Abstract
Purpose
To identify non-invasive clinical parameters to predict urodynamic bladder outlet obstruction (BOO) in patients with benign prostatic hyperplasia (BPH) using causal Bayesian networks (CBN).
Subjects and Methods
From October 2004 to August 2013, 1,381 eligible BPH patients with complete data were selected for analysis. The following clinical variables were considered: age, total prostate volume (TPV), transition zone volume (TZV), prostate specific antigen (PSA), maximum flow rate (Qmax), and post-void residual volume (PVR) on uroflowmetry, and International Prostate Symptom Score (IPSS). Among these variables, the independent predictors of BOO were selected using the CBN model. The predictive performance of the CBN model using the selected variables was verified through a logistic regression (LR) model with the same dataset.
Results
Mean age, TPV, and IPSS were 6.2 (±7.3, SD) years, 48.5 (±25.9) ml, and 17.9 (±7.9), respectively. The mean BOO index was 35.1 (±25.2) and 477 patients (34.5%) had urodynamic BOO (BOO index ≥40). By using the CBN model, we identified TPV, Qmax, and PVR as independent predictors of BOO. With these three variables, the BOO prediction accuracy was 73.5%. The LR model showed a similar accuracy (77.0%). However, the area under the receiver operating characteristic curve of the CBN model was statistically smaller than that of the LR model (0.772 vs. 0.798, p = 0.020).
Conclusions
Our study demonstrated that TPV, Qmax, and PVR are independent predictors of urodynamic BOO.
Introduction
Urodynamic study (UDS) is considered the gold standard for clinical assessment of bladder outlet obstruction (BOO) in patients with benign prostatic hyperplasia (BPH) [1]. Patients with urodynamic BOO show higher efficacy after transurethral surgery [2]. In this respect, BOO is helpful in stratifying BPH patients eligible for surgical treatment. However, UDS has significant limitations in terms of invasiveness, cost, and morbidity [3].
Numerous attempts have been made to substitute non-invasive clinical parameters for UDS to predict BOO; however, individual variables, including symptom score [4], prostate specific antigen (PSA) level [5], free uroflowmetry (UFM) [6], post-void residual (PVR) urine volume [7], and prostate size [8], have shown a poor to weak correlation with BOO.
To improve prediction ability, combinations of non-invasive clinical parameters have been investigated to predict BOO [9]–[17]. The statistical methods used for combinations were diverse from the cumulative scoring system [9], to the construction of a formula by logistic regression analysis [10]–[13], to the artificial neural network (ANN) models [14]–[17]. However, these attempts had limited predictive performance. Moreover, the need to use numerous clinical parameters makes clinical application difficult. Furthermore, some predictive models [14]–[17] could not explain which variables are comparatively important for BOO owing to their ‘black box’ nature [18].
Causal Bayesian networks (CBN) have emerged as an advanced alternative to conventional statistical models in the medical field [19]. The benefit of this model is that it can visualize the interaction of causes and rule out indirect causes of events [20]. Hence, we aimed to identify non-invasive clinical parameters to predict BOO using a CBN model. To the best of our knowledge, this study is the first to test CBN model for BOO prediction.
Materials and Methods
I. Data collection
The Institutional Review Board of Seoul National University Hospital (SNUH) approved the protocol of this study. A database of 2,492 patients that were older than 45 and that had lower urinary tract symptoms (LUTS) was created from records dated between October 2004 and August 2013. The data were retrieved from the urodynamic database registry and Electronic Medical Records System of SNUH. All information was anonymised and de-identified prior to analysis. Patients with a history of previous genitourinary surgery, pelvic radiation therapy, urinary tract infection, urethral stricture, interstitial cystitis, and neuropathy suggesting neurogenic bladder or incomplete evaluations were excluded. Thus, after excluding 1,111 such patients (44.6%), the data from 1,381 patients were analyzed.
Clinical parameters of the subjects, including history, physical examination, International Prostatic Symptom Score (IPSS) [21], UFM, PVR, PSA, prostate volume (PV) measured by transrectal ultrasonography, and UDS results were retrieved. UFM (Flowmaster, Medical Measurement System, Enschede, Netherlands) results were obtained as free flow, whenever voided volume was less than 120 ml, and fails were repeated. PVR was measured after UFM using an ultrasound bladder scanner (BladderScan BVI 3000, Verathon Inc., WA, USA). All UDS were performed using a multichannel video system (UD-2000, Medical Measurement System) according to International Continence Society (ICS) recommendations [22]. The BOO index, which is equal to detrusor pressure at maximal flow rate (PdetQmax)−2×maximal flow rate (Qmax), was used to determine BOO [23]. Patients with BOO Index ≥40 were considered as obstructed.
II. Database characteristics
The patient demographics characteristics are shown in Table 1. The mean age of patients was 66.2 (±7.3, SD) years. The TPV and PSA were 48.5 (±25.9) ml and 2.71 (±3.53) ng/ml, respectively. The IPSS-total, IPSS-storage, IPSS-emptying, and IPSS-QoL were 17.9 (±7.9), 7.1 (±3.5), 10.8 (±5.5), and 3.9 (±1.2), respectively. The Mean BOO index was 35.1 (±25.2), and 477 patients (34.5%) were classified as having BOO.
Table 1. Characteristics of 1,381 patients.
| Total subjects (N = 1381) | |
| Age (years) | 66.2±7.3 |
| Prostate volume (ml) | |
| Total prostate volume | 48.5±25.9 |
| Transitional zone volume | 24.1±22.4 |
| Prostate specific antigen (ng/ml) | 2.71±3.53 |
| International Prostatic Symptom Score (IPSS) | |
| IPSS-total | 17.9±7.9 |
| IPSS-storage | 7.1±3.5 |
| IPSS-emptying | 10.8±5.5 |
| IPSS-quality of life | 3.9±1.2 |
| Uroflowmetry parameters | |
| Maximum flow rate (ml/sec) | 11.6±4.9 |
| Post-void residual volume (ml) | 58.1±77.8 |
| Urodynamic study parameters | |
| Maximal urethral closure pressure (cmH2O) | 74.3±26.8 |
| Functional urethral length (mm) | 72.0±20.6 |
| First desire (ml) | 203.0±90.1 |
| Normal desire (ml) | 284.9±108.2 |
| Strong desire (ml) | 371.7±108.3 |
| Compliance (ml/cmH2O) | 67.3±50.8 |
| PdetQmax (cmH2O) | 52.6±21.7 |
| Opening pressure (cmH2O) | 54.3±25.8 |
| Bladder outlet obstruction index | 35.1±25.2 |
PdetQmax, detrusor pressure at maximum flow rate.
III. Statistical methods for BOO prediction
To predict BOO, the following two statistical methods were applied.
Logistic regression (LR) analysis. A backward stepwise regression analysis [24] was utilized. Age, total prostate volume (TPV), transition zone volume (TZV), PSA, Qmax, PVR and IPSS were entered into LR model as variables for BOO prediction. Relative risk (Exp(β)) of BOO was calculated, with each non-invasive parameter increasing by one unit.
Causal Bayesian networks (CBN). If event A causes events B and C, and these events directly influence event D, the probability of event D depends on each of the possible values of events B and C. The probability of event D can be expressed in the equation, P (event D| event B, event C). In that case, events A and D are in the causal Markov condition [25]. This means that event A is not a direct cause of event D. If the probabilities of direct causes (events B and C) are conditioned, event A does not influence the probability of event D. The causal Markov condition can be visually identified in a CBN, which has a relationship of two or more degrees between nodes. Considering that events A and D have a two degree relationship, we can easily infer that these two events are conditionally independent.
The causal Markov condition permits the joint distribution of the n variables in a CBN to be factored as in the following equation:
where xi denotes a state of variable Xi, πi denotes a joint state of the parents of Xi, and K denotes background knowledge [20].
IV. Identification and verification of the independent parameters
CBN was applied to identify the independent predictors of BOO. The causal relationships and their interactions were visualized by established CBN. The variables only directly linked to BOO were selected as the independent predictors. The weights of each selected variable were estimated using the Spearman's correlation test. The accuracy of BOO prediction model using the selected variables was compared with that of the LR model. To compare the predictive performance, the comparison of receiver operating characteristic (ROC) curves by DeLong et al. [26] was applied.
A p-value <0.05 was considered significant. A CBN model to predict BOO was established using the Banjo version 2.2.0 software (Duke University, Durham, NC, USA; non-commercially available at: http://www.cs.duke.edu/~amink/software/banjo/). Highlights of the settings are limiting the number of parents to five and running the analysis for up to 6 hours (the Banjo setup file is presented in Appendix S1). The commercial statistical program package SPSS version 18.0 (Chicago, IL, USA) was used for LR, Spearman correlation, and other descriptive statistical analyses. MedCalc version 12.4.0 (Ostend, Belgium) was applied for the comparison of ROC curves.
Results
Identification of non-invasive BOO predictors using CBN
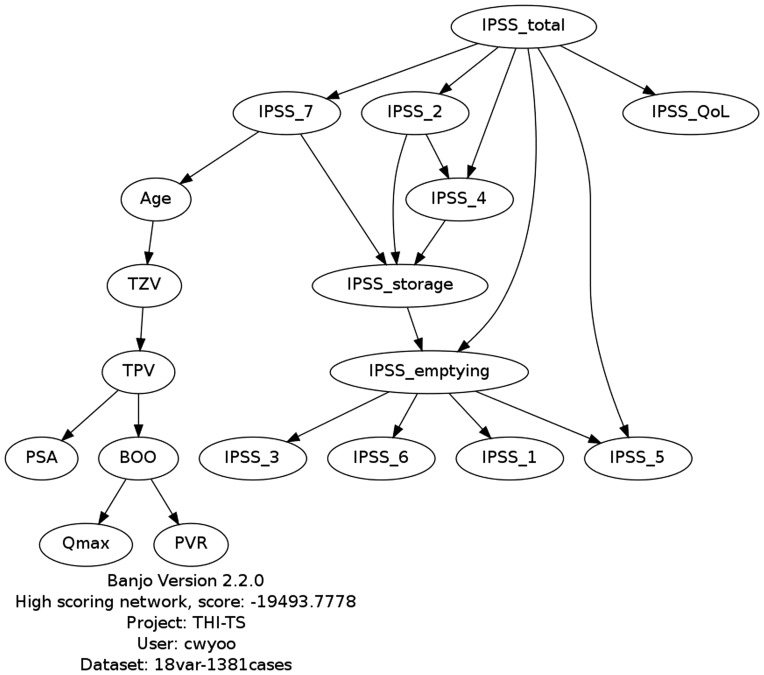
Based on the BPH patient data, the best network structure was selected/learned using the CBN model (Fig. 1). TPV, Qmax, and PVR exhibited direct relationships with BOO. Therefore, those three variables were selected as non-invasive independent predictors of BOO. The correlation coefficient was the highest for TPV (R = 0.391 p<0.001), followed by Qmax (R = −0.253, p<0.001) and PVR (R = 0.214, p<0.001).
Figure 1. Causal Bayesian network model for bladder outlet obstruction.
TPV, total prostate volume; TZV, transitional zone volume; PSA, prostatic specific antigen; BOO, bladder outlet obstruction; Qmax, maximum flow rate; PVR, post-void residual volume; IPSS, International Prostate Symptom Score.
Verification of BOO prediction
Sensitivity, specificity, and accuracy of BOO predictions with the aforementioned three variables by CBN were 51.4%, 85.2%, and 73.5%, respectively (Table 2). In the LR model, age (Exp(β) = 0.981, p = 0.046), Qmax (Exp(β) = 0.890, p<0.001), PVR (Exp(β) = 1.003, p<0.001), TPV (Exp(β) = 1.014, p = 0.049), TZV (Exp(β) = 1.039, p<0.001), PSA (Exp(β) = 1.051, p = 0.039) IPSS item 2 (frequency) (Exp(β) = 0.866, p = 0.007), and IPSS item 4 (Urgency) (Exp(β) = 1.227, p<0.001) were selected as significant predictive variables. In the LR model, the sensitivity, specificity, and accuracy were 51.6%, 90.4%, and 77.0%, respectively.
Table 2. Predictive value of two predictive models for bladder outlet obstruction.
| Predicted BOO | Urodynamic BOO | Total | Sensitivity | Specificity | Accuracy | |||
| (+) | (−) | |||||||
| LR model | Total (N = 1381) | (+) | 246 | 87 | 333 | 246/477 (51.6%) | 817/904 (90.4%) | (246+817)/1381 (77.0%) |
| (−) | 231 | 817 | 1048 | |||||
| Total | 477 | 904 | 1381 | |||||
| CBN model | Total (N = 1381) | (+) | 245 | 134 | 379 | 245/477 (51.4%) | 770/904 (85.2%) | (245+770)/1381 (73.5%) |
| (−) | 232 | 770 | 1002 | |||||
| Total | 477 | 904 | 1381 | |||||
LR, logistic regression; ANN, artificial neural networks; CBN, causal Bayesian networks; BOO, bladder outlet obstruction.
To compare the predictive power of the three selected non-invasive clinical parameters, a comparison of ROC curves was performed. The area under ROC curve (AUROC) of CBN and the LR models were 0.772 and 0.798, respectively (p = 0.020; figures not presented).
Discussion
Because individual variables have a very low correlation with BOO, many researchers have built statistical prediction methods that combine multiple variables [9]–[13]. For this purpose, they have used diverse variables, including Qmax, PVR, IPSS, PSA, and PV. However, no one has established a specific independent predictor of BOO [9]–[13]. Some differences in detailed variables have been suggested for prediction models. Moreover, the number of variables used in these predictions is too many to be feasible for real-life practice with BPH patients.
Previous studies seeking to identify non-invasive predictors of BOO have encountered two major difficulties. The first is the non-linear relationship between the variables. Among the single non-processed variables, prostate size seems to be one of the most highly correlating variables with BOO (R range: 0.28–0.32, p<0.001) [8], [27]. However, Eckhardt et al. [27] have found that mean TPV decreased at the Schäfer grade of 5 and 6, contrary to general expectations. These non-linear conditions occur commonly in clinical medicine. The second difficulty stems from the fact that some clinical parameters have a co-variability, fiu., some clinical parameters interact with each other [19], so that the established model is capable of overestimating or underestimating the predictive power. Bell et al. [28] reported that increased PVR occurs in BOO patients. However, Eckhard et al. [27] pointed out that larger PVR may reflect detrusor underactivity rather than BOO. Yet, Kranse et al. [7] supported the findings that BOO and detrusor underactivity commonly cause a higher PVR.
ANN models are expected to be able to detect non-linear relationships and interactions between predictor variables. Sonke et al. [14] proposed the first ANN model for BOO prediction using 1903 patients. IPSS, Qmax, PVR, TPV, and PSA were used as the input variables. They reported that overall sensitivity and specificity were 71% and 69%. Wadie et al. [15] reported the higher predictive power of ANN compared to conventional statistical models among 460 subjects using only IPSS. However, the same group presented another ANN model considering average flow rate and Qmax on UFM, PVR, and TPV in variable conditions and showing only moderate performance with 76% accuracy [17]. Another study reported 82% and 77% sensitivity and specificity, respectively, using IPSS, TPV, PSA, and UFM results [16]. Comprehensive results show, however, that the predictive performance of ANN is not superior to that of the conventional linear models. Moreover, due to the ‘black box’ nature of ANN, the model cannot be easily interpreted [18]. Therefore, these models do not explain the relative contribution of non-invasive clinical parameters to urodynamic BOO.
In general, the advantage of CBNs is that they can identify conditional independence relationships and thus make it possible to confirm the only direct independent causes of the events. We expected that this advantage of the CBN model could confirm independent variables for the prediction of BOO. In this study, through the established CBN model, we found that TPV, Qmax, and PVR were important predictors of BOO (Fig. 1). Our BOO prediction model with only three independent variables (TPV, Qmax, and PVR) showed moderate predictive value (Table 2)
To compare the performance of the BOO prediction model using the three selected independent predictors, the LR model was established from the same dataset and the predictive powers of the two models were compared (Table 2). The LR model showed a predictive performance comparable with that reported in previous studies [10]–[13]. The predictive performance of the CBN model was statistically inferior to that of the LR model (AUROC: 0.772 vs. 0.798; p = 0.020). However, when only the three variables (TPV, Qmax, and PVR) were taken into account to predict BOO, the accuracy was not overly compromised compared with when using the complex equations (considering age, Qmax, PVR, TPV, TZV, PSA, IPSS item 2, and item 4 as predictive variables), which were derived from the LR model. These three non-invasive clinical parameters are also routinely evaluated in actual clinical practice for BPH patients.
Indeed, our CBN model comprised categorized values of clinical parameters due to in nature characteristics of CBN model for clarifying interactions between the variables. It is not clear whether the lower performance of CBN originates from the elliptical non-invasive clinical parameters or from transforming the clinical parameters into categorical variables to estimate the CBN models. However, it is interesting that the BOO can be predicted moderately with only three non-invasive clinical parameters. This study was unable to conclude whether the other variables that show conditional independence can be excluded for BOO prediction.
Our data showed that TPV was the most important predictive factor for BOO (R = 0.391), followed by, Qmax (R = −0.253), and PVR (0.214) in that order. Our results are consistent with those of previous studies which reported that TPV had a higher correlation with BOO compared to the other non-invasive clinical parameters [10]–[13]. These results suggest that TPV is the most important clinical parameter for BOO prediction in real clinical practice and that TZV and PSA do not need to be considered as predictors.
Qmax and PVR also had a moderate correlation with BOO(|R| range: 0.214–0.253). CBN showed that these variables are independent predictors of BOO. Therefore, these clinical parameters should be considered in BOO prediction. Previous studies considered various combinations of UFM results, such as Qmax, average flow rate (Qavg), and PVR in prediction models [9]–[13], but it has not yet been concluded which variables are the more important predictors of BOO. Our CBN model showed that Qmax and PVR are important for BOO prediction.
It is interesting that all IPSS items showed conditional independence (not independent predictors) for BOO prediction (Fig. 1). Previous studies excluded the IPSS from the BOO prediction model [10]–[13], and van Venrooij et al. [4] reported that IPSS has no statistical correlation with urodynamic obstruction grade; these are in agreement with our CBN results. However, in our LR model IPSS item 2 (frequency) and IPSS item 4 (urgency) were predictors of BOO (p range: <0.001 to 0.007).
The strength of this study is that we made our non-missing dataset of 1,381 patients large enough to support the construction of the CBN model. Moreover, in our study, all of the UDS were performed uniformly using the same protocol following ICS recommendations [22]. However, our current study has some limitations. Our model was unable to account for the weight of each independent predictor. Therefore, the relative importance of predictors should be identified by means of indirect correlation analysis. Second, our CBN model is built from a cross-sectional database; hence, in a strict sense, our model did not show cause-effect relationships between parameters, but showed simple correlations or interactions. It is thus impossible to confirm variables that precede the cause. Finally, the predictive power of CBN model was too low for the model to be considered to be useful in clinical practice. We believe that additional well-designed and in-depth researches into the CBN model are needed.
Conclusions
Our results show that TPV, Qmax, and PVR are independent non-invasive predictors of BOO. Among them, TPV is the most important clinical parameter for predict of BOO.
Supporting Information
Banjo setting file used for causal Bayesian network model of this study.
(TXT)
Acknowledgments
Yu-Kyung Lee assisted with database management.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data contains identifying human information that is unsuitable for public deposition. Requests for the data may be sent to the corresponding author.
Funding Statement
This study was supported by grant No. 04-2010-1080 from the Seoul National University Hospital Research Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Abrams P (1995) Objective evaluation of bladder outlet obstruction. Br J Urol 76: 11–15. [PubMed] [Google Scholar]
- 2. Min DS, Cho HJ, Kang JY, Yoo TK, Cho JM (2013) Effect of transurethral resection of the prostate based on the degree of obstruction seen in urodynamic study. Korean J Urol 54: 840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Porru D, Madeddu G, Campus G, Montisci I, Scarpa RM, et al. (1999) Evaluation of morbidity of multi-channel pressure-flow studies. Neurourol Urodyn 18 6: 647–652. [DOI] [PubMed] [Google Scholar]
- 4. van Venrooij GEPM, Boon TA, de Gier RPE (1995) International prostate symptom score and quality of life assessment versus urodynamic parameters in men with benign prostatic hyperplasia symptoms. J Urol 153 5: 1516–1519. [DOI] [PubMed] [Google Scholar]
- 5. Laniado ME, Ockrim JL, Marronaro A, Tubaro A, Carter SS (2004) Serum prostate-specific antigen to predict the presence of bladder outlet obstruction in men with urinary symptoms. BJU Int 94 9: 1283–1286. [DOI] [PubMed] [Google Scholar]
- 6. Jensen KM, Jørgensen JB, Mogensen P (1988) Urodynamics in prostatism. I. Prognostic value of uroflowmetry. Scand J Urol Nephrol Suppl 114: 63–71. [PubMed] [Google Scholar]
- 7. Kranse R, van Mastrigt R (2003) Weak correlation between bladder outlet obstruction and probability to void to completion. Urology 62 4: 667–671. [DOI] [PubMed] [Google Scholar]
- 8. Rosier PFWM, Rosette JJMCH (1995) Is there a correlation between prostate size and bladder outlet obstruction? World J Urol 13 1: 9–13. [DOI] [PubMed] [Google Scholar]
- 9. Rosier PFWM, de Wildt MJAM, Wijkstra H, Debruyne FFMJ, de la Rosette JJMCH (1996) Clinical diagnosis of bladder outlet obstruction in patients with benign prostatic enlargement and lower urinary tract symptoms: development and urodynamic validation of a clinical prostate score for the objective diagnosis of bladder outlet obstruction. J Urol 155 5: 1649–1654. [PubMed] [Google Scholar]
- 10. Madersbacher S, Klingler HC, Djavan B, Stulnig T, Schatzl G, et al. (1997) Is obstruction predictable by clinical evaluation in patients with lower urinary tract symptoms? Br J Urol 80 1: 72–77. [DOI] [PubMed] [Google Scholar]
- 11. Ockrim JL, Laniado ME, Patel A, Tubaro A, St Clair Carter S (2001) A probability based system for combining simple office parameters as a predictor of bladder outflow obstruction. J Urol 166 6: 2221–2225. [PubMed] [Google Scholar]
- 12. van Venrooij GEPM, Boon TA (1996) The value of symptom score, quality of life score, maximal urinary flow rate, residual volume and prostate size for the diagnosis of obstructive benign prostatic hyperplasia: A urodynamic analysis. J Urol 155 6: 2014–2018. [DOI] [PubMed] [Google Scholar]
- 13. van Venrooij GEPM, Eckhardt MD, Boon TA (2004) Noninvasive assessment of prostatic obstruction in elderly men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Urology 63 3: 476–480. [DOI] [PubMed] [Google Scholar]
- 14. Sonke GS, Heskes T, Verbeek AL, de la Rosette JJ, Kiemeney LA (2000) Prediction of bladder outlet obstruction in men with lower urinary tract symptoms using artificial neural networks. J Urol 163 1: 300–305. [PubMed] [Google Scholar]
- 15. Wadie BS, Badawi AM, Ghoneim MA (2001) The relationship of the international prostate symptom score and objective parameters for diagnosing bladder outlet obstruction. Part II: The potential usefulness of artificial neural networks. J Urol 165 1: 35–37. [DOI] [PubMed] [Google Scholar]
- 16. Djavan B, Fong YK, Harik M, Milani S, Reissigl A, et al. (2004) Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology 64 6: 1144–1148. [DOI] [PubMed] [Google Scholar]
- 17. Wadie BS, Badawi AM, Abdelwahed M, Elemabay SM (2006) Application of artificial neural network in prediction of bladder outlet obstruction: A model based on objective, noninvasive parameters. Urology 68 6: 1211–1214. [DOI] [PubMed] [Google Scholar]
- 18. Tu JV (1996) Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes. J Clin Epidemiol 49 11: 1225–1231. [DOI] [PubMed] [Google Scholar]
- 19. Nikovski D (2000) Constructing Bayesian networks for medical diagnosis from incomplete and partially correct statistics. IEEE Trans Neural Netw Learn Syst 12 4: 509–516. [Google Scholar]
- 20.Pearl J (1988) Bayesian Inference. In: Brachman RJ, editor. Probabilistic reasoning in intelligent systems: networks of plausible inference. San Francisco (CA): Morgan Kaufmann Publisher. pp. 29–75. [Google Scholar]
- 21. Choi HR, Chung WS, Shim BS, Kwon SW, Hong SJ, et al. (1996) Translation validity and reliability of I-PSS Korean version. Korean J Urol 37 6: 659–665. [Google Scholar]
- 22. Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, et al. (2002) Good urodynamic practices: Uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 21 3: 261–274. [DOI] [PubMed] [Google Scholar]
- 23. Griffiths D, Hofner K, Mastrigt RV, Rollema HJ, Spangberg A, et al. (1997) Standardization of terminology of lower urinary tract function: pressure-flow studies of voiding, urethral resistance, and urethral obstruction. Neurourol Urodyn 16 1: 1–18. [DOI] [PubMed] [Google Scholar]
- 24. Derksen S, Keselman HJ (1992) Backward, forward and stepwise automated subset selection algorithms: Frequency of obtaining authentic and noise variables. Br J Math Psychol 45 2: 265–282. [Google Scholar]
- 25.Spirtes P, Glymour C, Scheines R (2000) Direct vs. indirect causation. In: Causation, prediction, and search 2nd Ed. Cambridge (MA): MIT Press. pp.42–47. [Google Scholar]
- 26. DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44: 837–845. [PubMed] [Google Scholar]
- 27. Eckhardt MD, Venrooij GEPM, Boon TA (2001) Interactions between prostate volume, filling cystometric estimated parameters, and data from pressure-flow studies in 565 men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Neurourol Urodyn 20 5: 579–590. [DOI] [PubMed] [Google Scholar]
- 28. Ball AJ, Feneley RCL, Abrams PH (1981) The Natural History of Untreated “Prostatism”. Br J Urol 53 6: 613–616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Banjo setting file used for causal Bayesian network model of this study.
(TXT)
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data contains identifying human information that is unsuitable for public deposition. Requests for the data may be sent to the corresponding author.