Abstract
Background
Conflicting evidence exists on whether smoking acts as an effect modifier of the association between APOE genotype and risk of coronary heart disease (CHD).
Methods and results
We searched PubMed and EMBASE to June 11, 2013 for published studies reporting APOE genotype, smoking status and CHD events and added unpublished data from population cohorts. We tested for presence of effect modification by smoking status in the relationship between APOE genotype and risk of CHD using likelihood ratio test.
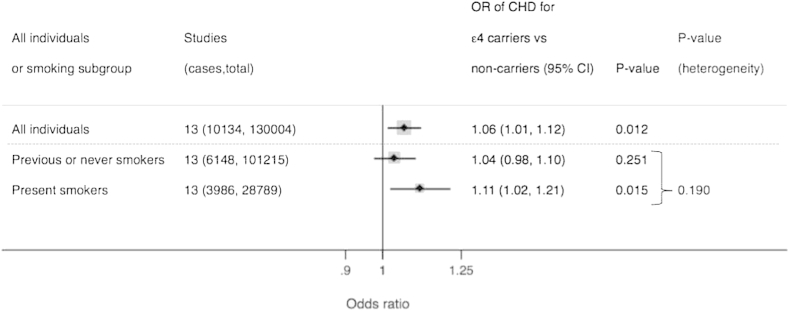
In total 13 studies (including unpublished data from eight cohorts) with 10,134 CHD events in 130,004 individuals of European descent were identified. The odds ratio (OR) for CHD risk from APOE genotype (ε4 carriers versus non-carriers) was 1.06 (95% confidence interval (CI): 1.01, 1.12) and for smoking (present vs. past/never smokers) was OR 2.05 (95%CI: 1.95, 2.14). When the association between APOE genotype and CHD was stratified by smoking status, compared to non-ε4 carriers, ε4 carriers had an OR of 1.11 (95%CI: 1.02, 1.21) in 28,789 present smokers and an OR of 1.04 (95%CI 0.98, 1.10) in 101,215 previous/never smokers, with no evidence of effect modification (P-value for heterogeneity = 0.19). Analysis of pack years in individual participant data of >60,000 with adjustment for cardiovascular traits also failed to identify evidence of effect modification.
Conclusions
In the largest analysis to date, we identified no evidence for effect modification by smoking status in the association between APOE genotype and risk of CHD.
Keywords: APOE genotype, Smoking, Coronary heart disease, Gene–environment interaction
Highlights
-
•
We examined evidence for an interaction between APOE genotype, smoking and risk of coronary heart disease.
-
•
This was conducted in the largest meta-analysis of published and unpublished data sets to date (>130,000 individuals).
-
•
Our analysis did not identify evidence of interaction.
-
•
These findings bring into question presence of a clinically meaningful interaction between APOE genotype and smoking.
1. Introduction
Cardiovascular diseases are the leading cause of death worldwide. Over recent decades, much research attention has focussed on investigating the genetic causes of coronary heart disease (CHD). Despite enormous advances in our understanding of the genetic basis, it is humbling that exposure to tobacco smoke remains a critically important, major, preventable cause of CHD [1].
One of the most widely studied genetic variants in CHD is APOE, variation in which encodes the three common isoforms of apolipoprotein E (apoE), ε2, ε3, and ε4, that have important roles in plasma lipid metabolism and transportation [2], [3]. APOE variants have a strong and consistent effect on the concentration of plasma lipids and on risk of CHD [4]. ApoE regulates multiple additional metabolic pathways and influences oxidative stress [5]. Previous studies have investigated the potential role of smoking as an effect modifier of the association between genotype and CHD risk [6], [7], [8]. Early studies identified that the association between carriers of the ε4 allele and CHD was greater amongst smokers than non-smokers [6] but this was not replicated in larger studies [7]. However, more recent studies that reported findings in support of effect modification have brought into question the underlying relationship [8], [9].
Effect modification is biologically plausible as in vitro studies show that recombinant ApoE ε4 is a poorer anti-oxidant than both ApoE ε2 and ε3 [10], and we previously reported that APOE ε4 carriers who smoked had lower anti-oxidant status [6]. Thus it is possible that in the presence of smoking, an impaired anti-oxidant diathesis would result in a greater risk of CHD. If true, the increase in CHD risk caused by smoking should be greater in individuals who carry the ε4 allele compared to individuals who do not carry the ε4 allele. However, an important argument against the plausibility of an anti-oxidant mediated APOE-by-smoking interaction is the fact that to date, no randomized trial of an anti-oxidant intervention has shown a reduction in the risk of CHD, which undermines the “oxidation hypothesis” of CHD [11].
It is therefore timely to conduct a large scale, rigorous investigation to address this question. To this end, we conducted a systematic review to identify studies reporting effect modification of APOE genotype on risk of CHD by smoking status and supplemented studies that met our inclusion criteria with de novo data from large population cohorts. Concern has been raised that the scientific evidence may be interpreted as suggesting that the sequelae of smoking could be less serious in individuals that did not carry the APOE ε4 allele [7]. The devastating consequences of smoking to human health is unquestionable, and the purpose of this scientific investigation was not to investigate the potential protection of smoking by certain variants of APOE genotype, but rather to investigate the potential for effect modification as a means of understanding the biological processes by which APOE increases the risk of CHD.
2. Methods
2.1. Literature search
We used the PRIMSA statement [12] as a guide and include a completed PRISMA checklist (Supplementary table S1) and flow diagram (Supplementary figure 1). An early analysis plan is included in the Supplementary material.
We searched PubMed and EMBASE from inception to June 11, 2013 for studies that included the keywords “APOE”, “genotype”, “smoking” and “CHD”. Full details of the search strategy are provided in the Supplementary material. Eligible studies reported CHD outcomes in relation to APOE genotype stratified by smoking status in individuals of European descent. The search was conducted by DM and a random subset of articles was double-checked by MVH. Discrepancies were resolved by consensus. We only included studies that reported the CHD outcome of myocardial infarction (MI) alone or in combination with angina or cardiac interventions (such as revascularization), thus studies reporting stroke or a composite of CHD and stroke were excluded. Furthermore, studies that reported angiographically-determined coronary artery stenosis but not clinical events were also excluded. To minimize human error, DM and MVH conducted data entry separately and checked for concordance between retrieved data.
We updated one previously reported study (NPHSII [6]) for CHD events and supplemented the retrieved articles from the search with eight additional studies: one randomized trial (Thrombosis Prevention Trial [13]), one nested case–control study (EPIC-Netherlands [14]), one cohort (ELSA [15]) and five general population cohorts: Copenhagen City Heart Study (CCHS) [16], Copenhagen General Population Study (CGPS) [17], Czech post-MONICA [18], EPIC-Norfolk [19] and HAPIEE-Czech [20]. Ethics Committees at the contributing centres approved use of new data for updated and unpublished studies.
For all studies, we tested the Hardy Weinberg Equilibrium for the genotypes determined by the two single nucleotide polymorphisms that encode the APOE isoforms. For published studies, we noted whether the original report stated presence of effect modification in the APOE–CHD relationship by smoking status.
2.2. Outcome classification
For the new cohorts (CCHS, CGPS, Czech post-MONICA, EPIC-Netherlands, HAPIEE-Czech), we used ICD codes specific for myocardial infarction (i.e. ICD-8: 410, ICD-9: 410, ICD-10: I21 and ICD-10: I22). For TPT, we used the primary outcome reported in the original randomized trial. Outcome definitions for all studies including those identified from the electronic search and new studies are reported in Table 1.
Table 1.
Characteristics of the studies included in the analysis.
| Ref/study | Study design | Country of origin | Number of study participant | Age, mean (SD) (controls in case–control studies) | Proportion male, % (controls in case-control studies) | Recruitment | Follow-up (years) | CHD outcomes | Outcome ascertainment | APOE SNPs in HWE? | Original report stated presence of effect modification?a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Published studies identified in the systematic review | |||||||||||
| Gustavsson et al. [9]/INTERGENE and SHEEP | Case–control | Sweden | 6389 | 54 (12) | 46.2 | Cases were patients with first or recurrent CHD and controls were randomly selected from the population | N/A | Acute MI, unstable angina, CHD exacerbations | MI: changes in blood levels of the enzymes CK and LDH, specified ECG-changes and autopsy findings according to the Swedish Association of Cardiologists in 1991 | Yes | Yes |
| Keavney et al. [7]/ISIS | Case–control | UK | 7385 | 46 (10) | 67.9 | Cases were patients with suspected AMI and controls selected from the relatives and spouses of the case group | N/A | Acute MI | Cardiac enzyme or electrocardiographic criteria, or both | Yes | No |
| Liu et al. [23]/Physicians' Health Study | Nested case–control | USA | 731 | 60 (9) | 100 | Male physicians registered with the American Medical Association | 12 | Fatal and nonfatal MI | WHO criteria for MI. Autopsies and deaths recorded for fatal MI diagnoses. | Yes | No |
| Talmud et al. [24]/Whitehall II | Prospective cohort | UK | 5380 | 57 (6) | 100 | Randomly selected among British civil servants | 5.8 | Fatal/nonfatal MI, angina (definitive or probable) | Fatal MI: national registries; nonfatal MI: MONICA criteria; angina: abnormal investigation such as angiography, exercise electrocardiography, stress imaging, study electrocardiogram or clinical confirmation | Yes | No |
| Published studies identified in the systematic review updated for incident CHD events | |||||||||||
| Humphries et al. [6]/NPHSII | Prospective cohort | UK | 2630 | 55.7 (3.2) | 100 | General practices, hospital clinics, coroner's offices | >10 | Fatal CHD (coronary deaths/fatal MI), nonfatal MI, coronary artery surgery and silent MI | WHO criteria | Yes | Yes |
| Studies not previously published | |||||||||||
| Copenhagen City Heart Study (CCHS) | Prospective cohort | Denmark | 8926 | 55.1(15.1) | 44 | Population-based | 34 | Fatal and nonfatal MI | ICD-8: 410; ICD-10: I21 and I22 | Yes | N/A |
| Copenhagen General Population Study (CGPS) | Prospective cohort | Denmark | 57,942 | 57.1(13.3) | 44 | Population-based | 34 | Fatal and nonfatal MI | ICD-8: 410; ICD-10: I21 and I22 | Yes | N/A |
| Czech post-MONICA | Prospective cohort | Czech Republic | 1875 | 55.0 (10.3) | 45 | Population-based | 6 | Nonfatal MI | ICD-10: I21 and I22 | Yes | N/A |
| ELSA | Cohort | UK | 5020 | 67.5 (9.8) | 46 | Respondents of Nationwide survey | 11 | Fatal and nonfatal CHD | Fatal CHD (ICD-10: I20–I25) and self-reported CHD | Yes | N/A |
| EPIC-Netherlands | Nested case–control | Netherlands | 2129 | 54.1 (10.1) | 22 | Population-based | 13 | Fatal and nonfatal MI | ICD-9: 410; ICD-10: I21, I22 | Yes | N/A |
| EPIC-Norfolk | Prospective cohort | UK | 22,838 | 59.2 (9.2) | 46 | Population-based | 10 | Fatal and nonfatal MI | ICD-10: I21 and I22 | Yes | N/A |
| HAPIEE-Czech | Prospective cohort | Czech Republic | 6256 | 58.3 (7.1) | 46 | Population-based | 7 | Fatal and nonfatal MI | ICD-10: I21 and I22 | Yes | N/A |
| MRC GP Research Framework Investigators, TPT trial [30] | Randomized clinical trial | UK | 2503 | 56.1 (6.7) | 100 | Hospital clinics | 9 | Coronary death, fatal and nonfatal MI | WHO criteria | Yes | N/A |
CHD, coronary heart disease; CK, creatine kinase; ECG, electrocardiogram; HWE, Hardy Weinberg Equilibrium; ICD, international classification of disease; LDH, lactate dehydrogenase; MI, myocardial infarction; N/A, not applicable; WHO, World Health Organization.
Effect modification by smoking status in the APOE–CHD relationship.
2.3. APOE genotype grouping
We conducted two separate genetic analyses: one simple, and one detailed. For the simple analysis, we stratified individuals into two groups, based on carriage of the ε4 allele of APOE genotype: ε4 carriers consisted of APOE genotypes ε3/ε4 or ε4/ε4; non-ε4 carriers consisted of APOE genotypes ε3/ε3, ε2/ε3 or ε2/ε2. Where possible, ε2/ε4 carriers were excluded by convention [6], [21].
For the detailed genetic analysis, we used the original APOE genotype groups and placed them into order according to the previously reported association with CHD [4] (i.e. from lowest to highest CHD risk: ε2/ε2, ε2/ε3, ε3/ε3, ε3/ε4 or ε4/ε4). Treating APOE genotype in this fashion yields a monotonic relationship between genotype group and risk of CHD [4].
2.4. Analysis
2.4.1. Smoking status: present versus past/never
Using tabulated data, we reconstructed the original data sets with the following variables: APOE genotype, smoking status (present, past or never) and CHD status. This enabled us to conduct “quasi-individual participant data” analyses to investigate the association between APOE genotype and CHD risk. The NPHSII study was stratified as previous studies have shown heterogeneity according to recruitment centre [22]. For the analyses described below, we used logistic regression with CHD status as the dependent variable; the principle summary measure was therefore an odds ratio (OR). In every analysis, we adjusted for study design using an unordered categorical variable with the “i.” prefix in Stata.
For the simple analysis, we initially tested the univariate association between smoking and APOE ε4 genotype carrier status (comparing ε4 carriers to non-carriers) individually with CHD, which served to validate our data set for the analysis of effect modification. Second, we stratified the association between APOE genotype and CHD by smoking status (into present vs. previous/never; this grouping was used as it was the most widely-reported in the identified studies and allowed us to include all studies). Finally, we tested for an interaction between APOE and smoking by fitting two statistical models: (i) a multivariate model with CHD status as the dependent variable, and smoking status and APOE genotype as the independent variables (adjusting for study design), and (ii) a model consisting of the same variables as model (i), but with an interaction term fitted between smoking status and APOE genotype. We used the Likelihood ratio test (LRT) to test the null hypothesis that the simpler model lacking the interaction term, i.e. model (i), explained the data better. We used a generous P-value threshold (<0.05) from the LRT as evidence against the null hypothesis (of no effect modification). To investigate whether there was a relationship between sample size and evidence for effect modification, we also conducted the LRT for effect modification between APOE genotype and smoking status in each study individually, and plotted the P-values derived from LRT against sample size, grouped by whether the original publication reported evidence of effect modification. We tested for evidence of a pair-wise correlation between sample size and LRT P-value by obtaining the Pearson’s correlation coefficient from the “pwcorr” command in Stata.
In the detailed genetic analysis, we investigated the association between APOE genotype with odds of CHD in the three large population cohorts (EPIC-Norfolk, CCHS and CGPS) in which outcomes were ICD codes for myocardial infarction, with detailed information on smoking (present, past, never) and APOE genotype. We arranged genotype groups in order according to the reported association with CHD risk in the largest genetic association reported to date [4]. Thus, individuals were assigned a numerical value of 1–5 depending on their APOE genotype (1 = ε2/ε2, 2 = ε2/ε3, 3 = ε3/ε3, 4 = ε3/ε4 or 5 = ε4/ε4). In all individuals (irrespective of smoking status), we first obtained the odds ratio of CHD for each individual group by conducting a categorical logistic regression analysis, using the ε3/ε3 genotype as the reference group. We then tested for the presence of a linear association between the APOE genotype groups using logistic regression, by treating the APOE genotype as a continuous trait (thus the beta coefficient on the log odds scale for this trait was the slope for an incremental increase in APOE genotype). Next, we stratified the analysis by smoking status into present, past or never and reconstructed the plots. For each of these three smoking groups, we generated a slope for the association between APOE genotype and log odds of CHD. Finally, we tested for a difference in these three slopes (of the linear estimates for APOE genotype by smoking status: present, past, never smoking) by fitting an interaction term between APOE genotype status and smoking status in the logistic regression model and using likelihood ratio test to investigate whether a simpler model (without the interaction term fitted) better explained the data.
2.4.2. Pack years and adjustment for other cardiovascular risk factors
In addition to the analysis of present vs. past/never smoker in all studies (including published and unpublished data), access to individual participant data in two large population-based studies (Copenhagen General Population Study and Copenhagen City Heart Study) with a pooled sample size of 68,177 facilitated the conduct of a more detailed analysis of smoking phenotype and permitted statistical adjustment for cardiovascular risk factors.
For this, we used pack years, a trait that encompasses both the number of cigarettes smoked per day and the duration of smoking (one pack year is equivalent to 20 cigarettes smoked every day for one year). We conducted logistic regression analyses using pack years as a categorical variable. In detail, the dependent variable was MI, and the independent variables were pack years (treated as an unordered categorical variable with individuals grouped into 0, >0 to <10, ≥10 to <20 and ≥20 pack years), and APOE ε4 genotype carrier status (dichotomized into ε4 carriers and non-carriers). This was conducted initially in the two studies separately, unadjusted for any other traits. The model was then repeated with an interaction term fitted between pack years and APOE genotype status and the Likelihood ratio test was used to test model fit. We then repeated these analyses with statistical adjustment for the following cardiovascular traits (that could act as confounders): (i) age (grouped into 10-year blocks), (ii) gender, (iii) hypertension (classified as SBP ≥140 mmHg or DBP >90 mmHg) and (iv) type 2 diabetes (T2D, ascertained from the following ICD codes: ICD-8: 250; ICD-10: E11, E13 and E14) to investigate whether an interaction between pack years and APOE genotype emerged. Finally, a fully adjusted model was fitted that included all cardiovascular variables.
All analyses were conducted using Stata version 13.1 (StataCorp, College Station, Texas 77845 USA).
3. Results
Of the 425 articles retrieved from the search, five studies met our inclusion criteria (Supplementary figure 1) [6], [7], [9], [23], [24]. To this, we updated data from one cohort and added data from eight new studies (Supplementary figure 1). This yielded a total of 13 studies with 10,134 CHD events in 130,004 individuals of European descent of whom 28,789 were present smokers.
Study characteristics including age and gender are provided in Table 1. Twenty-two per cent of individuals in the pooled data set were current smokers and 28% were carriers of the APOE ε4 allele (study-level characteristics are reported in Supplementary Table S2). The genotypes of the SNPs comprising the APOE isoforms were in Hardy Weinberg Equilibrium in all contributing studies. Two of the five previously published studies reported evidence of effect modification of the association between APOE genotype and CHD risk by smoking status [6], [9]. In these two studies, the outcomes were a composite that included angina, silent MI or coronary artery surgery.
3.1. Univariate association of APOE genotype and smoking status with CHD
Carriers of the ε4 allele had an OR of CHD of 1.06 (95%CI 1.01, 1.12; P = 0.01) compared to individuals who did not carry the ε4 allele. Individuals who currently smoked had an OR of CHD of 2.05 (95%CI: 1.95, 2.14; P = 9.7 × 10−196) compared to individuals that did not currently smoke (including previous or never smokers).
3.1.1. Association of APOE ε4 allele genotype with CHD stratified by smoking status and test for interaction
In analysis of 6148 CHD cases in 101,215 individuals who did not currently smoke, carriers of the ε4 allele had an OR of CHD of 1.04 (95%CI 0.98, 1.10; P = 0.25) compared to individuals who did not carry the ε4 allele. In 28,789 current smokers with 3986 CHD events, carriers of the ε4 allele had an OR of CHD of 1.11 (95%CI 1.02, 1.21; P = 0.02) compared to individuals who did not carry the ε4 allele.
When tested formally using likelihood ratio test, we identified no evidence of an interaction between APOE genotype and smoking (Parameter estimate = 0.07; 95%CI: −0.03, 0.17; χ2 1.72; (df = 1); P = 0.19) (Fig. 1).
Fig. 1.
Association between APOE genotype and CHD in all individuals and stratified by smoking status. P-value for heterogeneity obtained from testing whether an interaction term between APOE and smoking represents the data better than no interaction term (tested using likelihood ratio test).
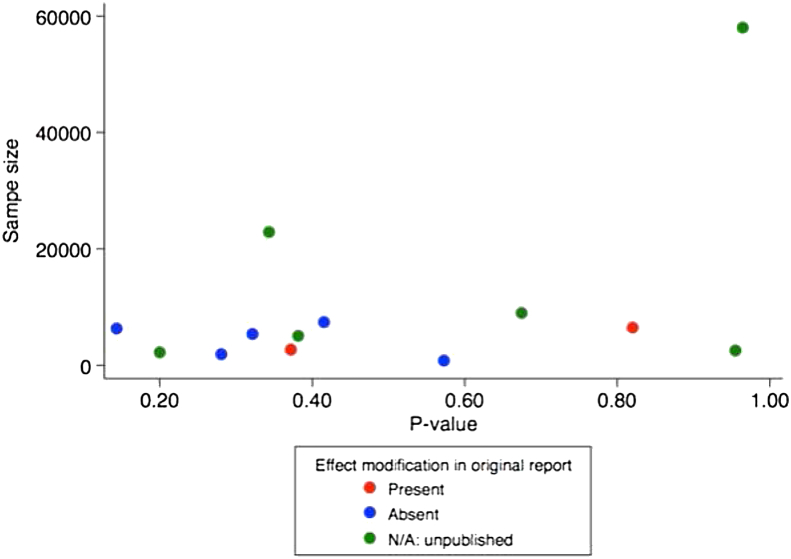
An investigation into the relationship between sample size and P-value for effect modification in each study, categorized according to whether the original publication reported presence of effect modification, did not identify a relationship between sample size and P-value for LRT (Pearson's r = 0.45; P-value for correlation = 0.13). Interestingly, using our analytical approach, we did not reproduce the small P-values for effect modification in either of the two previously reported studies, both of which had fewer than 7000 participants. In contrast, the largest study in our analysis, with a sample size of 57,942 (and which used ICD codes specific for MI), had the largest P-value for interaction (P = 0.96; Fig. 2).
Fig. 2.
Scatter plot of the P-value for interaction and sample size in the 13 studies. P-value obtained from testing whether an interaction term between APOE and smoking represents the data better than no interaction term (tested using likelihood ratio test). The correlation between P-value and sample size was Pearson's r = 0.45 (P-value for correlation = 0.13).
3.2. Detailed analysis
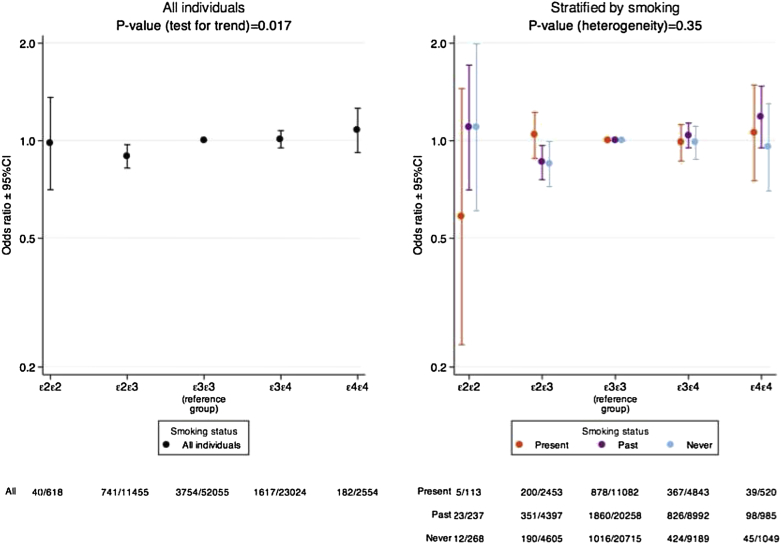
When we investigated the association between APOE genotype status and CHD regardless of smoking status, we found strong evidence for a linear association between APOE genotype and CHD status when individuals were arranged in the following order: ε2/ε2, ε2/ε3, ε3/ε3, ε3/ε4 or ε4/ε4 with the log odds of CHD increasing by 0.046 (SE 0.019; P = 0.017) for each incremental increase in APOE genotype status (Fig. 3).
Fig. 3.
Detailed association between APOE genotype and CHD overall (left) and stratified by smoking status (right).
When we stratified this analysis by smoking status into three groups (present, past or never smokers), and tested for evidence of heterogeneity in the association between APOE genotype and odds of CHD according to smoking status, we found no evidence to support effect modification (P for heterogeneity of slopes by smoking status = 0.35; Fig. 3 and Supplementary Table S3).
3.2.1. Analysis of pack years in two large prospective cohorts with MI outcomes
In 59,349 and 8828 individuals in CGPS and CCHS, respectively, when an interaction term was fitted between APOE genotype (ε4 allele carriers vs. non-carriers) and pack years, no evidence for an interaction for risk of MI was identified (P-values for interaction = 0.54 and 0.65 for CGPS and CCHS, respectively) (Supplementary Tables S4 and S5). This finding did not alter with subsequent adjustment for age, gender, hypertension or T2D (Supplementary Tables S6 and S7).
4. Discussion
This study represents the largest investigation to date into the role of smoking in the association between APOE genotype status and risk of CHD. We conducted a systematic review and supplemented identified studies with unpublished and updated data, and found that the association between APOE genotype and CHD was not meaningfully different when stratified by smoking status. Our interpretation is that there is no evidence to support the hypothesis that smoking is an effect modifier of the association between APOE genotype and CHD events.
In clinical trials, the standard method of testing effect modification is to perform analyses stratified by the potential modifier [25], and investigate for differences between the treatment effects amongst strata. Owing to the random allocation of alleles at gametogenesis, genetic studies share many of the features of randomized trials [26], thus a similar strategy can be used for investigating gene–environment interactions. This can be conducted by comparing summary estimates from meta-analysis stratified by the potential effect modifier, or by re-constructing individual participant data (from summary tabulated data, as we did in this analysis) and conducting a likelihood ratio test with and without an interaction term fitted between the exposure (in this case APOE genotype) and potential modifier (smoking status). A large P-value on likelihood ratio test (as identified in our analysis) makes the presence of effect modification unlikely.
This study adds to the growing list of examples of initial reports of effect modification in individual small studies that were subsequently refuted by large-scale evidence [27], a form of “winner's curse” [28]. Spurious effect modification is a concern when sample sizes are small [29]. In our analysis, we had over 10,000 CHD cases in over 130,000 individuals, meaning that the absence of evidence for heterogeneity in the estimates of APOE genotype with odds of CHD when stratified by smoking status indicates that a clinically relevant difference between smoking subgroups is very unlikely. Although it is tempting to interpret the estimate for CHD seen in non-smoking ε4 carriers as suggestive that this subgroup does not have an elevated risk of CHD compared to non-ε4 carriers (since the 95%CI includes the null), this is likely due to reduced power within strata of subgroups, and the salient feature is the lack of evidence for interaction between APOE genotype and smoking status.
It is curious that we did not replicate the presence of effect modification for two previous studies included in our analysis. This could be accounted for by updating one study (NPHSII) [6] for incident CHD events and in the other [9], effect modification was stronger in women than men. Furthermore, lack of access to individual participant data, and alternative analytical strategies in previous studies such as use of never smokers as the baseline group and comparing present and past smokers stratified by APOE genotype to this baseline, could also account for the discrepancy. It is noteworthy that both individual studies were relatively small and that the largest single study in this analysis (with a sample size over 6-fold greater than the combined sample size of the two previous studies with presence of effect modification) had the largest P-value for interaction.
There are several limitations to this study. Firstly, although we conducted a “quasi-individual participant data” analysis, we did not have access to individual participant data for all included studies, and therefore were not able to conduct multivariate analyses taking into account potential confounders such as age, gender and social status. However, we were able to conduct a detailed analysis in the large data sets that we had access to individual data. Second, we did not have markers of oxidative stress that we could use for a more detailed investigation into oxidative pathways as potential mediators of the association between APOE genotype and risk of CHD.
Our study also has several advantages. First, this is the largest analysis to date, incorporating new data from three very large prospective cohorts and five other studies. Second, using the data sets with refined data on APOE genotype status and in which outcome ascertainment was conducted using ICD codes to make our outcome specific to myocardial infarction, we were able to conduct a more detailed analysis by investigating evidence for a difference in the slope of the APOE–CHD relationship by smoking status. Third, we were able to examine a more detailed smoking phenotype (pack years) in two large prospective cohorts with a combined sample size of 68,177. Both the simple and detailed genetic and smoking analyses failed to identify evidence to support the hypothesis that smoking status acts as an effect modifier of the relationship between APOE genotype with CHD risk.
In conclusion, in the largest analysis to date including new data from large population-based cohorts, we identified no evidence to support the hypothesis that smoking status modifies the association between APOE genotype and risk of CHD. Regardless of these findings, as the leading cause of preventable disease and death in the world, smoking should be actively discouraged in all individuals.
Financial disclosure
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgements
MVH was supported by an MRC Population Health Scientist Fellowship (G0802432). FWA is supported by a clinical fellowship from the Netherlands Organisation for Health Research and Development (ZonMw grant 90700342). PH was supported by ESRC fellowship.
The EPIC-NL study was funded by ‘Europe against Cancer’ Programme of the European Commission (SANCO); the Dutch Ministry of Public Health, Welfare and Sports (formerly Ministry of Welfare, Public Health and Culture); the Dutch Cancer Society; ZonMW the Netherlands Organisation for Health Research and Development; and World Cancer Research Fund (WCRF). We thank GBA, Statistics Netherlands, and the institute PHARMO for providing data on vital status, cause of death and occurrence of cancer and other chronic diseases. The Copenhagen City Heart Study and The Copenhagen General Population Study were supported by The Danish Heart Foundation and The Danish Medical Research Council.
EPIC-Norfolk: We thank the participants and general practitioners who took part in EPIC-Norfolk. EPIC-Norfolk is supported by programme grants from the Cancer Research Campaign and Medical Research Council with additional support from the Stroke Association, British Heart Foundation, Department of Health, and the Wellcome Trust.
Genetic analyses in Czech post-MONICA are supported by Ministry of Health, Czech Republic – conceptual development of research organization (Institute for Clinical and Experimental Medicine – IKEM, IN 00023001).
The HAPIEE study was supported by the Wellcome Trust and the US National Institute of Health.
Footnotes
Supplementary material related to this article can be found at http://dx.doi.org/10.1016/j.atherosclerosis.2014.07.038.
Supplementary material
The following are the supplementary material related to this article:
References
- 1.Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahley R.W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 3.Angelopoulos T.J., Lowndes J. ApoE genotype: impact on health, fitness and nutrition. World Rev Nutr Diet. 2008;98:77–93. doi: 10.1159/000152923. [DOI] [PubMed] [Google Scholar]
- 4.Bennet A.M., Di Angelantonio E., Ye Z., Wensley F., Dahlin A., Ahlbom A. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298(11):1300–1311. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 5.Strittmatter W.J., Bova Hill C. Molecular biology of apolipoprotein E. Curr Opin Lipidol. 2002;13(2):119–123. doi: 10.1097/00041433-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Humphries S.E., Talmud P.J., Hawe E., Bolla M., Day I.N., Miller G.J. Apolipoprotein E4 and coronary heart disease in middle-aged men who smoke: a prospective study. Lancet. 2001;358(9276):115–119. doi: 10.1016/S0140-6736(01)05330-2. [DOI] [PubMed] [Google Scholar]
- 7.Keavney B., Parish S., Palmer A., Clark S., Youngman L., Danesh J. Large-scale evidence that the cardiotoxicity of smoking is not significantly modified by the apolipoprotein E 2/3/4 genotype. Lancet. 2003;361(9355):396–398. doi: 10.1016/S0140-6736(03)12386-0. [DOI] [PubMed] [Google Scholar]
- 8.Grammer T.B., Hoffmann M.M., Scharnagl H., Kleber M.E., Silbernagel G., Pilz S. Smoking, apolipoprotein E genotypes, and mortality (the Ludwigshafen RIsk and Cardiovascular Health study) Eur Heart J. 2013;34(17):1298–1305. doi: 10.1093/eurheartj/eht001. [DOI] [PubMed] [Google Scholar]
- 9.Gustavsson J., Mehlig K., Leander K., Strandhagen E., Bjorck L., Thelle D.S. Interaction of apolipoprotein E genotype with smoking and physical inactivity on coronary heart disease risk in men and women. Atherosclerosis. 2012;220(2):486–492. doi: 10.1016/j.atherosclerosis.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Jolivalt C., Leininger-Muller B., Bertrand P., Herber R., Christen Y., Siest G. Differential oxidation of apolipoprotein E isoforms and interaction with phospholipids. Free Radic Biol Med. 2000;28(1):129–140. doi: 10.1016/s0891-5849(99)00232-4. [DOI] [PubMed] [Google Scholar]
- 11.Myung S.K., Ju W., Cho B., Oh S.W., Park S.M., Koo B.K. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;346:f10. doi: 10.1136/bmj.f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meade T.W., Wilkes H.C., Stirling Y., Brennan P.J., Kelleher C., Browne W. Randomized controlled trial of low dose warfarin in the primary prevention of ischaemic heart disease in men at high risk: design and pilot study. Eur Heart J. 1988;9(8):836–843. doi: 10.1093/oxfordjournals.eurheartj.a062576. [DOI] [PubMed] [Google Scholar]
- 14.Beulens J.W., Monninkhof E.M., Verschuren W.M., van der Schouw Y.T., Smit J., Ocke M.C. Cohort profile: the EPIC-NL study. Int J Epidemiol. 2010;39(5):1170–1178. doi: 10.1093/ije/dyp217. [DOI] [PubMed] [Google Scholar]
- 15.Netuveli G., Wiggins R.D., Hildon Z., Montgomery S.M., Blane D. Quality of life at older ages: evidence from the English longitudinal study of aging (wave 1) J Epidemiol Commun Health. 2006;60(4):357–363. doi: 10.1136/jech.2005.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordestgaard B.G., Agerholm-Larsen B., Wittrup H.H., Tybjaerg-Hansen A. A prospective cardiovascular population study used in genetic epidemiology. The Copenhagen City Heart Study. Scand J Clin Lab Invest Suppl. 1996;226:65–71. [PubMed] [Google Scholar]
- 17.Nordestgaard B.G., Benn M., Schnohr P., Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 18.Cifkova R., Skodova Z., Bruthans J., Adamkova V., Jozifova M., Galovcova M. Longitudinal trends in major cardiovascular risk factors in the Czech population between 1985 and 2007/8. Czech MONICA and Czech post-MONICA. Atherosclerosis. 2010;211(2):676–681. doi: 10.1016/j.atherosclerosis.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Day N., Oakes S., Luben R., Khaw K.T., Bingham S., Welch A. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer. 1999;80(Suppl. 1):95–103. [PubMed] [Google Scholar]
- 20.Peasey A., Bobak M., Kubinova R., Malyutina S., Pajak A., Tamosiunas A. Determinants of cardiovascular disease and other non-communicable diseases in Central and Eastern Europe: rationale and design of the HAPIEE study. BMC Public Health. 2006;6:255. doi: 10.1186/1471-2458-6-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lussier-Cacan S., Bolduc A., Xhignesse M., Niyonsenga T., Sing C.F. Impact of alcohol intake on measures of lipid metabolism depends on context defined by gender, body mass index, cigarette smoking, and apolipoprotein E genotype. Arteriosclerosis Thromb Vascu Biol. 2002;22(5):824–831. doi: 10.1161/01.atv.0000014589.22121.6c. [DOI] [PubMed] [Google Scholar]
- 22.Chen X.H., Rodriguez S., Hawe E., Talmud P.J., Miller G.J., Underhill P. Evidence of admixture from haplotyping in an epidemiological study of UK Caucasian males: implications for association analyses. Human Heredity. 2004;57(3):142–155. doi: 10.1159/000079245. [DOI] [PubMed] [Google Scholar]
- 23.Liu S., Ma J., Ridker P.M., Breslow J.L., Stampfer M.J. A prospective study of the association between APOE genotype and the risk of myocardial infarction among apparently healthy men. Atherosclerosis. 2003;166(2):323–329. doi: 10.1016/s0021-9150(02)00335-0. [DOI] [PubMed] [Google Scholar]
- 24.Talmud P.J., Lewis S.J., Hawe E., Martin S., Acharya J., Marmot M.G. No APOE4 effect on coronary heart disease risk in a cohort with low smoking prevalence: The Whitehall II study. Atherosclerosis. 2004;177(1):105–112. doi: 10.1016/j.atherosclerosis.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Yusuf S., Wittes J., Probstfield J., Tyroler H.A. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA. 1991;266(1):93–98. [PubMed] [Google Scholar]
- 26.Hingorani A., Humphries S. Nature's randomised trials. Lancet. 2005;366(9501):1906–1908. doi: 10.1016/S0140-6736(05)67767-7. [DOI] [PubMed] [Google Scholar]
- 27.Sleight P. Debate: subgroup analyses in clinical trials: fun to look at - but don't believe them! Curr Control Trials Cardiovasc Med. 2000;1(1):25–27. doi: 10.1186/cvm-1-1-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young N.S., Ioannidis J.P., Al-Ubaydli O. Why current publication practices may distort science. PLoS Med. 2008;5(10):e201. doi: 10.1371/journal.pmed.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willke R.J., Zheng Z., Subedi P., Althin R., Mullins C.D. From concepts, theory, and evidence of heterogeneity of treatment effects to methodological approaches: a primer. BMC Med Res Methodol. 2012;12:185. doi: 10.1186/1471-2288-12-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thrombosis prevention trial randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council's General Practice Research Framework. Lancet. 1998;351(9098):233–241. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.