Abstract
Basic fibroblast growth factor 2 (bFGF) is a potent mitogen for mesenchymal cells, and the local application of recombinant bFGF accelerates bone union and defect repair. However, repeated dosing is required for sustained therapeutic effect as the efficacy of bFGF decreases rapidly following its diffusion from bone defect sites. Here, we attempted to develop a collagen-based bone formation system using a fusion protein (collagen binding-bFGF, CB-bFGF) consisting of bFGF and the collagen-binding domain (CBD) of Clostridium histolyticum collagenase. The addition of the CBD to bFGF did not modify its native biological activity, as shown by the capacity of the fusion protein to promote the in vitro proliferation of periosteal mesenchymal cells. The affinity of the fusion protein towards collagen and demineralized bone matrix (DBM) was also confirmed by collagen-binding assays. Moreover, in vivo periosteal bone formation assays showed that the combination of CB-bFGF with a collagen sheet induced periosteal bone formation at protein concentrations lower than those required for bFGF alone. In addition, grafts of DBM loaded with CB-bFGF accelerated new bone formation in rat femurs compared to the same concentration of bFGF administered alone. Taken together, these properties suggest that the CB-bFGF/collagen composite is a promising material for bone repair in the clinical setting. © 2013 Wiley Periodicals, Inc. J Biomed Mater Res Part A: 102A: 1737–1743, 2014.
Keywords: basic fibroblast growth factor, bone tissue engineering, bone repair, collagen, collagen-binding domain
INTRODUCTION
Repair of bone defects and fractures is one of the major therapeutic goals in the field of orthopedic tissue engineering. During the bone repair process, intramembranous and endochondral ossifications in the periosteum are required for the formation of new bone.1,2 Therefore, a bioactive agent that stimulates ossification in the periosteum is expected to promote bone repair in the clinical setting.
Fibroblast growth factors (FGFs) form a family of 23 structurally related polypeptides that play critical roles in angiogenesis3 and mesenchymal cell mitogenesis.4 Among FGF family members, FGF-2 (basic FGF) accumulates most abundantly in the bone matrix and is expressed in periosteum during the early stages of bone formation.1,5,6 Several animal model studies have reported that locally applied recombinant human bFGF (rhbFGF) has osteogenic properties for regenerating bone fractures and defects, and osteoporotic bone.7–12 Moreover, recent clinical trials have revealed that bFGF accelerates bone union of osteotomy and tibial shaft fractures.13,14 Based on these properties, bFGF is an effective growth factor for promoting bone regeneration and has therapeutic potential in the clinical setting.
Despite the osteogenic potential of bFGF, its efficiency decreases rapidly following its diffusion in body fluid from the bone defect site. Thus, large doses or repeated administration of bFGF are required for sustained therapeutic effect, making such treatment clinically impractical and expensive. In addition, high doses of bFGF can lead to adverse side effects, including thrombocytopenia, renal toxicity, and malignant cell activation.15,16 Due to these properties, bFGF should ideally be used in combination with a carrier to promote retention at wound sites.17–21 Although a number of different natural and synthetic carriers for bFGF have been evaluated,17–21 currently available carriers are restricted to demineralized bone matrix (DBM) and collagen, and are therefore the most frequently used scaffolds for bone repair in the clinical setting. For this reason, the development of a local delivery system for bFGF based on DBM or collagen is expected to facilitate the repair of bone fractures and defects.
We previously constructed a fusion protein (collagen binding-bFGF, CB-bFGF) consisting of bFGF and the CBD of Clostridium histolyticum collagenase22 and demonstrated that the subcutaneous injection of CB-bFGF without carrier into nude mice had more potent skin fibroblast growth-promoting effects at the injection site than native bFGF. Moreover, we revealed that CBD binds to several types of tissues containing collagen, including tendon, aorta, skin, and cartilage,23 suggesting that CB-bFGF construct with collagen or DBM can be utilized for the long-term retention of bFGF in wound sites.
Here, we investigated the stimulating effect of the CB-bFGF construct on in-vivo bone formation in rats. The pre-clinical evaluation of CB-bFGF for bone repair was performed in rats because the surgical procedure and grafting of materials are more easily performed and reproducible than using mice.
MATERIALS AND METHODS
Preparation of bFGF and CB-bFGF
Recombinant human bFGF was provided by Kaken Pharmaceuticals (Tokyo, Japan). Recombinant CB-bFGF was produced by similar methods as described previously.22 Briefly, a nucleotide fragment encoding a C-terminal fragment (Glu767–Arg981) of C. histolyticum class II collagenase (ColH) was inserted at the SmaI site of a GST-fusion vector, pGEX-4T-2 (GE Healthcare). A second nucleotide fragment, encoding an 18 kDa isoform of human bFGF (Met134–Ser288), was amplified by PCR using cDNA prepared from the human osteosarcoma cell line OST-1-PF as the template and was then inserted between the BamHI and EcoRI sites of the modified pGEX-4T-2 plasmid. Escherichia coli BL21 CodonPlus RIL (Agilent, Santa Clara, CA) was transformed with the resultant plasmid, and grown in 2 l 2YT-G medium containing 16 g/L Bacto Tryptone (BD, Sparks, MD), 10 g/L Bacto Yeast extract (BD), 5 g/L NaCl, 2% glucose, and 50 µg/mL ampicillin and 30 µg/mL chloramphenicol. Expression of the GST fusion protein was induced by the addition of 1 mM isopropyl-thio-beta-d-galactopyranoside, purified from the cleared lysate using 10 mL of glutathione-Sepharose beads (GE Healthcare), and cleaved by thrombin protease (GE Healthcare). The obtained fraction was mixed with 2 mL of Heparin-Sepharose beads (GE Healthcare), and CB-bFGF was eluted from the beads with a salt gradient (0.5–2.0M NaCl, total of 20 mL) in 50 mM Tris-HCl (pH 7.5). CB-bFGF was purified to near homogeneity at a yield of approximately 4.5 mg.
Cell culture
All procedures involving the handling of animals were performed in accordance with the guidelines of the animal ethics committee of Kitasato University. A specific pathogen-free colony of Wistar rats was maintained at Nippon Charles River Laboratories (Kanagawa, Japan). The rats were housed in a semi-barrier system with a controlled environment (temperature, 23 ± 2°C; humidity, 55% ± 10%; lighting, 12-h light/dark cycle) throughout the study. All rats were fed a diet of standard rodent chow (CRF-1; Oriental Yeast Co., Tokyo, Japan). The periosteum was excised from the femurs of 10-week-old male rats, as previously described.24 The excised tissue was minced, digested for 2 h at 37°C with type I collagenase (0.2%; Sigma, Lakewood, NJ), and then passed through a 40-μm filter (Becton Dickinson, Franklin Lakes, NJ) to yield single-cell suspensions. Cell numbers were counted using a hemocytometer. Nucleated cells isolated from the periosteum were plated at 1 × 104 cells/cm2 in 6-well plates containing α-minimum essential medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin, and were then incubated at 37°C in 5% CO2 for 7 days as Passage 0 (P0) cells. Expression of the mesenchymal cell markers CD29, CD54, and CD90 in P0 cells was confirmed by flow cytometry, as previously described.25 Briefly, P0 cells were resuspended in 500 μL phosphate-buffered saline (PBS) containing labeled antibodies against CD45 (fluorescein isothiocyanate, FITC), CD29 (Phycoerythrin, PE), CD54 (PE), or CD90 (Peridinin Chlorophyll Protein, PERCP; Biolegend, San Diego, CA), After incubation for 30 min at 4°C, the cells were washed once with PBS and then resuspended in 1 mL PBS for analysis. Cell fluorescence was evaluated by flow cytometry using a FACSCalibur instrument (Becton Dickinson), and the generated data were analyzed using CellQuest software (Becton Dickinson).
Proliferation assay
P0 cells were detached from culture dishes by treatment with 0.25% trypsin and 1 mM EDTA for 5 min and then seeded at 1.25 × 103/well in 96-well plates. Cells were then treated with bFGF or CB-bFGF (0, 0.0058, 0.058, 0.29, and 0.58 pmol) for 3 days. Cell proliferation was assessed by the water-soluble tetrazolium (WST) assay using a commercial WST kit (Cell Count Reagent SF; Nacalai Tesque, Kyoto, Japan), as previously described.26
Collagen-binding assay
Collagen-binding activity was assayed as previously described.22 Briefly, proteins dissolved in 400 µL of 50 mM Tris-HCl (pH 7.5) were incubated at 4°C for 30 min in the absence and presence of 8 mg DBM derived from rat femurs or 1.6 mg of a porcine-derived insoluble type I collagen sheet (5 × 5 × 0.2 mm; Nippi Research Institute of Biomatrix, Tokyo, Japan). Following centrifugation at 13,000×g for 5 min, the resulting supernatants were analyzed for collagen binding by direct enzyme-linked immunosorbent assay (ELISA). Briefly, each well of a Costar EIA plate (Costar, Cambridge, MA) was coated overnight at 4°C with 100 μL supernatant. After blocking with a 1% bovine serum albumin–PBS–Tween 20 solution, plates were incubated with anti-bFGF antibody (Santa Cruz Biotechnology, CA) for 60 min. The plates were then incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG for 1 h. The colorimetric reaction was developed using 100 μL 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonate peroxidase substrate (KPL, Gaithersburg, MA) per well for 15 min at room temperature. After the reaction was stopped with 100 μL stopping buffer, the optical density at 405 nm (OD405) was detected using a Spectrafluor Plus plate reader (Tecan, Durham, NC).
Preparation of graft material
To prepare material for grafting, an average of 1.6 mg of a porcine-derived insoluble type I collagen sheet (5 × 5 × 0.2 mm; Nippi Research Institute of Biomatrix, Tokyo, Japan) or 8 ± 1 mg DBM derived from rat femurs were added into a 1.5-mL centrifuge tube for each rat, and then incubated with bFGF or CB-bFGF (0, 0.0058, 0.058, and 0.58 nmol) at 4°C for 30 min. Following centrifugation at 13,000×g for 5 min, supernatants were removed and the graft materials were immediately used for the in vivo periosteal bone-formation assay.
In vivo periosteal bone-formation assay
To prepare rat femurs for receiving grafts, leg hair was shaved and a skin incision was then made in the center of the thigh to expose the right femur of the hind leg. A collagen sheet or DBM incubated with PBS, bFGF, or CB-bFGF was then grafted onto the periosteum of the anterior surface of the femur. DBM was grafted onto the femur above the periosteum in a 5 × 5 mm2 area. To evaluate the effects of carrier on periosteal bone-formation, 100 μL of PBS, and 0.58 nmol bFGF or CB-bFGF solutions without carrier were injected onto the periosteum of the anterior surface of the femur.
After bone-grafting surgery, the rats were allowed to use their hind legs without restriction. To assess new bone formation, eight rats in each group were sacrificed with excess CO2 gas 2 weeks after performing the graft, because new bone volume and mineral content had reached their maximum values in all test groups by this time point (data not shown).
Quantification of new bone volume and bone mineral content
After sacrificing rats, femurs were excised with the surrounding muscle. The femurs were placed in 4% paraformaldehyde, stored at 4°C for 48 h, and then transferred to PBS. Micro-CT images were obtained using a microfocus X-ray CT system (inspeXio SMX-90CT; Shimadzu Co., Tokyo, Japan). Images were obtained using the following settings: acceleration voltage, 90 kV; current, 110 mA; voxel size, 25 μm/pixel; matrix size, 1024 × 1024. Images of the whole femur were obtained and 10-mm regions of interest (400 slices) were defined at the mid-femur. New bone volume and bone mineral content were measured using three-dimensional (3D) image analysis software (Tri-3D-Bon; Ratoc System Engineering Co., Tokyo, Japan) as previously described.1
Statistical analysis
All statistical analyses were performed using SPSS software (Version 11.0; SPSS, Chicago, IL). One-way ANOVA with Tukey's multiple comparison test was used to examine differences among the control, bFGF, and CB-bFGF groups. The unpaired t-test was used to examine differences between dose-matched bFGF and CB-bFGF groups. A p value of <0.05 was considered statistically significant.
RESULTS
In vitro biological activities of bFGF and CB-bFGF
The biological activities of purified bFGF and CB-bFGF were evaluated using a proliferation assay with rat periosteal mesenchymal cells. Isolated periosteal cells were positive for the mesenchymal stem cell markers CD29, CD54, and CD90, and were negative for the hematopoietic cell marker CD45 [Fig. 1(A)]. Following treatment with bFGF and CB-bFGF, the number of periosteal mesenchymal cells had significantly increased after three days of culture [Fig. 1(B)]. No differences in cell number were detected between the bFGF- and CB-bFGF-treated groups.
Figure 1.
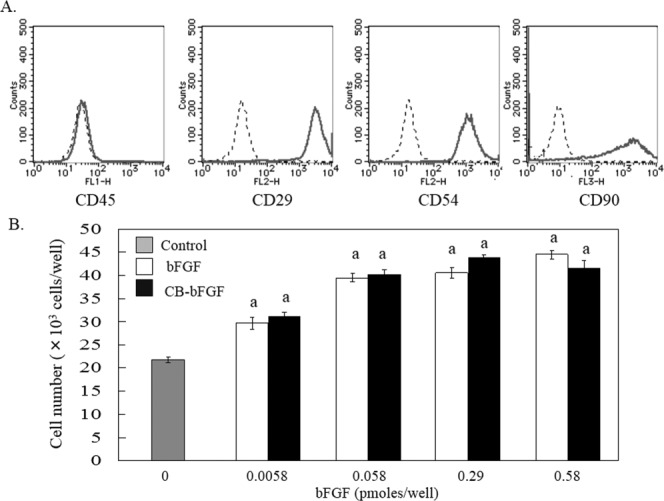
In vitro proliferation activity of bFGF and CB-bFGF. A: Flow cytometry analysis of mesenchymal cell markers in isolated periosteal cells. Dotted line: stained sample. Solid line: non-staining sample. B: Dose-dependent induction of periosteal mesenchymal cell proliferation by bFGF and CB-bFGF. Cell numbers were quantified three days after the treatment. Data are presented as the mean ± S.E. (n = 8). a: p < 0.05 compared with the untreated control group.
Collagen-binding ability of CB-bFGF to collagen sheet and DBM
The in vitro collagen-binding ability of CB-bFGF was evaluated by incubating various amounts of CB-bFGF with a collagen sheet or DBM, and then performing a direct ELISA with the supernatant of these suspensions. When 0.058 nmol CB-bFGF was added to 1.6 mg collagen sheet or 8 mg DBM, the amount of CB-bFGF in the supernatant was below the detection limit of the ELISA. However, when the amount of CB-bFGF added to the collagen sheet or DBM was increased to 0.58 nmol, 0.16 ± 0.015 and 0.07 ± 0.028 nmol CB-bFGF, respectively, were detected in the supernatant samples. Based on the results of the collagen-binding assay, it was estimated that 0.42 and 0.51 nmol CB-bFGF bound to the collagen sheet and DBM, respectively.
In-vivo periosteal bone formation induced by locally injected CB-bFGF
New bone volume and bone mineral content in the femurs of rats locally injected with 0.58 nmol bFGF and CB-bFGF were significantly higher than those in the PBS-injection group [Fig. 2(A–C)]. However, no differences in these parameters were detected between the bFGF and CB-bFGF injection groups.
Figure 2.
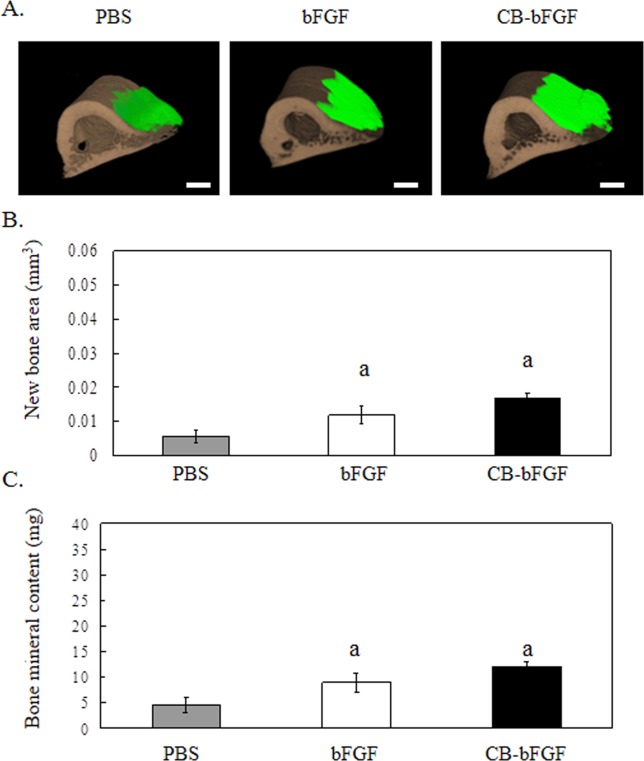
3D micro-CT analysis of rat femurs two weeks after local injection. A: 3D micro-CT image, A-1: PBS, A-2: 0.58 nmol bFGF, A-3: 0.58 nmol CB-bFGF. Green color: new bone; brown color: existing bone. The scale bars indicate 1 mm. B: New bone area, and C: Bone mineral content after local injection of 0.58 nmol bFGF or CB-bFGF. Data are presented as the mean ± S.E. (n = 8). a: p < 0.05 compared with the control group. b: p < 0.05 compared with the dose-matched group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In vivo periosteal bone formation induced by CB-bFGF-loaded collagen sheets
Periosteal bone formation in rat femurs grafted with collagen sheets in PBS (PBS/CS) or collagen sheets incubated with bFGF (bFGF/CS) or CB-bFGF (CB-bFGF/CS) was evaluated by micro-CT image analysis 2 weeks after performing the grafts. Femurs grafted with CB-bFGF/CS prepared using 0.58 nmol CB-bFGF had markedly higher levels of bone formation and bone mineral content compared to that of femurs grafted with similarly prepared bFGF/CS [Fig. 3(B,C)]. In addition, the new bone volume and bone mineral content in femurs grafted with CB-bFGF/CS (0.058 nmols CB-bFGF) were higher than those of femurs grafted with PBS/CS [Fig. 3(B)]. In contrast, no differences in the amount of bone formation were observed between the PBS/CS- and bFGF/CS-treated femurs [0.058 nmol bFGF; Fig. 3(B)].
Figure 3.
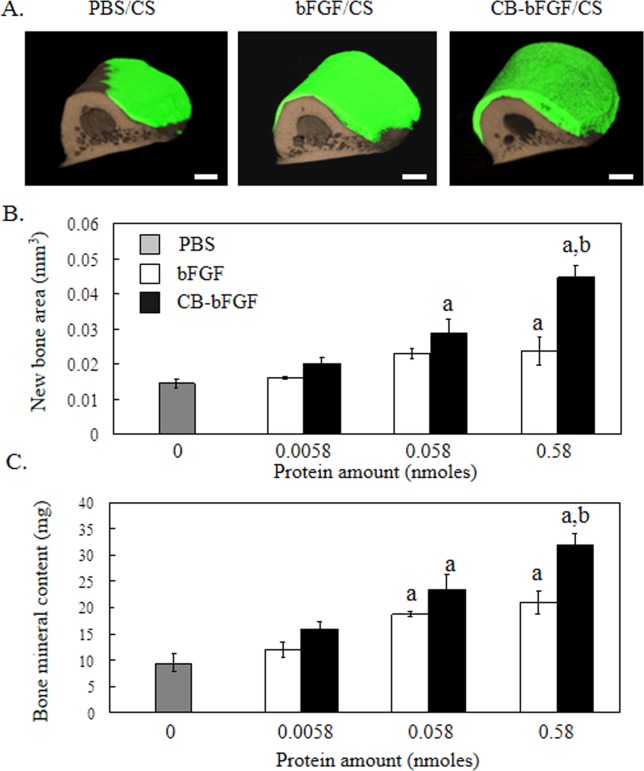
3D micro-CT analysis of rat femurs two weeks after grafting collagen sheets loaded with CB-bFGF. A: 3D micro-CT image, A-1: PBS, A-2: 0.58 nmol bFGF, A-3: 0.58 nmol CB-bFGF. Green color: new bone; brown color: existing bone. The scale bars indicate 1 mm. B: New bone area, and C: Bone mineral content after grafting collagen loaded with various amounts of either bFGF or CB-bFGF. Data are presented as the mean ± S.E. (n = 8). a: p < 0.05 compared with the control group. b: p < 0.05 compared with the dose-matched group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In-vivo periosteal bone formation induced by CB-bFGF-loaded DBM
Two weeks after being grafted onto rat femurs, bFGF/DBM and CB-bFGF/DBM (0.058 nmol bFGF protein) had significantly stimulated periosteal bone formation compared to PBS/DBM [Fig. 4(B)]. In the CB-bFGF/DBM-treated group, bone formation increased in a dose-dependent manner, whereas a plateau in bone formation was reached for bFGF/DBM at 0.058 nmol. New bone volume and bone mineral content in femurs treated with CB-bFGF/DBM (0.29 and 0.58 nmol CB-bFGF) were significantly higher than those of femurs treated with similarly prepared bFGF/DBM [Fig. 4(A–C)].
Figure 4.
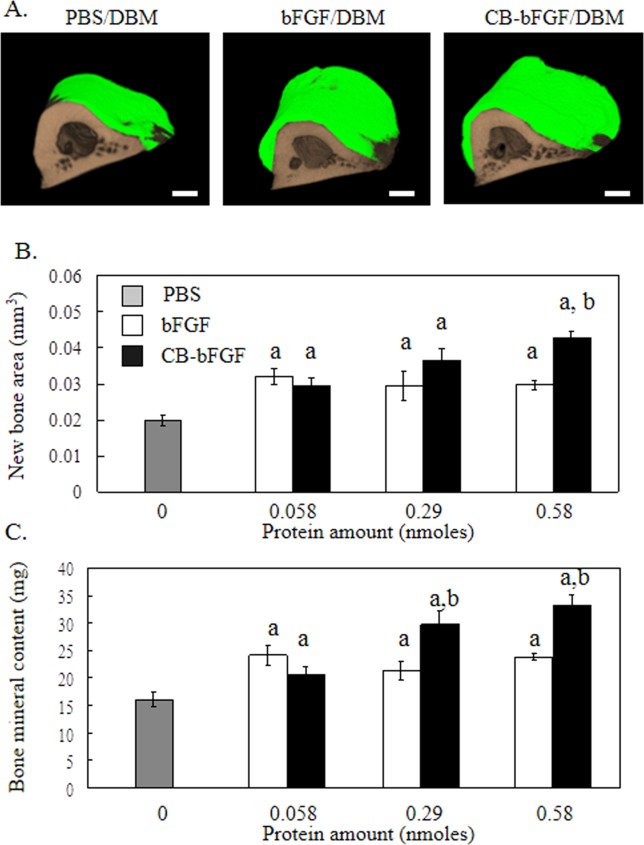
3D micro-CT analysis of rat femurs two weeks after grafting DBM loaded with CB-bFGF. A: 3D micro-CT image, A-1: PBS, A-2: 0.58 nmol bFGF, A-3: 0.58 nmol CB-bFGF. Green color: new bone; brown color: existing bone. The scale bars indicate 1 mm. B: New bone area, and C: Bone mineral content after grafting DBM with various amounts of either bFGF or CB-bFGF. Data are presented as the mean ± S.E. (n = 8). a: p < 0.05 compared with the control group. b: p < 0.05 compared with the dose-matched group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
In this study, we have demonstrated that the addition of a CBD to bFGF does not modify its native biological activity in vitro, as shown by the capacity of the CB-bFGF fusion protein to promote the proliferation of periosteal mesenchymal cells. The affinity of CB-bFGF towards collagen and DBM was also confirmed in collagen-binding assays. Moreover, an in vivo periosteal bone formation assay revealed that the combination of CB-bFGF with a collagen sheet induced periosteal bone formation at a lower protein concentration than that of the combination of bFGF with a collagen sheet. Grafts performed using DBM with CB-bFGF also accelerated new bone formation in rat femurs compared to the same concentration of bFGF added alone. Taken together, these findings suggest that CB-bFGF may be used in the clinical setting at lower concentrations than those required for bFGF when used in combination with either a collagen sheet or DBM.
To date, several growth factors have been modified with CBDs from von Willebrand factor (vWF), 27–29 mammalian collagenase,30 and fibronectin,31,32 with the recombinant proteins displaying increased collagen-binding activity without impacting cytokine activity. Here, the CBD derived from C. histolyticum collagenase did not modify the in vitro biological activity of bFGF, as shown by its capacity to promote periosteal mesenchymal cell proliferation.
Collagen is considered to be one of the best carriers for bone growth proteins because it has high versatility and biocompatibility, and low immunogenicity. DBM is also a useful bone-filling material, as it has excellent bone morphogenetic protein 2 (BMP2) retention/liberation properties.33 Here, grafting of a collagen sheet and DBM alone or in combination with either bFGF or CB-bFGF improved bone formation and mineralization compared to locally injected PBS. In addition, 0.58 nmol bFGF and bFGF-CBD had no marked effects when injected without collagen or DBM; however, when bFGF and CB-bFGF at this dose were co-administered with collagen and DBM, greater bone-promoting effects were observed. These results suggest that collagen sheet and DBM not only improve bone formation at graft sites, but are also good carriers for bFGF.
Recent studies have shown that bFGF fused with the CBD derived from vWF enhances wound repair of bladders and abdominal walls compared to native bFGF.34,35 In this study, quantitative micro-CT analysis demonstrated that bFGF fused with the CBD derived from C. histolyticum collagenase markedly enhances bone formation when loaded onto collagen sheets. Furthermore, in vivo bone formation induced by CB-bFGF was achieved at a lower concentration than that by native bFGF. Unger et al.16 reported that intracoronary injection of bFGF into humans at doses of 3–30 μg/kg was generally tolerated in subjects with stable angina, whereas those administered higher doses of bFGF had higher incidences of sustained hypotension and bradycardia. Therefore, due to the potential adverse effects of bFGF, particularly when administered at high doses, the use of CS/CB-FGF composite may be safer to limit bFGF exposure at wound sites.
The attempted reconstruction of large skeletal defects by implanting DBM alone has been shown to result in non-union.36,37 To overcome this problem, Lu et al.18 mixed DBM with bFGF and demonstrated that this combination accelerates bone formation in allogeneic intramembranous bone grafts. In our present study, DBM combined with CB-bFGF accelerated bone formation compared to DBM loaded with native bFGF.
Several studies have reported that DBM loaded with CBD-BMP2 accelerates bone formation compared to native BMP-2.27,28 Our present concept differed from the addition of CBD-BMP2 to DBM to impart osteoinductive properties.27,28 For efficient bone repair, periosteal mesenchymal cells proliferate in the early phase and then undergo osteoblastic differentiation followed by the proliferation of osteoblasts in late phases for the synthesis of new bone. The combination of growth factors, such as BMP2 and bFGF, results in synergistic effects that further stimulate the complex cellular events and interactions that lead to new bone formation.38–40 For example, the combination of BMP-2 and bFGF enhances osteoblastic differentiation of cultured mesenchymal stromal cells,39 while Wang et al.40 reported that the combination of rhBMP-2 with bFGF synergistically promotes new bone formation in vivo. bFGF also stimulates osteoblast proliferation without affecting its inhibitory role in osteoblast differentiation.41 Consistent with this fact, we found that the bone mineral content of femurs grafted with DBM/CB-bFGF was increased. Thus, DBM loaded with CB-bFGF could stimulate periosteal mesenchymal cell proliferation in the early phase and osteoblastic differentiation and osteoblast proliferation in late phases, leading to efficient bone repair. The DBM/CB-bFGF composite is also a promising material for the acceleration of periosteal bone formation in the clinical setting.
CONCLUSIONS
We developed a collagen-based local delivery system for bFGF using human FGF-2 fused with the CBD derived from C. histolyticum collagenase. In combination with a collagenic carrier, the CB-bFGF fusion protein induced new bone formation when implanted above periosteum. These properties suggest that the CB-bFGF/collagen composite is a promising agent for promoting bone repair in the clinical setting.
REFERENCES
- Ueno M, Urabe K, Naruse K, Uchida K, Minehara H, Yamamoto T, Steck R, Gregory L, Wullschleger ME, Schuetz MA, Itoman M. Influence of internal fixator stiffness on murine fracture healing: Two types of fracture healing lead to two distinct cellular events and FGF-2 expressions. Exp Anim. 2011;60:79–87. doi: 10.1538/expanim.60.79. [DOI] [PubMed] [Google Scholar]
- Ueno M, Uchida K, Takaso M, Minehara H, Suto K, Takahira N, Steck R, Schuetz MA, Itoman M. Distribution of bone marrow-derived cells in the fracture callus during plate fixation in a green fluorescent protein-chimeric mouse model. Exp Anim. 2011;60:455–462. doi: 10.1538/expanim.60.455. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Nambu M, Ishizuka T, Hattori H, Kanatani Y, Takase B, Kishimoto S, Amano Y, Aoki H, Kiyosawa T, Ishihara M, Maehara T. Effect of controlled release of fibroblast growth factor-2 from chitosan/fucoidan micro complex-hydrogel on in vitro and in vivo vascularization. J Biomed Mater Res A. 2008;85:619–627. doi: 10.1002/jbm.a.31563. [DOI] [PubMed] [Google Scholar]
- Varkey M, Kucharski C, Haque T, Sebald W, Uludag H. In vitro osteogenic response of rat bone marrow cells to bFGF and BMP-2 treatments. Clin Orthop Relat Res. 2006;443:113–123. doi: 10.1097/01.blo.0000200236.84189.87. [DOI] [PubMed] [Google Scholar]
- Canalis E, Centrella M, McCarthy T. Effects of basic fibroblast growth factor on bone formation in vitro. J Clin Invest. 1988;81:1572–1577. doi: 10.1172/JCI113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SN, Bostrom MP, Lane JM. Bone growth factors. Orthop Clin North Am. 2000;31:375–388. doi: 10.1016/s0030-5898(05)70157-7. [DOI] [PubMed] [Google Scholar]
- Kato T, Kawaguchi H, Hanada K, Aoyama I, Hiyama Y, Nakamura T, Kuzutani K, Tamura M, Kurokawa T, Nakamura K. Single local injection of recombinant fibroblast growth factor-2 stimulates healing of segmental bone defects in rabbits. J Orthop Res. 1998;16:654–659. doi: 10.1002/jor.1100160605. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Nakamura K, Tabata Y, Ikada Y, Aoyama I, Anzai J, Nakamura T, Hiyama Y, Tamura M. Acceleration of fracture healing in nonhuman primates by fibroblast growth factor-2. J Clin Endocrinol Metab. 2001;86:875–880. doi: 10.1210/jcem.86.2.7199. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Kurokawa T, Hanada K, Hiyama Y, Tamura M, Ogata E, Matsumoto T. Stimulation of fracture repair by recombinant human basic fibroblast growth factor in normal and streptozotocin-diabetic rats. Endocrinology. 1994;135:774–781. doi: 10.1210/endo.135.2.8033826. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawaguchi H, Aoyama I, Hanada K, Hiyama Y, Awa T, Tamura M, Kurokawa T. Stimulation of bone formation by intraosseous application of recombinant basic fibroblast growth factor in normal and ovariectomized rabbits. J Orthop Res. 1997;15:307–313. doi: 10.1002/jor.1100150222. [DOI] [PubMed] [Google Scholar]
- Radomsky ML, Aufdemorte TB, Swain LD, Fox WC, Spiro RC, Poser JW. Novel formulation of fibroblast growth factor-2 in a hyaluronan gel accelerates fracture healing in nonhuman primates. J Orthop Res. 1999;17:607–614. doi: 10.1002/jor.1100170422. [DOI] [PubMed] [Google Scholar]
- Tabata Y, Yamada K, Hong L, Miyamoto S, Hashimoto N, Ikada Y. Skull bone regeneration in primates in response to basic fibroblast growth factor. J Neurosurg. 1999;91:851–856. doi: 10.3171/jns.1999.91.5.0851. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Jingushi S, Izumi T, Fukunaga M, Matsushita T, Nakamura T, Mizuno K, Nakamura T, Nakamura K. Local application of recombinant human fibroblast growth factor-2 on bone repair: A dose-escalation prospective trial on patients with osteotomy. J Orthop Res. 2007;25:480–487. doi: 10.1002/jor.20315. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Oka H, Jingushi S, Izumi T, Fukunaga M, Sato K, Matsushita T, Nakamura K. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: A randomized, placebo-controlled trial. J Bone Miner Res. 2010;25:2735–2743. doi: 10.1002/jbmr.146. [DOI] [PubMed] [Google Scholar]
- Epstein SE, Fuchs S, Zhou YF, Baffour R, Kornowski R. Therapeutic interventions for enhancing collateral development by administration of growth factors: Basic principles, early results and potential hazards. Cardiovasc Res. 2001;49:532–542. doi: 10.1016/s0008-6363(00)00217-0. [DOI] [PubMed] [Google Scholar]
- Unger EF, Goncalves L, Epstein SE, Chew EY, Trapnell CB, Cannon RO, III, Quyyumi AA. Effects of a single intracoronary injection of basic fibroblast growth factor in stable angina pectoris. Am J Cardiol. 2000;85:1414–1419. doi: 10.1016/s0002-9149(00)00787-6. [DOI] [PubMed] [Google Scholar]
- Kamo K, Miyakoshi N, Kasukawa Y, Sasaki H, Shimada Y. Effects of single and cyclical local injections of basic fibroblast growth factor on cancellous bone defects in rabbits. J Orthop Sci. 2009;14:811–819. doi: 10.1007/s00776-009-1403-2. [DOI] [PubMed] [Google Scholar]
- Lu M, Rabie AB. The effect of demineralized intramembranous bone matrix and basic fibroblast growth factor on the healing of allogeneic intramembranous bone grafts in the rabbit. Arch Oral Biol. 2002;47:831–841. doi: 10.1016/s0003-9969(02)00119-x. [DOI] [PubMed] [Google Scholar]
- Omata K, Matsuno T, Asano K, Hashimoto Y, Tabata Y, Satoh T. Enhanced bone regeneration by gelatin-beta-tricalcium phosphate composites enabling controlled release of bFGF. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1553. DOI: 10.1002/term.1553. [DOI] [PubMed] [Google Scholar]
- Zellin G, Linde A. Effects of recombinant human fibroblast growth factor-2 on osteogenic cell populations during orthopic osteogenesis in vivo. Bone. 2000;26:161–168. doi: 10.1016/s8756-3282(99)00252-5. [DOI] [PubMed] [Google Scholar]
- Zou GK, Song YL, Zhou W, Yu M, Liang LH, Sun DC, Li DH, Deng ZX, Zhu WZ. Effects of local delivery of bFGF from PLGA microspheres on osseointegration around implants in diabetic rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:284–289. doi: 10.1016/j.tripleo.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Andrades JA, Wu LT, Hall FL, Nimni ME, Becerra J. Engineering, expression, and renaturation of a collagen-targeted human bFGF fusion protein. Growth Factors. 2001;18:261–275. doi: 10.3109/08977190109029115. [DOI] [PubMed] [Google Scholar]
- Chen B, Lin H, Wang J, Zhao Y, Wang B, Zhao W, Sun W, Dai J. Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials. 2007;28:1027–1035. doi: 10.1016/j.biomaterials.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Chen B, Lin H, Zhao Y, Wang B, Zhao Y, Liu Y, Liu Z, Dai J. Activation of demineralized bone matrix by genetically engineered human bone morphogenetic protein-2 with a collagen binding domain derived from von Willebrand factor propolypeptide. J Biomed Mater Res A. 2007;80:428–434. doi: 10.1002/jbm.a.30900. [DOI] [PubMed] [Google Scholar]
- Kitajima T, Terai H, Ito Y. A fusion protein of hepatocyte growth factor for immobilization to collagen. Biomaterials. 2007;28:1989–1997. doi: 10.1016/j.biomaterials.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Shi C, Chen W, Zhao Y, Chen B, Xiao Z, Wei Z, Hou X, Tang J, Wang Z, Dai J. Regeneration of full-thickness abdominal wall defects in rats using collagen scaffolds loaded with collagen-binding basic fibroblast growth factor. Biomaterials. 2011;32:753–759. doi: 10.1016/j.biomaterials.2010.09.038. [DOI] [PubMed] [Google Scholar]
- Visser R, Arrabal PM, Becerra J, Rinas U, Cifuentes M. The effect of an rhBMP-2 absorbable collagen sponge-targeted system on bone formation in vivo. Biomaterials. 2009;30:2032–2037. doi: 10.1016/j.biomaterials.2008.12.046. [DOI] [PubMed] [Google Scholar]
- Nishi N, Matsushita O, Yuube K, Miyanaka H, Okabe A, Wada F. Collagen-binding growth factors: Production and characterization of functional fusion proteins having a collagen-binding domain. Proc Natl Acad Sci USA. 1998;95:7018–7023. doi: 10.1073/pnas.95.12.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima T, Matsushita O, Minami J, Nishi N, Okabe A, Itano T. Collagen-binding domain of a Clostridium histolyticum collagenase exhibits a broad substrate spectrum both in vitro and in vivo. Connect Tissue Res. 2001;42:281–290. doi: 10.3109/03008200109016842. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- Uchida K, Urabe K, Naruse K, Ujihira M, Mabuchi K, Itoman M. Comparison of the cytokine-induced migratory response between primary and subcultured populations of rat mesenchymal bone marrow cells. J Orthop Sci. 2007;12:484–492. doi: 10.1007/s00776-007-1159-5. [DOI] [PubMed] [Google Scholar]
- Onuma K, Urabe K, Naruse K, Uchida K, Itoman M. Allogenic serum improves cold preservation of osteochondral allografts. Clin Orthop Relat Res. 2012;470:2905–2914. doi: 10.1007/s11999-011-2182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FL, Kaiser A, Liu L, Chen ZH, Hu J, Nimni ME, Beart RW, Jr, Gordon EM. Design, expression, and renaturation of a lesion-targeted recombinant epidermal growth factor-von Willebrand factor fusion protein: Efficacy in an animal model of experimental colitis. Int J Mol Med. 2000;6:635–643. doi: 10.3892/ijmm.6.6.635. [DOI] [PubMed] [Google Scholar]
- de Souza SJ, Brentani R. Collagen binding site in collagenase can be determined using the concept of sense-antisense peptide interactions. J Biol Chem. 1992;267:13763–13767. [PubMed] [Google Scholar]
- Ishikawa T, Eguchi M, Wada M, Iwami Y, Tono K, Iwaguro H, Masuda H, Tamaki T, Asahara T. Establishment of a functionally active collagen-binding vascular endothelial growth factor fusion protein in situ. Arterioscler Thromb Vasc Biol. 2006;26:1998–2004. doi: 10.1161/01.ATV.0000233359.74484.77. [DOI] [PubMed] [Google Scholar]
- Chen W, Shi C, Yi S, Chen B, Zhang W, Fang Z, Wei Z, Jiang S, Sun X, Hou X, Xiao Z, Ye G, Dai J. Bladder regeneration by collagen scaffolds with collagen binding human basic fibroblast growth factor. J Urol. 2010;183:2432–2439. doi: 10.1016/j.juro.2010.02.042. [DOI] [PubMed] [Google Scholar]
- Peel SA, Hu ZM, Clokie CM. In search of the ideal bone morphogenetic protein delivery system: In vitro studies on demineralized bone matrix, purified, and recombinant bone morphogenetic protein. J Craniofac Surg. 2003;14:284–291. doi: 10.1097/00001665-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Tuli SM, Singh AD. The osteoninductive property of decalcified bone matrix. An experimental study. J Bone Joint Surg Br. 1978;60:116–123. doi: 10.1302/0301-620X.60B1.342532. [DOI] [PubMed] [Google Scholar]
- Wittbjer J, Palmer B, Rohlin M, Thorngren KG. Osteogenetic activity in composite grafts of demineralized compact bone and marrow. Clin Orthop Relat Res. 1983:229–238. [PubMed] [Google Scholar]
- Alam S, Ueki K, Marukawa K, Ohara T, Hase T, Takazakura D, Nakagawa K. Expression of bone morphogenetic protein 2 and fibroblast growth factor 2 during bone regeneration using different implant materials as an onlay bone graft in rabbit mandibles. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:16–26. doi: 10.1016/j.tripleo.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Maegawa N, Kawamura K, Hirose M, Yajima H, Takakura Y, Ohgushi H. Enhancement of osteoblastic differentiation of mesenchymal stromal cells cultured by selective combination of bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-2 (FGF-2) J Tissue Eng Regen Med. 2007;1:306–313. doi: 10.1002/term.41. [DOI] [PubMed] [Google Scholar]


