Abstract
Leaf senescence is the final stage of leaf development in which the nutrients invested in the leaf are remobilized to other parts of the plant. Whereas senescence is accompanied by a decline in leaf cytokinin content, exogenous application of cytokinins or an increase of the endogenous concentration delays senescence and causes nutrient mobilization. The finding that extracellular invertase and hexose transporters, as the functionally linked enzymes of an apolasmic phloem unloading pathway, are coinduced by cytokinins suggested that delay of senescence is mediated via an effect on source-sink relations. This hypothesis was further substantiated in this study by the finding that delay of senescence in transgenic tobacco (Nicotiana tabacum) plants with autoregulated cytokinin production correlates with an elevated extracellular invertase activity. The finding that the expression of an extracellular invertase under control of the senescence-induced SAG12 promoter results in a delay of senescence demonstrates that effect of cytokinins may be substituted by these metabolic enzymes. The observation that an increase in extracellular invertase is sufficient to delay leaf senescence was further verified by a complementing functional approach. Localized induction of an extracellular invertase under control of a chemically inducible promoter resulted in ectopic delay of senescence, resembling the naturally occurring green islands in autumn leaves. To establish a causal relationship between cytokinins and extracellular invertase for the delay of senescence, transgenic plants were generated that allowed inhibition of extracellular invertase in the presence of cytokinins. For this purpose, an invertase inhibitor was expressed under control of a cytokinin-inducible promoter. It has been shown that senescence is not any more delayed by cytokinin when the expression of the invertase inhibitor is elevated. This finding demonstrates that extracellular invertase is required for the delay of senescence by cytokinins and that it is a key element of the underlying molecular mechanism.
INTRODUCTION
Cytokinins are a group of plant hormones that promote cell division and play a major role in the regulation of various biological processes associated with active growth, metabolism, and plant development. Because these processes are associated with an enhanced demand for carbohydrates, a link to the regulation of assimilate partitioning (Brenner and Cheikh, 1995), sink strength (Kuiper, 1993), and source-sink relations (Roitsch and Ehness, 2000) has been suggested. This hypothesis is experimentally supported by the observation that radioactively labeled nutrients are preferentially transported and accumulated in cytokinin-treated tissue (Mothes and Engelbrecht, 1963), suggesting that the hormone creates a new source-sink relationship, thus causing nutrient mobilization. Higher plants consist of a mosaic of photosynthetically active source tissues, such as mature leaves, and photosynthetically less active or inactive sink tissues, such as seeds, flowers, roots, fruits, and tubers. The source-sink relations in mature plants are not static, and changes, with respect to the relative sink strength of various organs, number of sinks competing for a common pool of carbohydrates, and sink to source transitions, occur during plant development. The photoassimilates produced in the source organs are transported into the sink organs mostly in the form of sucrose. An apoplasmic phloem unloading of sucrose is mandatory in symplastically isolated tissues, such as embryos or stomata, and seems to be characteristic for actively growing tissues (Eschrich, 1980) and, thus, under conditions that may be under the control of cytokinins. In apoplastic unloading pathways, sucrose is released from the sieve elements of the phloem into the apoplast by a sucrose transporter, where it is irreversibly hydrolyzed by an extracellular invertase ionically bound to the cell wall. The resulting hexose monomers are then taken up by sink cells through monosaccharide transporters (Roitsch and Tanner, 1996). The extracellular invertase has a crucial function both in source-sink regulation and for supplying carbohydrates to sink tissues, being considered as a central modulator of sink activity (Tang et al., 1999; Goetz et al., 2001; Roitsch et al., 2003). It has been shown that extracellular invertases are upregulated by several stimuli that affect carbohydrate requirements, including growth stimulating phytohormones (Roitsch, 1999; Roitsch et al., 2003). The extracellular invertase activity is usually high in tissues with an elevated cytokinin concentration. A direct link between cytokinin and the function of invertases was suggested by the stimulation of invertase activity by cytokinins of in vitro cultivated Chicorium tissues (Lefebre et al., 1992). This was further substantiated by the finding that extracellular invertases from Chenopodium rubrum (Ehness and Roitsch, 1997) and Lycopersicon esculentum (Godt and Roitsch, 1997) were induced by physiological concentrations of different cytokinins. In addition, hexose transporters of C. rubrum (Ehness and Roitsch, 1997) were coinduced with the extracellular invertase by cytokinins. The coordinated induction of the two functionally linked key enzymes of an apoplasmic phloem unloading pathway was shown to result in a higher uptake of hexose sugars. These findings provided the groundwork for elucidating the molecular basis of the mode of cytokinin action, supporting the speculation that the upregulation of both extracellular invertase and hexose transporters may account for the phenomenon of transport of nutrients to cytokinin-treated tissue as observed by Mothes and coworkers in the 1960s.
The cytokinins are also a key component of plant senescence (Gan and Amasino, 1996, 1997; Buchanan-Wollaston, 1997; Nam, 1997). Leaf senescence is the final stage of leaf development but is also a recycling process in which the nutrients from these leaves are translocated to other parts of the plant, such as younger leaves, developing seeds, or storage tissues (Gan and Amasino, 1996). The senescing leaves, according to the model proposed for the initiation of leaf senescence, have a photosynthetic rate such that they no longer contribute fixed carbon to the rest of the plant (Hensel et al., 1993). The initiation of leaf senescence is subjected to regulation both by internal and environmental factors. In particular, phytohormones are assumed to be the main internal factors controlling this developmental process. Whereas abscisic acid and ethylene promote senescence, cytokinins typically inhibit senescence (Smart, 1994). Physiological studies have shown that in a variety of monocotyledonous and dicotyledonous plant species, exogenous cytokinin treatment results in delay of leaf senescence (Richmond and Lang, 1957), that the endogenous levels of cytokinins drop along the progression of leaf senescence (Gan and Amasino, 1996), and that differences in tobacco (Nicotiana tabacum) leaf senescence are related to differences in endogenous cytokinin content (Singh et al., 1992a, 1992b). The so-called green islands in autumn leaves, produced by specific species of caterpillar, fungi, or bacteria, have been shown to be caused by cytokinin secretion (Engelbrecht et al., 1969; Angra and Mandahar, 1993; Chen and Ertl, 1994). The enhanced expression of a bacterial isopentenyltransferase (ipt) gene encoding a cytokinin biosynthetic enzyme under the control of the senescence-activated promoter SAG12 (Noh and Amasino, 1999a, 1999b) in tobacco plants produced an efficient retardation in the process of leaf senescence in the mature leaves (Gan and Amasino, 1995), confirming the regulatory role of cytokinins on leaf senescence in tobacco. In addition, the senescence-specific expression of the maize (Zea mays) gene knotted1 encoding a transcription factor in tobacco caused an increase in cytokinin levels and a delayed-senescence phenotype (Ori et al., 1999). However, although the fact that cytokinins cause a delay in senescence was confirmed by elegant transgenic approaches, the underlying molecular basis for this cytokinin effect is still not understood.
In our attempt to elucidate the relationship between cytokinin and primary metabolism, the observed increase of extracellular invertase activity in the delayed-senescence leaves of SAG12:ipt tobacco plants supported a link among cytokinins, extracellular invertases, and carbohydrate partitioning for the delay of senescence. The suggested link between cytokinins and source-sink relations was further substantiated by complementing functional approaches in transgenic plants. The findings demonstrate that an increase in extracellular invertase activity is not only sufficient to cause a delay of senescence, but that this key enzyme of an apoplasmic phloem unloading pathway is an essential component of the molecular mechanism of delay of senescence by cytokinins.
RESULTS
Delay of Senescence by Cytokinins Correlates with an Increase in Extracellular Invertase Activity
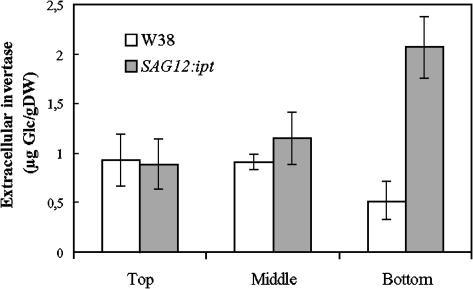
The observed induction of extracellular invertases from different plant species by cytokinin (Ehness and Roitsch, 1997) suggested that this regulatory mechanism may be responsible for the delay of senescence in leaves. The induction of sink activity would result in the attraction of metabolites, leading to a decreased remobilization from the senescing leaf into the stem. To further substantiate this hypothesis, we have analyzed plants with autoregulated delay of senescence. It has been shown that controlling the expression of ipt, a gene encoding isopentenyltransferase, catalyzing the rate limiting step in cytokinin biosynthesis, with the senescence-inducible promoter of the SAG12 gene results in the delay of leaf senescence in tobacco (Gan and Amasino, 1995) and lettuce (Lactuca sativa) (McCabe et al., 2001) and corolla senescence in petunia (Petunia hybrida) (Chang et al., 2003). The SAG12 gene from Arabidopsis thaliana encodes a putative Cys protease expressed in senescing leaves and stems (Noh and Amasino, 1999b; Grbic, 2002), and promoter regions responsible for the senescence-specific expression have been identified (Noh and Amasino, 1999a). The activity of the extracellular invertase isoenzyme has been determined in 17-week-old wild-type plants (N. tabacum cv Wisconsin 38 [W38]) and plants with autoregulated increase in cytokinins in senescing leaves (SAG12:ipt; Gan and Amasino, 1995). In the wild-type plants, the bottom leaves, and to a lesser extent the leaves in the middle of the plants, showed senescence symptoms, such as an evident degradation of chlorophyll and starting necroses, whereas the top leaves showed no apparent symptoms. By contrast, none of the leaves of the transgenic plants showed signs of senescence, and no apparent influence of the leaf position was evident. In control plants, the level of extracellular invertase activity was not significantly affected in middle leaves and reduced by 46% in the bottom leaves with respect to the extracellular invertase activity in the top leaves of these plants (Figure 1). By contrast, the leaves of the SAG12:ipt plants showed an inverse distribution of the extracellular invertase activity. The extracellular invertase activity of the SAG12:ipt plants was slightly higher in the middle leaves and 139% higher in the bottom leaves compared with the top leaves of these transgenic plants. Thus, the extracellular invertase activity in the bottom leaves of the SAG12:ipt plants was 400% higher compared with the activity in the bottom leaves of senescing wild-type plants. This finding demonstrates that the delay of leaf senescence in the SAG12:ipt plants correlates with an increase in extracellular invertase activity and supports the hypothesis that cytokinin induction of extracellular invertase contributes to the delay of senescence.
Figure 1.
Cytokinin-Mediated Delay in Senescence Correlates with an Increase in the Activity of Extracellular Invertase.
Extracellular invertase activity has been determined in top, middle, and bottom leaves of tobacco plants expressing the ipt gene under control of the senescence-activated promoter SAG12 (SAG12:ipt; Gan and Amasino, 1995) and of wild-type plants (W38). Bars represent the mean value of three independent replications ± se. DW, dry weight.
Senescence-Induced Expression of Extracellular Invertase Results in Delay of Senescence
To substantiate a link between the extracellular invertase activity and delay of senescence by cytokinins, it has been tested whether an increase in extracellular invertase activity may replace the effect of endogenously or exogenously applied cytokinins on the delay of senescence. For this purpose, an extracellular invertase was expressed under control of the SAG12 promoter in transgenic tobacco plants to test the effect of senescence-induced increase in extracellular invertase activity. Among the numerous extracellular invertases cloned so far, only the cDNA of Cin1 of C. rubrum was proven to encode a biologically active enzyme by heterologous expression (Roitsch et al., 1995) and functional analyses (Goetz and Roitsch, 1999). A SAG12:Cin1 construct was engineered and transformed into tobacco line W38. Approximately 50% of the 70 transgenic lines obtained were characterized by a delayed senescence phenotype both at the whole plant level as well as in senescence assays. For further analyses, the three transgenic lines, NT58-5, NT58-15, and NT58-69, were characterized in detail, and representative results are shown for line NT58-5. After 17 weeks of development, the SAG12:Cin1 plants showed a similar phenotype as the SAG12:ipt plants (Gan and Amasino, 1995) with a clear delay of senescence in the bottom leaves in comparison with the wild-type line (Figure 2A). Analysis of detached young leaves from line NT58-5 incubated in the light for 4 weeks showed that these leaves remained green in comparison with the senescent wild-type leaves (Figure 2B).
Figure 2.
Senescence-Induced Expression of Extracellular Invertase Cin1 Results in a Delay of Senescence.
(A) Phenotype of the transgenic SAG12:Cin1 and of the wild-type (W38) tobacco plants 17 weeks after sawing. The transgenic plants show a delay in senescence of mature leaves in respect to the loss of mature leaves in the wild-type plants.
(B) Delay in senescence of detached young leaves from the transgenic SAG12:Cin1 and wild-type (W38) tobacco plants incubated in the light for 4 weeks.
The results have been reproduced in five independent experiments with three independent transgenic lines, and representative results obtained with line NT58-5 are shown.
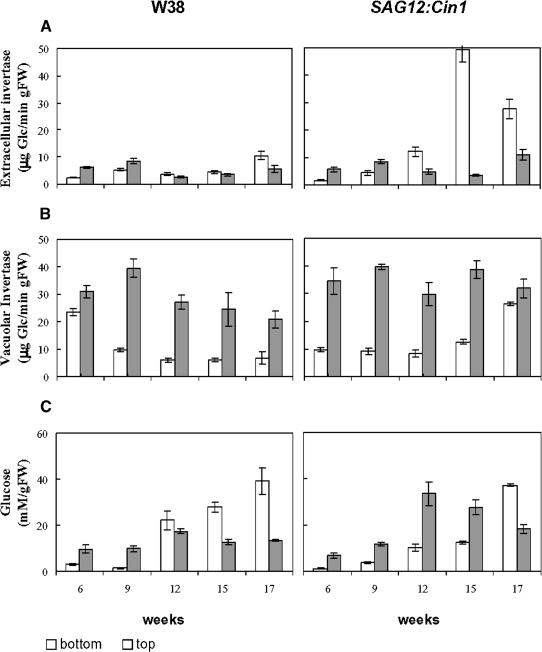
The analyses of the invertase activity during development revealed that the extracellular invertase activity specifically increased in the bottom leaves of the SAG12:Cin1 plants starting in the twelfth week of development and showing the highest levels of 50 μg Glc min−1 grams of fresh weight (FW)−1 at week 15 (Figure 3A). The onset of the increase in extracellular invertase activity coincided with the induction of the SAG12 promoter as revealed by the analysis of β-glucuronidase activity in SAG12:uid reporter gene plants (data not shown).
Figure 3.
The Increase in Extracellular Invertase Activity in Transgenic Tobacco Plants Expressing Extracellular Invertase under Control of the Senescence-Activated Promoter SAG12 Is Specific and Does Not Result in an Increased Glucose Concentration.
(A) Extracellular invertase activity measured in bottom and top leaves of SAG12:Cin1 and wild-type (W38) plants 17 weeks after sawing. Bars represent the mean value of three independent replications ± se.
(B) Vacuolar invertase activity measured in bottom and top leaves of SAG12:Cin1 and wild-type (W38) plants 17 weeks after sawing. Bars represent the mean value of three independent replications ± se.
(C) Glucose contents of bottom and top leaves of SAG12:Cin1 and wild-type (W38) plants 17 weeks after sawing. Bars represent the mean of three independent replication leaves ± se.
The results have been reproduced with three independent transgenic lines, and representative results obtained with line NT58-5 are shown.
The vacuolar invertase activity was higher in the top leaves compared with the bottom leaves both of W38 plants and of SAG12:Cin1 plants (Figure 3B). Whereas the vacuolar invertase activity of the control plants decreased during development, this intracellular invertase activity increased at weeks 15 and 17 in the SAG12:Cin1 plants.
Analyses of the concentration of soluble sugars revealed that the glucose content in the bottom and top leaves from SAG12:Cin1 and W38 plants showed an inverse distribution during the different stages of development (Figure 3C). The glucose levels in the senescing bottom leaves of the wild-type plants exceeded the level in the top leaves at the onset of senescence and then further increased, whereas the level remained relatively constant in the top leaves. By contrast, in line NT58-5, the onset of senescence resulted in an inverse distribution of the glucose content compared with the wild-type plants. At weeks 12 and 15, the glucose content in the top leaves of line NT58-5 was 150 and 105% higher, respectively, than in the bottom leaves. Only in samples harvested at week 17, characterized by senescence symptoms also in the bottom leaves of the transgenic line, the glucose content in the bottom leaves increased and exceeded the level in the top leaves. There were no apparent differences between wild-type plants and line NT58-5 with respect to the concentrations of fructose and sucrose (data not shown).
The results obtained with transgenic line NT58-5 have been reproduced in long-term experiments performed with the two independent transgenic lines NT58-15 and NT58-69 also expressing extracellular invertase Cin1 under control of the SAG12 promoter. Similar results have been obtained with respect to the delayed senescence at the whole plant level and in detached leaves, activities of extracellular and vacuolar invertase, and concentrations of soluble sugars (data not shown). In particular, these data support the unexpected finding that invertase activity and hexose levels are not positively correlated and that in SAG12:Cin1 plants, glucose levels are lower in bottom leaves compared with wild-type plants.
Localized Chemical Induction of Extracellular Invertase Expression Results in Ectopic Delay of Senescence
The conclusion derived from the senescence-induced expression of extracellular invertase that extracellular invertase may substitute the cytokinin stimulus was further verified by a complementing experimental approach involving an inducible promoter system. For this purpose, a tetracycline-inducible (derepression) system was chosen (Gatz and Lenk, 1998). Extracellular invertase has been engineered under control of the corresponding promoter and transformed into W38 tobacco plants expressing the required repressor gene. Approximately 40 transgenic plants have been obtained, the three transgenic lines NT17-1, NT27-5, and NT35-7 were analyzed in detail, and representative data obtained with line NT35-7 are shown.
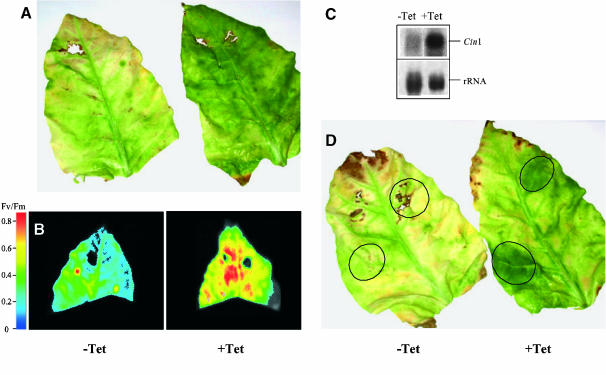
The effect of the chemical induction of the extracellular invertase expression was studied in leaf detachment assays. The induction of the Cin1 gene, by applying tetracycline to leaves by petiole feeding, produced a marked delay of senescence visible after 4 weeks of incubation when the control leaves showed a senesced phenotype (Figure 4A). This retarded-senescence phenotype was related to a higher photosynthetic capacity as determined by chlorophyll fluorescence measurements (Figure 4B) and a higher chlorophyll content (data not shown). In addition, the simultaneous application of tetracycline and kinetin resulted in a higher retardation phenotype and chlorophyll content (data not shown), possibly because of the simultaneous induction of an endogenous tobacco extracellular invertase.
Figure 4.
Chemical Induction of Extracellular Invertase Cin1 Results in Delay of Senescence.
(A) Effect of the infiltration of tetracycline into detached leaves of a transgenic line expressing the extracellular invertase Cin1 under control of the tetracycline-inducible TetR promoter. The left leaf was infiltrated with an MS control solution, whereas the right one was infiltrated with the same solution containing tetracycline at a final concentration of 10 μg/L.
(B) Chlorophyll fluorescence image of the leaves shown in (A).
(C) RNA gel blot showing the accumulation of the Cin1 transcripts in leaves after 2 h of treatment with tetracycline in comparison to control leaves.
(D) Effect of the localized induction of Cin1 by spotting chlorotetracycline onto transgenic leaves. An MS solution containing either 0.02% Silwet (left control leaf) or the detergent plus chlorotetracycline (10 μg/L) (right leaf) was spotted onto the marked zones.
The results have been reproduced in five independent experiments with three independent transgenic lines, and representative results obtained with line NT35-7 are shown.
To verify that the delayed senescence was because of the induction of Cin1, we performed RNA gel blot analysis. The results show a strong induction of the transcripts for the transgene already after 2 h of incubation with tetracycline (Figure 4C). Induction of the transgene resulted in an average 16-fold higher extracellular invertase activity in tetracycline-treated leaves showing a delay of senescence compared with senescing control leaves. Whereas the mean value of the activity of extracellular invertase of tetracycline-treated leaves was 159.8 milliunits/g FW (se ± 24.2), the corresponding value of control leaves was 12.3 milliunits/g FW (se ± 3.6) as determined in four different sets of samples. These results further support a positive correlation between extracellular invertase activity and delay of senescence and demonstrate that the induction of the expression of extracellular invertase can effectively substitute the cytokinin action on the delay of senescence.
The effect of tetracycline was further studied by local induction of gene expression in the transgenic leaves. The localized tetracycline application leads to the appearance of green islands similar to those observed in autumn leaves (Figure 4D). The amount of chlorophyll in the green areas was two to six times higher than in the corresponding zones of control leaves. A simultaneous increase of extracellular invertase activity in the treated zones was detected (data not shown), further supporting a causal relationship between the appearance of green islands and increased leaf invertase activity.
Senescence Is Not Delayed by Cytokinins When Extracellular Invertase Activity Is Inhibited
The results obtained with the previously described transgenic approaches prove that an increase in extracellular invertase is sufficient for delay of senescence. Because these data do not rule out the activation of a cytokinin-independent pathway, a further functional approach was performed to answer the question of whether extracellular invertase activity is essential for the delay of senescence by cytokinins. The rational for this approach was to inhibit the extracellular invertase activity in the presence of cytokinins by the expression of an invertase inhibitor under control of a cytokinin-inducible promoter. For this purpose, a tobacco apoplasmic invertase inhibitor (Greiner et al., 1998) was cloned behind the promoter of the cytokinin-inducible invertase gene Lin6 (Godt and Roitsch, 1997) and transformed into the tobacco cultivar W38. Approximately 40 transgenic plants have been obtained, the three transgenic lines NT71-9, NT71-25, and NT71-29 were analyzed in detail, and representative data obtained with line NT71-29 are shown.
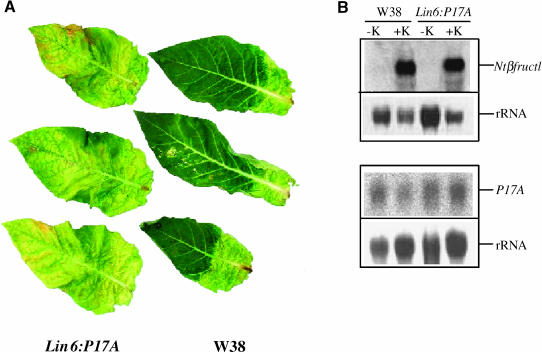
Leaf-detachment assays demonstrate that kinetin results in a marked delay of senescence in leaves of wild-type plants. By contrast, this is not the case in leaves of the transgenic line, where the senesced phenotype was observed both in the presence and absence of cytokinin (Figure 5A). The failure of cytokinin to delay senescence was related to the induction of the transgene, as shown by the RNA gel blot analysis. Invertase inhibitor transcripts were detected both in wild-type plants and line NT71-29 because of the fact that the transgene used in this construction corresponds to the endogenous tobacco gene. However, whereas in the wild-type leaves its expression is downregulated by kinetin, in the transgenic lines the levels of transcripts are increased, demonstrating an induction of the transgene under control of the cytokinin-inducible promoter (Figure 5B, bottom panel). Because the mRNA level of the endogenous invertase inhibitor gene is repressed by cytokinin, and thus inversely regulated than the transgene under control of the Lin6 promoter, the treatment results in a highly elevated level of the invertase inhibitor mRNA in the transgenic plant compared with the corresponding control incubation. To substantiate that cytokinin results in the assumed induction of an endogenous extracellular invertase of tobacco under the experimental conditions used, the mRNA level of tobacco extracellular invertase NtβFruct1 has been determined. Treatment with kinetin induced the expression of Ntβfruct1 in the wild type and transgenic line already after 1 d (Figure 5B, top panel), supporting the finding that an extracellular invertase is induced by cytokinin in tobacco. To analyze whether the induction of the invertase inhibitor gene under control of the cytokinin-inducible promoter indeed results in a posttranslational inhibition of the activity of the endogenous extracellular invertase coinduced by cytokinin, sugar levels have been determined. Table 1 shows that kinetin induction of the transgene results in an average reduction of the concentration of hexose sugars by 55%, whereas the concentrations in the control incubations were not significantly affected. This finding demonstrates that the products of the enzymatic reaction catalyzed by invertase are specifically reduced. Because the endogenous extracellular invertase NtβFruct1 was induced in wild-type leaves and leaves of the transgenic line to similar levels (Figure 5B), the data demonstrate a posttranslational inhibition of the invertase activity by the invertase inhibitor coinduced by cytokinin. Therefore, the results of this loss-of-function experiment provide further proof for a positive correlation between extracellular invertase activity and delay of senescence. When the extracellular invertase activity is inhibited, the delay of senescence phenotype is no longer observable despite the presence of cytokinin.
Figure 5.
Effect of the Inhibition of Extracellular Invertase Activity on the Delay of Senescence by Kinetin.
(A) Detached leaves of a transgenic tobacco line expressing the tobacco invertase inhibitor P17A under control of the cytokinin-inducible promoter Lin6 (Lin6:P17A) and wild-type plants (W38) were infiltrated with water containing kinetin at a final concentration of 30 μg/L.
(B) RNA gel blot analysis showing the accumulation of the transcripts for extracellular invertase Ntβfruct1 and the invertase inhibitor P17A in wild-type (W38) and transgenic (Lin6:P17A) plants after 1 and 3 d of treatment with kinetin (K), respectively.
The results have been reproduced in five independent experiments with three independent transgenic lines, and representative results obtained with line NT71-29 are shown.
Table 1.
Kinetin Induction of the Invertase Inhibitor P17A Results in Inhibition of Sucrose Cleavage
| Line | Time | Treatment | Glucose (mM/gFW) | Fructose (mM/gFW) | Sucrose (mM/gFW) |
|---|---|---|---|---|---|
| W38 | 1 d | None | 19.2 (2.4) | 13.5 (1.6) | 6.7 (1.2) |
| Kinetin | 19.7 (2.9) | 18.5 (2.8) | 9.1 (1.7) | ||
| 3 d | None | 42.5 (0.4) | 41.3 (1.2) | 9.0 (4.4) | |
| Kinetin | 47.5 (1.7) | 46.7 (1.7) | 9.1 (2.4) | ||
| Lin6:P17A | 1 d | None | 14.0 (6.1) | 11.6 (5.8) | 8.1 (2.1) |
| Kinetin | 6.0 (1.3) | 4.2 (1.6) | 6.6 (1.9) | ||
| 3 d | None | 46.5 (3.5) | 33.9 (2.4) | 8.8 (0.1) | |
| Kinetin | 19.9 (5.6) | 20.1 (5.4) | 9.1 (2.6) |
Detached leaves of a transgenic tobacco line expressing the tobacco invertase inhibitor P17A under control of the cytokinin-inducible promoter Lin6 (Lin6:P17A) and wild-type plants (W38) were infiltrated with water containing kinetin at a final concentration of 30 μg/L, and concentrations of soluble sugars have been determined. The values represent the mean value of three independent replications ± se obtained with line NT71-29. Similar results have been obtained with line NT71-9.
These data demonstrate that extracellular invertase is an essential component of the mechanism of delay of senescence by cytokinins. Cytokinins fail to delay senescence under conditions when invertase activity is inhibited.
DISCUSSION
Despite the importance of cytokinins for plant growth and development, this class of plant hormone is least understood with respect to the mode of action. Cytokinins are involved in many processes associated with active growth and enhanced metabolic activity. One particular cytokinin effect is the delay of senescence, and this study demonstrates that nutrient mobilization via an extracellular invertase is an essential component of the underlying regulatory mechanism. This demonstrates that the regulation of leaf senescence is related to changes in source-sink relations and that a direct link between cytokinin action and primary metabolism exists.
The induction of extracellular invertase by cytokinins in suspension cell cultures of C. rubrum was shown to be paralleled by a coordinated induction of a hexose transporter, which resulted in a twofold increase of sucrose uptake (Ehness and Roitsch, 1997). This greatly supported the hypothesis of an essential link between the molecular mechanism of this phytohormone and primary metabolism (Roitsch et al., 2003), which was further substantiated in this study. It has been shown that the delay of leaf senescence in transgenic plants with autoregulated cytokinin synthesis under control of the SAG12 promoter (Gan and Amasino, 1995) correlates with an increase of extracellular invertase activity. Thus, extracellular invertases could be direct mediators of cytokinin in the regulation of this process.
An Increase in Extracellular Invertase Activity Is Sufficient to Cause a Delay of Senescence
Because the cytokinin-mediated delay of senescence is paralleled by a change in the source-sink relationship by affecting extracellular invertase activity, the question arises whether extracellular invertases could replace the effect of endogenous increase or exogenous application of cytokinins. To test this hypothesis, transgenic tobacco plants that expressed the extracellular invertase gene from C. rubrum (Ehness and Roitsch, 1997) under control of the senescence-activated SAG12 promoter have been generated. The SAG12:Cin1 plants had a clear delayed-senescence phenotype, comparable to that observed in SAG12:ipt plants (Gan and Amasino, 1995). The bottom leaves of the 17-week-old transgenic lines were still green, whereas the wild-type plants displayed a clear progression of leaf senescence from bottom to top leaves. It has been shown previously that young healthy leaves detached from wild-type tobacco plants show symptoms of senescence already after 10 d of detachment. The analysis of the cell wall invertase activity in the leaves of the transgenic line showed an increase of activity specifically in the bottom leaves at the stage in which the activation of the promoter occurs, as shown by the analysis of reporter gene plants. However, no increase in extracellular invertase activity was detected in the leaves of wild-type plants at any of the stages analyzed. Thus, it is possible to substitute the cytokinin stimulus by a metabolic enzyme demonstrating a causal relationship. The increased sink strength of bottom leaves in the transgenic line, because of the enhanced expression of extracellular invertase, is sufficient to cause delay in senescence. As a secondary effect, this could account for the observed increase in vacuolar invertase activity. Furthermore, the hydrolysis of sucrose into the hexose monomers not only increases the local sink strength but will also exclude the sugar from phloem loading and transport or remobilization into the stem.
In general, source and sink metabolism is inversely regulated by various stimuli, including the metabolic regulation by sugars (Roitsch, 1999). An activation of sink metabolism via the induction of sink-specific enzymes, including extracellular invertase, is usually coupled to feedback inhibition of photosynthetic gene expression by carbohydrates (Ehness et al., 1997). The unexpected finding that an increase in extracellular invertase in bottom leaves of SAG12:Cin1 plants does not result in an increase in glucose steady state concentrations provides an explanation for why photosynthetic activity may be maintained despite the activation of sink metabolism. Therefore, the delay of senescence induced by extracellular invertases may be related to an activation of the metabolic carbohydrate flux. The resulting higher rate of sugar utilization causes a decrease of glucose content in the transgenic plants. This ensures that, despite the activation of sink metabolism, the hexose concentration does not reach the threshold level that would result in the feedback inhibition of photosynthetic gene expression. This provides a mechanism to uncouple the usually observed inverse and coordinated regulation of source and sink metabolism. The analyses of transgenic Arabidopsis plants with modulated hexokinase activity support the suggestion that the metabolic flux is related to the regulation of senescence (Xiao et al., 2000). Apparently there is a fine-tuned interaction and balance between extracellular hydrolysis by the cell wall–bound invertase and the metabolic flux of the sink cell to avoid the accumulation of carbohydrates. This could explain the contradictory findings that overexpressing a yeast invertase in transgenic Arabidopsis, tobacco, and tomato plants results in the accumulation of carbohydrates, an inhibition of photosynthesis, and symptoms that resemble premature senescence (Dickinson et al., 1991; Ding et al., 1993). The strong overexpression of the fungal invertase results in a disturbance of the delicate system that may not be counterbalanced by the plant metabolism because of the fact that the enzymatic and biochemical properties of the heterologous invertase is distinctly different from the plant extracellular invertases.
Sugars are not only substrates for heterotrophic growth but also important signals to regulate various processes in higher plants (Rolland et al., 2002). According to the generally accepted model, leaf senescence is initiated when the photosynthetic rate drops below a certain threshold. Because sugars are primary products of photosynthesis, it has been proposed that sugar levels could be part of the signaling system leading to senescence (Gan and Amasino, 1997; Quirino et al., 2000; Yoshida et al., 2002). However, the analyses of the regulation of leaf senescence by sugars so far have been inconclusive. A stimulation of senescence by high sugar levels is suggested by studies with tobacco plants that showed that the levels of glucose and fructose, but not sucrose, increase as the leaves progress through senescence (Wingler et al., 1998). Likewise, a relationship between elevated hexose levels and premature senescence has been concluded from the analyses of transgenic lettuce plants expressing a SAG12:ipt construct. By contrast, the activation of the senescence pathways by low sugar levels is supported by the repression of the senescence-activated SAG12 gene by exogenous application of sugars (Noh and Amasino, 1999a) and its induction because of sugar deprivation (Quirino et al., 2000). This hypothesis is supported by the accelerated leaf senescence of transgenic tomato plants overexpressing the Arabidopsis hexokinase with reduced contents of glucose or fructose in comparison with wild-type leaves (Dai et al., 1999). This study and the references cited above indicate that there is mounting evidence that sugar levels can influence senescence, but more studies are required to determine the causal relationship and variation among species. A solution to the contradictory data with respect to the relation between sugars and senescence are provided by the conclusion that two types of leaf senescence occur in maize leaves: senescence because of assimilate starvation and senescence because of excessive assimilate accumulation (Tollenaar and Daynard, 1982). In addition, the interaction between sugar and hormone signaling (Leon and Sheen, 2003) or the relative ratio between hexose sugars and sucrose rather than the absolute concentration (Wobus and Weber, 1999) may be important as signals to initiate senescence.
It has been observed that the longevity of cut flowers is improved by feeding sugar solutions (Nichols, 1973) and that this effect has been related to an inhibition of the enzymatic activities required for ethylene biosynthesis (Mayak and Borochov, 1984). Ethylene could provide a feedback loop to carbohydrate metabolism because it has been shown that inhibition of ethylene biosyntheses results in the elevation of soluble sugars and improvement of vase life of cut flowers (Ichimura and Hisamatsu, 1999; Zhang and Leung, 2001), that invertase expression is repressed by ethylene (Linden et al., 1996), and that cross talk between sugar and ethylene signaling exists (Zhou et al., 1998; Yanagisawa et al., 2003).
In a complementing functional approach addressing the role of invertases in the cytokinin-induced delay of senescence, transgenic plants harboring C. rubrum cell wall invertase under the control of a tetracycline-inducible promoter were generated. The delay of senescence induced by the application of tetracycline to detached leaves provides independent experimental evidence that extracellular invertase can effectively substitute the cytokinin action. However, the detection of higher chlorophyll contents and more evident senescence-delayed phenotypes in leaves treated simultaneously with kinetin plus tetracycline suggest the existence of additional components in the cytokinin-induced delay of senescence. In addition, the use of this chemically inducible promoter allowed study of the effect of localized induction of extracellular invertase that results in a specific delay of senescence in the treated zones in comparison with the rest of the leaf. Thus, the higher sink strength of these areas because of the expression of extracellular invertase would result in a nutrient mobilization to these zones. The green islands of autumn leaves have been shown to be caused by cytokinin secretion (Engelbrecht et al., 1969; Angra and Mandahar, 1993; Chen and Ertl, 1994). Because radioactively labeled nutrients are preferably transported to cytokinin-treated areas (Mothes and Engelbrecht, 1963), an altered sink-source relation has been suggested to be responsible for the formation of green islands. The observation that a localized increase of extracellular invertase produces zones with a delay of senescence supports this hypothesis. The detection of increased invertase activity in green islands of autumn leaves of different species (Z. Novalic and T. Roitsch, unpublished data) confirms this assumption.
Extracellular Invertase Is Essential for Cytokinin-Mediated Delay of Senescence
Current investigations of the kinetics of expression of senescence activated genes (SAG) under natural senescence and under induction of accelerated senescence have suggested that multiple pathways exist that activate a distinct set of genes, forming a regulatory network for leaf senescence. Certain genes are likely to be shared by different pathways and may be involved in the execution of senescence, whereas others seem to be unique to specific pathways and may be upstream regulatory genes that affect components of the senescence program. This plasticity of leaf senescence implies that blocking a particular pathway may not necessarily have a significant effect on the progression of senescence (Gan and Amasino, 1997; Chandlee, 2001). To address the question of whether extracellular invertase is an essential component delaying senescence induced by cytokinin, transgenic plants were generated that allowed cytokinin-inducible inhibition of extracellular invertase activity. Transgenic tobacco plants harboring the invertase inhibitor gene from tobacco under the control of the cytokinin-induced promoter of extracellular invertase Lin6 from tomato (Godt and Roitsch, 1997) were generated. The presence of putative invertase inhibitor proteins has been shown for several sink tissues (Krausgrill et al., 1998), including carnation petals of natural senescing flowers (Halaba and Rudnicki, 1989), where the enzyme has been postulated to control the translocation of sucrose to other organs of the flower (Halaba and Rudnicki, 1988). Recently, the inhibitor from tobacco was cloned, and its functionality and specificity of binding to extracellular invertases was demonstrated (Greiner et al., 1998). The effective inhibition of tobacco cell wall invertase reported for the recombinant protein represents a potent tool to study the effect of inhibiting extracellular invertase activity on the cytokinin-induced delay of senescence. The incubation of detached leaves with kinetin should (1) activate the endogenous cytokinin pathway for the delay of senescence, (2) induce the expression of endogenous cytokinin-inducible extracellular invertase, and (3) simultaneously result in the inhibition of extracellular invertase activity by the invertase inhibitor expressed under control of the cytokinin-inducible promoter. The finding that senescence is not any longer delayed in the transgenic plants in the presence of cytokinin demonstrates that an increase in extracellular invertase activity is required for the cytokinin-mediated delay in senescence. The presence of transcripts for the invertase inhibitor in detached young leaves is in agreement with the results obtained by Greiner et al. (1998), where transcripts of this gene are detected in source leaves of 7- and 15-week-old plants. The results reported show a decrease in expression of the invertase inhibitor in leaves from the bottom to the top of the plant, being high in the old source leaves and reaching the highest levels in senescent leaves (Greiner et al., 1998). This distribution and the findings of this study that the endogenous invertase inhibitor gene is repressed by cytokinin indirectly support a requirement for extracellular invertase activity under conditions of delayed senescence. In addition, the observed repression of the invertase inhibitor demonstrates the regulation of an invertase inhibitor by a plant hormone.
Whereas this study demonstrates the importance of extracellular invertase for only one particular effect of cytokinins, this key enzyme of apolasmic phloem unloading pathways also could be involved in other cytokinin-mediated responses. The active growth of tissues depends on the function of the cell division cycle. Because it has been shown that specific D-type cyclins, as important regulators of the cell cycle, are carbohydrate responsive, the extracellular invertase may have a dual function. It provides substrate to satisfy the increased carbohydrate demand of actively growing tissues and generates a metabolic signal to stimulate the cell cycle. Thus, invertases could contribute to the increase in sink size by inducing cell division via a sugar signaling mechanism.
The reported induction of extracellular invertases by sugars (Roitsch et al., 1995; Krausgrill et al., 1996; Godt and Roitsch, 1997; Tymowska-Lalanne and Kreis, 1998; Sinha et al., 2002) could provide a feed forward mechanism to amplify or maintain the cytokinin signal. An initial upregulation of the extracellular invertase will result in an increased sugar concentration that will then further induce the invertase or keep the invertase induced even if the initial stimulus is not present anymore.
Senescence is a type of programmed cell death that constitutes the final phase of leaf development. Although there has been extensive research focused on whole plant/leaf senescence, the molecular events that induce or contribute to the process have been investigated only recently (Chandlee, 2001). As a result, only a partial picture of the molecular basis for the regulation and progress of this process has emerged, and many questions remained answered. A further understanding of the underlying mechanisms will provide fundamental information about aspects of cell differentiation and the regulation of cellular events through the action of plant hormones and other signals (Gan and Amasino, 1996; Grbic, 2002). The potential regulation of the delay of leaf senescence would provide practical benefits, maximizing the crop yield and minimizing the postharvest and postproduction losses of fruits, vegetables, flowers, and other crops.
METHODS
Plasmid Construction
A construct for senescence-inducible expression of extracellular invertase Cin1 (SAG12:Cin1) was generated in two steps. The Cin1 cDNA was amplified by PCR from plasmid pMB3 (Roitsch et al., 1995) by primers CIN125Noc (5′-TGCATCGATCAAGTCGATGT-3′) and CIN1SacNco (5′-GCAATTGACTGTAATTCGTACTAATT-3′) that generated NcoI sites at both ends and a SacI at the 5′-end of the cDNA. After cutting with NcoI, the fragment was cloned into the corresponding site of plasmid pSG499 (Gan and Amasino, 1995) to generate plasmid pRE1171. The expression cassette was subcloned into the binary vector pBI101+ (Goetz et al., 2001) as SalI-SacI fragment to generate plasmid pRE1186-27.
A construct for tetracycline-inducible expression of extracellular invertase Cin1 (TetR:Cin1) was generated by initially releasing the complete cDNA of extracellular invertase Cin1 from plasmid pMB3 as EcoRI fragment, filling in the 5′-overhangs by Klenow polymerase, and cloning the fragment into the SmaI site of pUC18 to generate pMH06/11. The Cin1 cDNA was then subcloned as a KpnI/XbaI fragment into the corresponding sites of the binary vector pBinHygTx (Gatz and Lenk, 1998) to generate pRE697-17.
A fusion between the cytokinin-inducible promoter of extracellular invertase Lin6 and the apoplasmic invertase inhibitor P17A (Lin6:P17A) from tobacco (Nicotiana tabacum) was generated by subcloning the invertase inhibitor cDNA as BamHI/XhoI from plasmid pBK-CMV/P17A (Greiner et al., 1998) and plasmid pR6-11 (R. Proels and T. Roitsch, unpublished data) to generate plasmid pTF3-11.
Plant Transformation
The constructs were transferred to tobacco (N. tabacum cv Wisconsin 38) using standard Agrobacterium tumefaciens transformation procedures (Horsch et al., 1985). The T1 generation of transgenic plants that was heterozygous for the different transgenes was used for all experiments.
Senescence Assay on Detached Leaves
Detached young leaves from tobacco plants were incubated in a water bath at 49.2°C for 1 min and 30 s and then transferred to a flask with tap water. For experiments with transgenic lines expressing the tobacco invertase inhibitor P17A under control of the cytokinin-inducible Lin6 promoter, the water was supplemented with either 30 μg/L of kinetin or an equivalent amount of the solvent (NaOH). The leaves were incubated in a plant growth chamber with a 15-h-light/9-h-dark cycle and a constant temperature of 23°C.
For experiments with transgenic lines expressing the extracellular invertase Cin1 under control of the tetracycline-inducible TetR promoter, expression of the transgene was induced by infiltration of a solution containing MS medium (Duchefa, Haarlem, The Netherlands), pH 6.0, 0.02% Silwet, and 10 mg/L of chlorotetracycline. For localized induction, a small area was gently treated with glass paper and then repetitively painted with a brush wetted in the chlorotetracycline-containing solution. For mock inoculations, the chlorotetracycline was omitted.
The leaves were observed for 4 weeks, and photographs were taken. At least five independent replications with three independent lines were performed.
Chlorophyll Determinations
Three frozen leaf disks of each sample analyzed, corresponding to ∼0.033 g of fresh weight material, were extracted and homogenized in 1 mL of 80% acetone and kept thereafter at 4°C. After homogenization, the samples were centrifuged at 10,000g, 2 min at 4°C, and 1 mL of the supernatant was used for spectrophotometric determination. The concentration of chlorophyll a/b as well as the major carotenoides, comprising xantophyll and carotene, was calculated as described by Lichtenthaler (1987). Results were expressed as micrograms of chlorophyll or carotenoids per milliliter. The chlorophyll content of individual samples was determined at least in duplicate, and at least three independent experiments were analyzed.
Determination of Invertase Activity
The activity of extracellular invertase was determined at pH 4.5 as described previously (Roitsch et al., 1995). A Glc test kit (Roche, Indianapolis, IN) was used to determine the amount of Glc liberated. Control reactions were performed using the same volume of extract and water in the reaction mixture instead of sucrose. The concentration of protein in the extracts was determined using the procedure of Bradford (1976). The invertase activity of individual samples was determined at least in triplicate, and at least three independent experiments were analyzed.
Soluble Sugar Determination
For the determination of soluble sugars, 100 mg of ground frozen material were resuspended in 900 μL of distilled water, and after centrifugation at 13,000 rpm for 10 min at 4°C, the supernatant was heated at 105°C for 3 min to denature the enzymes. The resulting supernatant was used for the quantification of soluble sugars using a high-pressure liquid chromatography system coupled with pulsed amperometry detection (Dionex 4500i; Dionex Softron, Germering, Germany). The sugar content of individual samples was determined at least in duplicate, and at least three independent experiments were analyzed.
Isolation of RNA and RNA Gel Blot Analyses
RNA was obtained in a scaled-down extraction procedure. The material was frozen in liquid nitrogen, and after grinding in a mortar, two replicates of 100 mg of material were used for RNA isolation. Total RNA was isolated according to the methods of Chomczynski and Sacchi (1987). To remove the high content of carbohydrates of the RNA-containing samples, the pellets were treated with 500 μL of peqGold Optipure solution (PeqLab, Erlangen, Germany) and maintained at vigorous shaking at 20°C overnight. The samples were then centrifuged at 20,000g for 30 min at room temperature, and the RNA-containing pellet was dispersed in 200 μL of SDS 0.5% by vigorous shaking at 55°C for 2 h. The samples were extracted with one volume of chloroform, and the upper phase was precipitated by the addition of one volume of isopropanol and a final concentration of 0.2 M sodium acetate and incubated at −20°C overnight. The RNA was collected by centrifugation (20,000g) washed with 85% ethanol and dried by vacuum centrifugation. The pellet was dissolved in 20 μL of diethyl pyrocarbonate–treated water, and the concentration of RNA was calculated from the A260.
For RNA gel blot analysis, 15 μg of RNA samples containing ethidium bromide were subjected to electrophoresis on 1.3% agarose formaldehyde gels and transferred to nitrocellulose filters by capillary transfer, and the cDNA probes were labeled by random priming (MBI Fermentas, St. Leon-Rot, Germany). Hybridization was performed in 50% formamide, 5× SSC (750 mM NaCl and 75 mM sodium citrate, brought to pH 7.0 with HCl), 0.1% SDS, and 5× Denhardt's solution (0.1% Ficoll 400, 0.1% BSA, and 0.1% polyvinylpyrrolidone) at 42°C for 18 h. The membranes were washed with decreasing salt concentration at 42°C. A final washing step was performed in 0.2× SSC and 0.1% SDS.
Samples from at least three different experiments were analyzed by RNA gel blot analyses.
Supplementary Material
Acknowledgments
This paper is dedicated to Harry Beevers on the occasion of his 80th birthday in January 2004, who passed away in April 2004. We thank Michal Popik and Lada Nedbal (University of South Bohemia, Nove Hrady, Czech Republic) for contributing the chlorophyll fluorescence image, Lee Garret and Michal Davey (University of Nottingham, UK) for providing leaf material from SAG12:ipt plants, Steffen Greiner and Thomas Rausch (University of Heidelberg, Germany) for providing plasmid pBK-CMV/P17A, and Richard Amasino for providing plasmid pSG499 and seeds of the transgenic tobacco line PSAG12:IPT. We also thank G. Stühler, P. Schittko, E. Herold, A. Taffner, and C. Hampp for excellent technical assistance and G. Peissig and A. Schmidt for taking care of the plants. Financial support by the Deutsche Forschungsgemeinschaft (to W.T. and T.R., TA 36/15-1), the Alexander von Humboldt Foundation (to M.E.B.L.), the Studienstiftung des Deutschen Volkes (to R.P.), the Federation of European Biochemical Society (to M.C.G.G.), and the Deutsche Akademische Austauschdienst (to T.F.) is also gratefully acknowledged.
Online version contains Web-only data.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Thomas Roitsch (roitsch@biozentrum.uni-wuerzburg.de).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.018929.
References
- Angra, S.R., and Mandahar, C.L. (1993). Involvement of carbohydrates and cytokinins in pathogenicity of Helminthosporium carbonum. Mycopathologia 121, 91–99. [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the determination of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Brenner, M.L., and Cheikh, N. (1995). The role of phytohormones in photosynthate partitioning and seed filling. In Plant Hormones, P.J. Davies, ed (Dordrecht, The Netherlands: Kluwer Academic Press), pp. 649–670.
- Buchanan-Wollaston, V. (1997). The molecular biology of leaf senescence. J. Exp. Bot. 48, 181–199. [Google Scholar]
- Chandlee, J.M. (2001). Current molecular understanding of the genetically programmed process of leaf senescence. Physiol. Plant 113, 1–8. [Google Scholar]
- Chang, H., Jones, M., Banowetz, G.M., and Clark, D.G. (2003). Overproduction of cytokinins in petunia flowers transformed with PSAG12-IPT delays corolla senescence and decreases sensitivity to ethylene. Plant Physiol. 132, 2174–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.-M., and Ertl, J.R. (1994). Cytokinin biosynthetic enzymes in plants and slime mold. In Cytokinins: Chemistry, Activity and Function, D.W.S. Mok and M.C. Mok, eds (Boca Raton, FL: CRC Press), pp. 81–85.
- Chomczynski, P., and Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159. [DOI] [PubMed] [Google Scholar]
- Dai, N., Schaffer, A., Petreikov, M., Shahak, Y., Giller, Y., Ratner, K., Levine, K., and Granot, D. (1999). Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell 11, 1253–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, C.D., Altabella, T., and Chrispeels, M.J. (1991). Slow-growth phenotype of transgenic tomato expressing apoplastic invertase. Plant Physiol. 95, 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, B., Haudenshield, J.S., Willmitzer, L., and Lucas, W.J. (1993). Correlation between arrested secondary plasmodesmal development and onset of accelerated leaf senescence in yeast acid invertase transgenic tobacco plants. Plant J. 4, 179–189. [DOI] [PubMed] [Google Scholar]
- Ehness, R., Ecker, M., Godt, D., and Roitsch, T. (1997). Glucose and stress independently regulate source/sink relations and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 9, 1825–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehness, R., and Roitsch, T. (1997). Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J. 11, 539–548. [DOI] [PubMed] [Google Scholar]
- Engelbrecht, L., Orban, U., and Heese, W. (1969). Leaf-miner caterpillars and cytokinins in the “green islands” of autumn leaves. Nature 223, 319–321. [Google Scholar]
- Eschrich, W. (1980). Free space invertase, its possible role in phloem unloading. Ber. Dtsch. Bot. Ges. 93, 363–378. [Google Scholar]
- Gan, S., and Amasino, R.M. (1995). Inhibition of leaf senescence by autorregulated production of cytokinin. Science 270, 1986–1988. [DOI] [PubMed] [Google Scholar]
- Gan, S., and Amasino, R.M. (1996). Cytokinins in plant senescence: From spray and spray to clone and play. Bioessays 18, 557–565. [Google Scholar]
- Gan, S., and Amasino, R.M. (1997). Making sense of senescence. Plant Physiol. 113, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz, C., and Lenk, I. (1998). Promoters that respond to chemical inducers. Trends Plant Sci. 3, 352–358. [Google Scholar]
- Godt, D.E., and Roitsch, T. (1997). Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isozymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol. 115, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz, M., Godt, D.E., Guivarch, A., Kahmann, U., Chriqui, D., and Roitsch, T. (2001). Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. Proc. Natl. Acad. Sci. USA 98, 6522–6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz, M., and Roitsch, T. (1999). The different pH-optima and substrate specificities of extracellular and vacuolar invertases are determined by a single amino acid substitution. Plant J. 20, 707–711. [DOI] [PubMed] [Google Scholar]
- Grbic, V. (2002). Spatial expression pattern of SAG12:GUS transgene in tobacco (Nicotiana tabacum). Physiol. Plant 116, 416–422. [Google Scholar]
- Greiner, S., Krausgrill, S., and Rausch, T. (1998). Cloning of a tobacco apoplasmic invertase inhibitor: Proof of function of the recombinant protein and expression analysis during plant development. Plant Physiol. 116, 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaba, J., and Rudnicki, R.M. (1988). Invertase inhibitor-control of sucrose transport from carnation petals to other flower parts. Plant Growth Reg. 7, 193–200. [Google Scholar]
- Halaba, J., and Rudnicki, R.M. (1989). Invertase inhibitor in wilting flower petals. Sci. Hortic. 40, 83–90. [Google Scholar]
- Hensel, L.L., Grbic, V., Baumgarten, D.A., and Bleecker, A.B. (1993). Developmental and age-related processes that influence the longevity and senescence of photosynthetic tissues in Arabidopsis. Plant Cell 5, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch, R.B., Fry, J.B., Hoffmann, N.L., Wallroth, M., Eichholtz, D., Rogers, S.G., and Fraley, R.T. (1985). A simple and general method for transferring genes into plants. Science 227, 1229–1231. [DOI] [PubMed] [Google Scholar]
- Ichimura, K., and Hisamatsu, T. (1999). Effects of continuous treatment with sucrose on the vase life, soluble carbohydrate concentrations, and ethylene production of cut snapdragon flowers. J. Japan Soc. Hortic. Sci. 68, 61–66. [Google Scholar]
- Krausgrill, S., Greiner, S., Köster, U., Vogel, R., and Rausch, T. (1998). In transformed tobacco cells the apoplasmic invertase inhibitor operates as a regulatory switch of cell wall invertase. Plant J. 13, 275–280. [Google Scholar]
- Krausgrill, S., Sander, A., Greiner, S., Weil, M., and Rausch, T. (1996). Regulation of cell-wall invertase by a proteinaceous inhibitor. J. Exp. Bot. 47, 1193–1198. [DOI] [PubMed] [Google Scholar]
- Kuiper, D. (1993). Sink strength: Established and regulated by plant growth regulators. Plant Cell Environ. 16, 1025–1026. [Google Scholar]
- Lefebre, R., Vasseur, J., Backoula, E., and Coullerot, J.P. (1992). Participation of carbohydrate metabolism in the organogenic orientation of Chicorium intybus tissues cultivated in vitro. Can. J. Bot. 70, 1897–1902. [Google Scholar]
- Leon, P., and Sheen, J. (2003). Sugar and hormone connections. Trends Plant Sci. 8, 110–116. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler. (1987). Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J. Plant Physiol. 131, 101–110. [Google Scholar]
- Linden, J.C., Ehness, R., and Roitsch, T. (1996). Regulation by ethylene of apoplastic invertase expression in Chenopodium rubrum tissue culture cells. Plant Growth Regul. 19, 219–222. [Google Scholar]
- Mayak, S., and Borochov, A. (1984). Nonosmotic inhibition by sugars of the ethylene-forming activity associated with microsomal membranes from carnation petals. Plant Physiol. 76, 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe, M.S., Garratt, L., Schepers, F., Jordi, W.J.R.M., Stoopen, G.M., Davelaar, E., van Rhijn, J.H.A., Power, J.B., and Davey, M. (2001). Effects of PSAG12-IPT gene expression on development and senescence in transgenic lettuce. Plant Physiol. 127, 505–516. [PMC free article] [PubMed] [Google Scholar]
- Mothes, K., and Engelbrecht, L. (1963). On the activity of a kinetin-like root factor. Life Sci. 11, 852–857. [Google Scholar]
- Nam, H.G. (1997). The molecular genetic analysis of leaf senescence. Curr. Opin. Biotechnol. 8, 200–207. [DOI] [PubMed] [Google Scholar]
- Nichols, R. (1973). Senescence of cut carnation flower: Respiration and sugar status. J. Hortic. Sci. 48, 111–121. [Google Scholar]
- Noh, Y.-S., and Amasino, R.M. (1999. a). Identification of a promoter region responsible for the senescence-specific expression of SAG12. Plant Mol. Biol. 41, 181–194. [DOI] [PubMed] [Google Scholar]
- Noh, Y.-S., and Amasino, R.M. (1999. b). Regulation of developmental senescence is conserved between Arabidopsis and Brassica napus. Plant Mol. Biol. 41, 195–206. [DOI] [PubMed] [Google Scholar]
- Ori, N., Juarez, M.T., Jackson, D., Yamaguchi, J., Banowetz, G.M., and Hake, S. (1999). Leaf senescence is delayed in tobacco plants expressing the maize homeobox gene knotted1 under the control of a senescence-activated promoter. Plant Cell 11, 1073–1080. [PMC free article] [PubMed] [Google Scholar]
- Richmond, A.E., and Lang, A. (1957). Effect of kinetin on protein content and survival of detached Xanthium leaves. Science 125, 650–651.13421662 [Google Scholar]
- Rolland, F., Moore, B., and Sheen, J. (2002). Sugar sensing and signaling in plants. Plant Cell 14 (suppl.), S185–S205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch, T. (1999). Source-sink regulation by sugars and stress. Curr. Opin. Plant Biol. 2, 198–206. [DOI] [PubMed] [Google Scholar]
- Roitsch, T., Balibrea, M.E., Hofmann, M., Proels, R., and Sinha, A.K. (2003). Extracellular invertase: Key metabolic enzyme and PR protein. J. Exp. Bot. 54, 513–524. [DOI] [PubMed] [Google Scholar]
- Roitsch, T., Bittner, M., and Godt, D.E. (1995). Induction of apoplastic invertase of Chenopodium rubrum by D-glucose and a glucose analog and tissue-specific expression suggest a role in sink-source regulation. Plant Physiol. 108, 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch, T., and Ehness, R. (2000). Regulation of source/sink relations by cytokinins. Plant Growth Regul. 32, 359–367. [Google Scholar]
- Roitsch, T., and Tanner, W. (1996). Cell wall invertase: Bridging the gap. Bot. Acta 109, 90–93. [Google Scholar]
- Quirino, B.F., Noh, Y.-S., Himelblau, E., and Amasino, R.M. (2000). Molecular aspects of leaf senescence. Trends Plant Sci. 5, 278–282. [DOI] [PubMed] [Google Scholar]
- Singh, S., Letham, D.S., and Palni, L.M.S. (1992. a). Cytokinin biochemistry in relation to leaf senescence. V. Endogenous cytokinin levels and metabolism of zeatin riboside in leaf discs from green and senescent tobacco (Nicotiana rustica) leaves. J. Plant Physiol. 139, 279–283. [Google Scholar]
- Singh, S., Letham, D.S., and Palni, L.M.S. (1992. b). Cytokinin biochemistry in relation to leaf senescence. VII. Endogenous cytokinin levels and exogenous applications of cytokinins in relation to sequential leaf senescence of tobacco. Physiol. Plant 86, 398–406. [Google Scholar]
- Sinha, A.K., Römer, U., Köckenberger, W., Hoffmann, M., Elling, L., and Roitsch, T. (2002). Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiol. 128, 1480–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart, C.M. (1994). Gene expression during leaf senescence. New Phytol. 126, 419–448. [DOI] [PubMed] [Google Scholar]
- Tang, G.Q., Lüscher, M., and Sturm, A. (1999). Antisense repression and vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose partitioning. Plant Cell 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollenaar, M., and Daynard, T.B. (1982). Effect of source sink ratio on dry matter accumulation and leaf senescence of Zea mays. Can. J. Plant Sci. 62, 855–860. [Google Scholar]
- Tymowska-Lalanne, Z., and Kreis, M. (1998). Expression of the Arabidopsis thaliana invertase gene family. Planta 207, 259–265. [DOI] [PubMed] [Google Scholar]
- Wingler, A., Schaewen, A., Leegood, R.C., Lea, P.J., and Quick, W.P. (1998). Regulation of leaf senescence by cytokinins, sugars, and light. Plant Physiol. 116, 329–335. [Google Scholar]
- Wobus, U., and Weber, H. (1999). Sugars as signal molecules in plant seed development. Biol. Chem. 380, 937–944. [DOI] [PubMed] [Google Scholar]
- Xiao, W., Sheen, J., and Jang, J.C. (2000). The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol. Biol. 44, 451–461. [DOI] [PubMed] [Google Scholar]
- Yanagisawa, S., Yoo, S.D., and Sheen, J. (2003). Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425, 521–525. [DOI] [PubMed] [Google Scholar]
- Yoshida, S., Ito, M., Nishida, I., and Watanabe, A. (2002). Identification of a novel gene HYS1/CPR5 that has a repressive role in the induction of leaf senescence and pathogen-defence responses in Arabidopsis thaliana. Plant J. 29, 427–437. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., and Leung, D. (2001). Elevation of soluble sugar levels by silver thiosulfate is associated with vase life improvement of cut gentian flowers. J. Appl. Bot. 75, 85–90. [Google Scholar]
- Zhou, L., Jang, J.C., Jones, T.L., and Sheen, J. (1998). Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc. Natl. Acad. Sci. USA 95, 10294–10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.