-galactosidase level in CC101 is over-estimated (by at least 100-fold), and therefore the fold difference of the ΔmutT, and all the other mutT-compromised strains, over the CC101 level is under-estimated. We note that our CC101 ΔmutT ΔmutY dnaE941 cultures contain over 500-fold more Lac+ revertants than the wild-type CC101 strain, and although these CC101 ΔmutT ΔmutY dnaE941 cultures exhibit β-galactosidase levels that can be completely accounted for by Lac+ revertants [M(lac+) is 3079 Miller units; see Table 3], they exhibit only a 2.7-fold increase in β-galactosidase units over CC101. Figure 4 provides the answer to this conundrum, namely, that the CC101 ΔmutT ΔmutY dnaE941 strain is being effectively assayed for β-galactosidase content in the culture, while the CC101 strain is not being effectively assayed for β-galactosidase content in the culture, and the correction for this mis-monitoring would be over 100-fold.
We demonstrate that our observed β-galactosidase levels in CC101 ΔmutT cultures can be accounted for by the Lac+ revertant numbers we found in the same cultures, unlike the initial study, where the β-galactosidase levels observed were stated to be too high to be accounted for by Lac+ revertant numbers (18). It is difficult to address this discrepancy, but our reconstruction test results would suggest that the initial study may have contained more Lac+ revertants than appreciated in the ΔmutT cultures, and the authors did not realize that the purported CC101 β-galactosidase readings are beyond the range of the β-galactosidase assay (Figure 4). We do note that in a recent study (35), it was found that using a similar system that also monitored partial phenotypic suppression using a different lacZ amber mutation, a 1.5-fold increase in leakiness was observed in ΔmutT cultures compared to wild-type cultures, a result more in line with what we observe here (5.3- to 2.7-fold), and not what was previously observed (∼30-fold) in the CC101 background (18,39). This discrepancy in lacZamber leakiness (1.5- versus the 30-fold increase observed before at codon 461 of lacZ in a ΔmutT background) was not addressed by Inokuchi et al. (35). The system of Inokuchi et al. utilized a single base substitution in the glutamine codon (CAG) at the position 1456 of the lacZ gene, creating an amber nonsense mutation at codon 486; incorporation of 8-oxo-GTP opposite the A of the nonsense codon on the transcribed DNA strand would allow the incorporation of a glutamic acid residue at this site that will also provide essentially the same β-galactosidase activity as the wild-type enzyme (35). Therefore, we did not find an unaccountable increase in β-galactosidase levels in a ΔmutT strain at site 461 in lacZamber as found previously (18,39), and Inokuchi et al. did not find any significant increase at another site, 486 in lacZ, in a system that was created to specifically assess leakiness due to 8-oxo-GTP incorporation in the lacZ transcript (35). As was noted before (26), other trpE and tyrA auxotrophs with ochre mutations do not demonstrate any leakiness in a ΔmutT background (40).
Finally, we note that the high spontaneous level of A:T → C:G mutations in mutT strains is completely suppressed when the mutT cells are cultured in anaerobic conditions, indicating the essential role of oxygen in mutT-mediated mutagenesis (41–43). It was also observed that anaerobic conditions reduced transcriptional leakiness in a mutT strain almost to the wild-type level, consistent with the involvement of reactive oxygen species (18). It has been previously argued (18) that mutation and leakiness in mutT cultures exhibited differential responses during anaerobiosis (mutation was not reduced; leakiness was reduced) and therefore this would be consistent with the idea that mutT-mediated mutations are not responsible for the observed increase in β-galactosidase levels in a mutT aerobic culture; in light of other studies (41–43) this argument is no longer tenable.
Fluctuation analysis of β-galactosidase appearance
To qualitatively monitor β-galactosidase enzyme activity in growing cultures, Xgal (0.5 mg/ml) was added to LB media (39), and a fluctuation test was performed. We seeded ∼200 cells to wells of a microtiter dish and monitored growth (OD600) and cleavage of Xgal (OD615) to observe the nature of the appearance of β-galactosidase activity during growth of the CC101 and ΔmutT compromised cultures (Figure 5A). As the burden of mutT-mediated mutagenesis is attenuated from ΔmutT to ΔmutT ΔmutY or ΔmutT dnaE941 to ΔmutT ΔmutY dnaE941, the resulting cultures become less uniformly blue, and only some cultures turn blue whereas other cultures of the same strain remain clear. Indeed, in the CC101 ΔmutT ΔmutY dnaE941 strain, the great majority of wells were clear and similar in appearance to the wild-type parent CC101 (as shown in the OD615 tracings; Figure 5A bottom panel). Therefore, although the amounts of 8-oxo-dGTP and 8-oxo-GTP remain the same in all these ΔmutT strains, the phenotypic consequences of these oxidized nucleotides are decreased; we emphasize that it is only the mutational consequences of 8-oxo-dGTP that are precluded, since DNA polymerase III and MutY do not incorporate 8-oxo-GTP nor act on 8-oxo-GTP incorporation, respectively.
Figure 5.
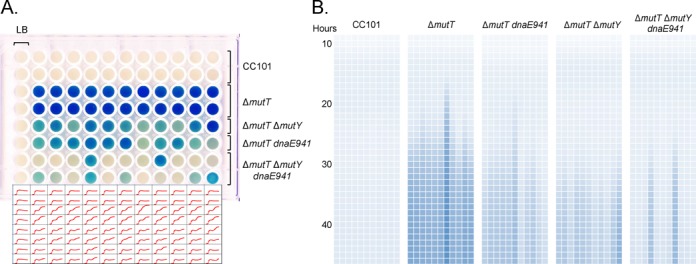
A fluctuation test analysis of Lac+ revertants in CC101 and ΔmutT compromised strains. Overnight LB cultures of each strain were diluted and ∼200 cells were seeded into fresh LB media containing 0.5 mg/ml Xgal in a microtiter dish (200 μl per well) and grown at 37°C with shaking in an automated plate reader. Readings were recorded every hour for 46 h (OD600 to monitor cell growth; OD615 to monitor Xgal cleavage). (A) False color microtiter plate after 46 h growth. Rows 1 and 2, CC101; rows 2 and 3, CC101 ΔmutT; row 5, CC101 ΔmutT ΔmutY; row 6, CC101 ΔmutT dnaE941; rows 7 and 8, CC101 ΔmutT ΔmutY dnaE941. The first column is an LB control (cells but no Xgal; OD600 readings from these wells were subtracted from OD615 readings from wells containing the same strain with Xgal to normalize readings to account for cell growth as shown in (B)); all other wells contain LB plus Xgal. The corresponding OD615 traces are found beneath the microtiter plate. (B) Heat map representation of the OD615 scans over time showing the fluctuation nature of the observance of Lac+ mutation. Each row represents normalized hourly OD615 readings starting at hour 9 and ending at hour 46. First panel shows CC101 results from row 1 in (A); second panel shows CC101 ΔmutT results from row 3 in (A); third panel shows CC101 ΔmutT dnaE941 results from row 6 in (A); fourth panel shows CC101 ΔmutT ΔmutY results from row 5 in (A); last panel shows CC101 ΔmutT ΔmutY dnaE941 from row 7 in (A).
When the time course of β-galactosidase enzyme activity in growing cultures is monitored (Figure 5B), the fluctuation distribution of β-galactosidase enzyme activity appearance becomes readily apparent. All ΔmutT wells exhibit an abrupt appearance of blue with the timing of color change varying from well to well. The timing of blue appearance becomes delayed and the numbers of wells that exhibit blue decreases as the mutational consequence of mutT-mediated mutagenesis is decreased. This is seen most clearly for the CC101 ΔmutT ΔmutY dnaE941 strain when singular stochastic events in a few cultures produce markedly different end results from the majority of similar cultures (another independent experiment giving identical results is described in Supplementary Figure S3).
We observe a fluctuation type of distribution of β-galactosidase appearance in a growing culture, consistent with Lac+ revertant events, and not a gradual constant production of β-galactosidase that should arise from chronic incorporation of 8-oxo-GTP into the lacZ transcript that would transiently produce long-lived β-galactosidase molecules that would progressively accumulate (newly created revertant Lac+ mRNA being offset by dilution of β-galactosidase by cell division); moreover, this gradual accumulated appearance should be similar for all ΔmutT compromised cultures, since the amount of 8-oxo-GTP is the same for all such strains. However, we see no evidence for a steady increase in blue color. Therefore, these results are completely consistent with the quantitative assay of mutation and β-galactosidase enzyme activity (Figure 3 and Supplementary Table S2).
The CC101 mutT background has been used to great effect in the screening of mammalian cDNAs to identify genes that can prevent A:T → C:G mutations by cleaning up the nucleotide pool (44,45). For example, hMTH1 (44) and hNUDT5 (45), despite different substrate specificities, can replace MutT function: when the cDNA for each human protein was expressed in CC101 mutT E. coli, the mutator phenotype was completely suppressed (99.4 and 99.9% reduction in lacZ amber reversion rate, respectively). However, other studies have used the suppression of blue-ness in a CC101 ΔmutT background grown in LB plus Xgal, or in a more quantitative β-galactosidase enzyme activity assay, to screen for mammalian (39) or plant (46) cDNA functions that are considered to prevent transcriptional errors caused by oxidative damage. Although both DNA and RNA errors will contribute to the β-galactosidase enzyme activity in the E. coli cultures, it was assumed that the increased β-galactosidase enzyme activity in mutT cultures was due solely to RNA errors, an idea that we challenge based on our results. We suggest that the findings of Ishibashi et al. (39) and Yoshimura et al. (46) can be explained by DNA errors arising through incorporation of 8-oxo-dGTP during DNA replication since there is a perfect correlation between β-galactosidase enzyme activity and mutation rate that they observe in their cultures (suppression of mutation by the cDNA gives clear cultures indicative of low β-galactosidase enzyme activity; or incomplete suppression of mutation by the cDNA gives blue cultures indicating higher β-galactosidase enzyme activity). This eliminates any requirement for errors arising through incorporation of 8-oxo-GTP during transcription.
MutT and transcription errors
The idea that persistent 8-oxo-GTP in the ribonucleotide pool, due to the absence of MutT function, can have phenotypic consequences for the cell is provocative (18,26). When we utilized an epigenetic switch system that can convert transient stochastic transcription error events into a heritable phenotype, via positive feed-back, we do not observe any increase in epigenetic switch frequency in a ΔmutT strain. When we decreased the mutational burden of ΔmutT in strain CC101 (although the levels of 8-oxo-dGTP and 8-oxo-GTP remain the same in all cells) we do not see any enhanced phenotypic effect attributable to RNA errors. Mutation fluctuation patterns become more pronounced as we decrease the mutational burden of mutT-mediated mutagenesis. Lac+ revertants can explain the increase in β-galactosidase enzyme activity in the ΔmutT cultures. Therefore, our results suggest that 8-oxo-dGTP can have ∼500× more relative DNA mutational impact during DNA replication than the RNA mutational impact of 8-oxo-GTP during RNA transcription.
It has been estimated that the content of 8-oxo-G in the DNA of mutT cells due to the incorporation of 8-oxo-dGTP is about four per 106 guanine residues (47). If the relative amounts of 8-oxo-dGTP to dGTP and 8-oxo-GTP to GTP in the pools are the same, and the propensity of DNA polymerase to incorporate 8-oxo-dGTP is the same as the propensity of RNA polymerase to incorporate 8-oxo-GTP relative to the non-oxidized nucleotide, then there should also be about four 8-oxo-G per 106 guanine residues in total RNA (26). Concerning the mutational consequences of such oxidized nucleotide incorporation, one also needs to consider the specificity of incorporation by the polymerase opposite A (mutagenic outcome) or C (non-mutagenic outcome) bases residing in the template. While it has been shown that E. coli DNA polymerase III can use 8-oxo-dGTP as efficiently as dGTP (14), 8-oxo-GTP is incorporated into RNA by E. coli RNA polymerase at a rate of only 4% of that for GTP (48). Moreover, while the incorporation of 8-oxo-GTP opposite template A and C occurs with similar efficiencies with RNA polymerase (49), DNA pol III incorporates 8-oxo-dGTP 20× more efficiently opposite template A compared with template C (15). Therefore, 8-oxo-dGTP should have ∼500× more relative DNA mutational impact during DNA replication than the RNA mutational impact of 8-oxo-GTP during RNA transcription. Due to the lower efficiency of RNA polymerase 8-oxo-GTP incorporation, and the lower mutagenic potential of such incorporation, we suggest that mutT-mediated RNA errors during transcription do not significantly increase the rate of ∼10−5 transcription errors per residue observed in wild-type E. coli (1,2). In fact, the level of 8-oxo-G in cellular RNA increases from one 8-oxo-G per 105 guanine residues to ten 8-oxo-G per 105 guanine residues during exposure to 5-mM H2O2, a treatment that kills 50% of the cells (50). This measurement takes into account both 8-oxo-G incorporation into RNA and also oxidation of G after incorporation into RNA. Thus, the difference in the relative mutational impact of 8-oxo-dGTP and 8-oxo-GTP in the absence of MutT function would explain the results we observe in this study, namely, ΔmutT creates a strong DNA mutator but does not equally create a strong RNA mutator.
Therefore, we find little evidence to support the idea that the absence of MutT function at the level of transcription produces any significant transient (phenotypic suppression) or heritable (epigenetic switching) consequences for the phenotype of the cell, and instead suggest that the observed increase in β-galactosidase levels in mutT strains is due to mutT-mediated mutagenesis. Although we have shown that transient transcription errors can have phenotypic consequences for the cell (5,6), and we continue to assess RNA mutator candidates, we do not find the ΔmutT situation to act as an RNA mutator.
A high level of reactive oxygen species can be detrimental for cell survival because of damage to DNA, RNA, protein and lipids. Whereas the removal of oxidized dGTP by MutT is critical to reduce DNA replication errors, it remains unclear how the persistence of oxidized GTP in the nucleotide pool can trigger aminoglycoside antibiotic cytotoxicity (20) or increase protein mistranslation in bacteria (19).
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
Acknowledgments
We thank Roel Schaaper (US National Institute of Environmental Health Sciences, USA) and Susan Rosenberg (Baylor College of Medicine, USA) for bacterial strains; Ralf Nehring and Hang Dai for technical assistance; Ralf Nehring, Priya Sivaramakrishnan and Miroslav Radman for reading the manuscript; and Francois Taddei for discussion and suggesting experiments. We thank Joel M. Sederstrom of the Cytometry and Cell Sorting Core at Baylor College of Medicine for expert assistance.
Footnotes
The authors wish it to be known that, in their opinion, these two authors contributed equally to this work.
FUNDING
US National Institutes of Health [1RO1GM88635 to C.H., R01 GM082837 to I.G., AI036211, CA125123 and RR024574 to Cytometry and Cell Sorting Core at Baylor College of Medicine]; US National Science Foundation [MCB1022327 to C.H., 082265 (CPLC) and PHY-1147498 (CAREER) to I.G.]; Welch Foundation [Q-1759 to I.G.]. Funding for open access charge: US National Institutes of Health [1RO1GM88635].
Conflict of interest statement. None declared.
REFERENCES
- 1.Ninio J. Connections between translation, transcription and replication error-rates. Biochimie. 1991;73:1517–1523. doi: 10.1016/0300-9084(91)90186-5. [DOI] [PubMed] [Google Scholar]
- 2.Imashimizu M., Oshima T., Lubkowska L., Kashlev M. Direct assessment of transcription fidelity by high-resolution RNA sequencing. Nucleic Acids Res. 2013;41:9090–9104. doi: 10.1093/nar/gkt698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedberg E.C., Walker G.C., Seide W., Wood R.D., Schultz R.A., Ellenberger T. DNA Repair and Mutagenesis. Washington DC: American Society for Microbiology Press; 2006. [Google Scholar]
- 4.Viswanathan A., You H.J., Doetsch P.W. Phenotypic change caused by transcriptional bypass of uracil in nondividing cells. Science. 1999;284:159–162. doi: 10.1126/science.284.5411.159. [DOI] [PubMed] [Google Scholar]
- 5.Gordon A.J.E., Halliday J.A., Blankschien M.D., Burns P.A., Yatagai F., Herman C. Transcriptional infidelity promotes heritable phenotypic change in a bistable gene network. PLoS Biol. 2009;7:e44. doi: 10.1371/journal.pbio.1000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon A.J.E., Satory D., Halliday J.A., Herman C. Heritable change caused by transient transcription errors. PLoS Genet. 2013;9:e1003595. doi: 10.1371/journal.pgen.1003595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gout J.-F., Thomas W.K., Smith Z., Okamoto K., Lynch M. Large-scale detection of in vivo transcription errors. Proc. Natl. Acad. Sci. U.S.A. 2013;110:18584–18589. doi: 10.1073/pnas.1309843110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novick A., Weiner M. Enzyme induction as an all-or-none phenomenon. Proc. Natl. Acad. Sci. U.S.A. 1957;43:553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozbudak E.M., Thattai M., Lim H.N., Shraiman B.I., van Oudenaarden A. Multistability in the lactose utilization network of Escherichia coli. Nature. 2004;427:737–740. doi: 10.1038/nature02298. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert W., Müller-Hill B. Isolation of the lac repressor. Proc. Natl. Acad. Sci. U.S.A. 1966;56:1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai L., Friedman N., Xie X.S. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006;440:358–362. doi: 10.1038/nature04599. [DOI] [PubMed] [Google Scholar]
- 12.Treffers H.P., Spinelli V., Belser N.O. A factor (or mutator gene) influencing mutation rates in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 1954;40:1064–1071. doi: 10.1073/pnas.40.11.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanofsky C., Cox E.C., Horn V. The unusual mutagenic specificity of an E. coli mutator gene. Proc. Natl. Acad. Sci. U.S.A. 1966;55:274–281. doi: 10.1073/pnas.55.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maki H., Sekiguchi M. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature. 1992;355:273–275. doi: 10.1038/355273a0. [DOI] [PubMed] [Google Scholar]
- 15.Yamada M., Shimizu M., Katafuchi A., Grúz P., Fujii S., Usui Y., Fuchs R.P., Nohmi T. Escherichia coli DNA polymerase III is responsible for the high level of spontaneous mutations in mutT strains. Mol. Microbiol. 2012;86:1364–1375. doi: 10.1111/mmi.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler R.G., White S.J., Koyama C., Moore S.C., Dunn R.L., Schaaper R.M. Interactions among the Escherichia coli mutT, mutM, and mutY damage prevention pathways. DNA Repair (Amst.) 2003;2:159–173. doi: 10.1016/s1568-7864(02)00193-3. [DOI] [PubMed] [Google Scholar]
- 17.Cupples C.G., Miller J.H. A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc. Natl. Acad. Sci. U.S.A. 1989;86:5345–5349. doi: 10.1073/pnas.86.14.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taddei F., Hayakawa H., Bouton M., Cirinesi A., Matic I., Sekiguchi M., Radman M. Counteraction by MutT protein of transcriptional errors caused by oxidative damage. Science. 1997;278:128–130. doi: 10.1126/science.278.5335.128. [DOI] [PubMed] [Google Scholar]
- 19.Dukan S., Farewell A., Ballesteros M., Taddei F., Radman M., Nyström T. Protein oxidation in response to increased transcriptional or translational errors. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5746–5749. doi: 10.1073/pnas.100422497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foti J.J., Devadoss B., Winkler J.A., Collins J.J., Walker G.C. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336:315–319. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller J.H. A Short Course in Bacterial Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 22.Datsenko K.A., Wanner B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K.A., Tomita M., Wanner B.L., Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006;2 doi: 10.1038/msb4100050. 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.So L.-H., Ghosh A., Zong C., Sepúlveda L.A., Segev R., Golding I. General properties of transcriptional time series in Escherichia coli. Nat. Genet. 2011;43:554–560. doi: 10.1038/ng.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skinner S.O., Sepúlveda L.A., Xu H., Golding I. Measuring mRNA copy number in individual Escherichia coli cells using single-molecule fluorescent in situ hybridization. Nat. Protoc. 2013;8:1100–1113. doi: 10.1038/nprot.2013.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bridges B.A. MutT prevents leakiness. Science. 1997;278:78–79. doi: 10.1126/science.278.5335.78. [DOI] [PubMed] [Google Scholar]
- 27.Blank A., Gallant J.A., Burgess R.R., Loeb L.A. An RNA polymerase mutant with reduced accuracy of chain elongation. Biochemistry. 1986;25:5920–5928. doi: 10.1021/bi00368a013. [DOI] [PubMed] [Google Scholar]
- 28.Müller-Hill B., Crapo L., Gilbert W. Mutants that make more lac repressor. Proc. Natl. Acad. Sci. U.S.A. 1968;59:1259–1264. doi: 10.1073/pnas.59.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calos M.P. DNA sequence for a low-level promoter of the lac repressor gene and an up promoter mutation. Nature. 1978;274:762–765. doi: 10.1038/274762a0. [DOI] [PubMed] [Google Scholar]
- 30.Raj A., van Oudenaarden A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell. 2008;135:216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satory D., Gordon A.J.E., Halliday J.A., Herman C. Epigenetic switches: can infidelity govern fate in microbes? Curr. Opin. Microbiol. 2011;14:212–217. doi: 10.1016/j.mib.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Fowler R.G., Schaaper R.M. The role of the mutT gene of Escherichia coli in maintaining replication fidelity. FEMS Microbiol. Rev. 1997;21:43–54. doi: 10.1111/j.1574-6976.1997.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 33.Miller J.H., Ganem D., Lu P., Schmitz A. Genetic studies of the lac repressor. I. Correlation of mutational sites with specific amino acid residues: construction of a colinear gene-protein map. J. Mol. Biol. 1977;109:275–298. doi: 10.1016/s0022-2836(77)80034-x. [DOI] [PubMed] [Google Scholar]
- 34.Gordon A.J.E., Burns P.A., Fix D.F., Yatagai F., Allen F.L., Horsfall M.J., Halliday J.A., Gray J., Bernelot-Moens C., Glickman B.W. Missense mutation in the lacI gene of Escherichia coli: Inferences on the structure of the repressor protein. J. Mol. Biol. 1988;200:239–251. doi: 10.1016/0022-2836(88)90237-9. [DOI] [PubMed] [Google Scholar]
- 35.Inokuchi H., Ito R., Sekiguchi T., Sekiguchi M. Search for proteins required for accurate gene expression under oxidative stress: roles of guanylate kinase and RNA polymerase. J. Biol. Chem. 2013;288:32952–32962. doi: 10.1074/jbc.M113.507772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vidmar J.J., Cupples C.G. MutY repair is mutagenic in mutT− strains of Escherichia coli. Can. J. Microbiol. 1993;39:892–894. doi: 10.1139/m93-133. [DOI] [PubMed] [Google Scholar]
- 37.Schaaper R.M. Suppressors of Escherichia coli mutT: antimutators for DNA replication errors. Mutat. Res. 1996;350:17–23. doi: 10.1016/0027-5107(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 38.Cupples C.G., Miller J.H. Effects of amino acid substitutions at the active site in Escherichia coli β-galactosidase. Genetics. 1988;120:637–644. doi: 10.1093/genetics/120.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishibashi T., Hayakawa H., Ito R., Miyazawa M., Yamagata Y., Sekiguchi M. Mammalian enzymes for preventing transcriptional errors caused by oxidative damage. Nucleic Acids Res. 2005;33:3779–3784. doi: 10.1093/nar/gki682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bridges B.A. Elevated mutation rate in mutT bacteria during starvation: evidence for DNA turnover? J. Bacteriol. 1996;178:2709–2711. doi: 10.1128/jb.178.9.2709-2711.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fowler R.G., Erickson J.A., Isbell R.J. Activity of the Escherichia coli mutT mutator allele in an anaerobic environment. J. Bacteriol. 1994;176:7727–7729. doi: 10.1128/jb.176.24.7727-7729.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakai A., Nakanishi M., Yoshiyama K., Maki H. Impact of reactive oxygen species on spontaneous mutagenesis in Escherichia coli. Genes Cells. 2006;11:767–778. doi: 10.1111/j.1365-2443.2006.00982.x. [DOI] [PubMed] [Google Scholar]
- 43.Setoyama D., Ito R., Takagi Y., Sekiguchi M. Molecular actions of Escherichia coli MutT for control of spontaneous mutagenesis. Mutat. Res. 2011;707:9–14. doi: 10.1016/j.mrfmmm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Furuichi M., Yoshida M.C., Oda H., Tajiri T., Nakabeppu Y., Tsuzuki T., Sekiguchi M. Genomic structure and chromosome location of the human mutT homologue gene MTH1 encoding 8-oxo-dGTPase for prevention of A:T to C:G transversion. Genomics. 1994;24:485–490. doi: 10.1006/geno.1994.1657. [DOI] [PubMed] [Google Scholar]
- 45.Ishibashi T., Hayakawa H., Sekiguchi M. A novel mechanism for preventing mutations caused by oxidation of guanine nucleotides. EMBO Rep. 2003;4:479–483. doi: 10.1038/sj.embor.embor838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshimura K., Ogawa T., Ueda Y., Shigeoka S. AtNUDX1, an 8-oxo-7, 8-dihydro-2 -deoxyguanosine 5 -triphosphate pyrophosphohydrolase, is responsible for eliminating oxidized nucleotides in Arabidopsis. Plant Cell Physiol. 2007;48:1438–1449. doi: 10.1093/pcp/pcm112. [DOI] [PubMed] [Google Scholar]
- 47.Tajiri T., Maki H., Sekiguchi M. Functional cooperation of MutT, MutM and MutY proteins in preventing mutations caused by spontaneous oxidation of guanine nucleotide in Escherichia coli. Mutat. Res. 1995;336:257–267. doi: 10.1016/0921-8777(94)00062-b. [DOI] [PubMed] [Google Scholar]
- 48.Sekiguchi T., Ito R., Hayakawa H., Sekiguchi M. Elimination and utilization of oxidized guanine nucleotides in the synthesis of RNA and its precursors. J. Biol. Chem. 2013;288:8128–8135. doi: 10.1074/jbc.M112.418723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamiya H., Suzuki A., Yamaguchi Y., Handa H., Harashima H. Incorporation of 8-hydroxyguanosine (8-oxo-7, 8-dihydroguanosine) 5′-triphosphate by bacterial and human RNA polymerases. Free Radic. Biol. Med. 2009;46:1703–1707. doi: 10.1016/j.freeradbiomed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Liu M., Gong X., Alluri R.K., Wu J., Sablo T., Li Z. Characterization of RNA damage under oxidative stress in Escherichia coli. Biol. Chem. 2012;393:123–132. doi: 10.1515/hsz-2011-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


