Abstract
The pathogenic mechanisms by which physical exercise influences atherosclerotic lesion formation remain poorly understood. Because vigorous physical training increases oxidative stress, this study tested the hypothesis that graduated and moderate physical exercise together with metabolic intervention (l-arginine and antioxidants) may contribute to increased vascular protection. Exercise training in mice was induced by graduated swimming. In hypercholesterolemic male mice on an atherogenic high-cholesterol diet, graduated and moderate exercise lowered plasma cholesterol and decreased atherosclerotic lesions compared with sedentary control mice. Antioxidants (1.0% vitamin E added to the chow and 0.05% vitamin C added to the drinking water) and l-arginine (6% in drinking water) supplementation to exercising hypercholesterolemic mice further and synergistically reduced atherosclerosis compared with untreated exercised mice. Arterial oxidation-specific epitopes and systemic oxidative stress were reduced by metabolic intervention. Graduated chronic exercise elicited an increase in production of nitric oxide through increased endothelial nitric oxide synthase expression and ameliorated scavenger activities. Thus, metabolic intervention with l-arginine and antioxidants together with graduated and moderate exercise training reduce atherosclerotic lesion formation.
Keywords: catalase, nitric oxide synthase, vitamin E, oxidative stress, low-density lipoprotein
Physical exercise is a deterrent of cardiovascular disease and atherogenesis in animal models (1–5). Exercise can also positively influence classical risk factors that are associated with coronary heart disease such as diabetes, hypertension, obesity, dyslipidemias, and endothelial dysfunction (6–8). The higher respiration rate during severe physical exercise leads to the generation of more free radicals than the endogenous antioxidant systems can scavenge (9), whereas moderate intensity aerobic exercise enhances endothelium dependent vasodilation in humans (10), and attenuates exercise-induced peroxidation (11) and cardiovascular mortality (12) in the elderly. Moreover, there is an inverse association between intensity of physical activity and the risk of coronary heart disease events (13). A metaanalysis of 51 controlled trials evaluated the effects of exercise-based cardiac rehabilitation on cardiac events (14), demonstrating that total mortality decreases 27% and cardiac mortality decreases 31%. Similarly, exercise training improved effort tolerance in heart failure patients enrolled in 15 controlled trials (15).
There is a plethora of data demonstrating a pivotal role for oxidation-sensitive mechanisms in endothelial dysfunction and atherogenesis (16–23). Increased aerobic metabolism during massive exercise is a source of oxidative stress (9, 24, 25). However, progressive adaptations to exercise may decrease systemic oxidative stress, thereby improving vascular function (10–12). These protective mechanisms could include increased antioxidant defenses, reduced basal production of oxidants, and reduction of radical leak during oxidative phosphorylation (10–12, 24, 25). Physical training is also an effective treatment for patients with peripheral arterial atherosclerotic disease and claudication (26, 27). Paradoxically, exercise-induced oxidative stress could be potentially beneficial for vascular dysfunction and atherogenesis through the induction of arterial antioxidant responses. This induction of scavenger enzymes would not only minimize oxidative damage but also reduce in situ the generation of oxidants in different species, including humans (28–33). Exercise can also stimulate endothelial NO synthase (eNOS) and increase NO bioavailability, representing an important mechanism by which exercise conveys benefit in vasculoprotection (34, 35). Nevertheless, strenuous exercise may initiate an immune and vascular proinflammatory milieu (36). There is induction of catalase and eNOS in the aorta by vigorous exercise in hypercholesterolemic mice, and vitamin E supplementation in the exercised group does not appear to affect atherosclerosis (32). In contrast, several studies showed clear beneficial effects of vitamin E on lesion progression in hypercholesterolemic mice (37–40). Consistently, there is increased atherosclerosis in hypercholesterolemic mice deficient in α-tocopherol transfer protein and vitamin E (41). There is also vulnerable atherosclerotic plaque morphology in hypercholesterolemic mice that are unable to make vitamin C (42). These effects may explain why vitamins E and C can exert synergistic beneficial effects in preventing the development of atherosclerosis (43). Several small-scale studies have demonstrated that l-arginine augments endothelial function and improves exercise ability in cardiovascular patients by enhancing vasodilation and reducing monocyte adhesion (44). l-arginine normalizes aerobic capacity (45) and reduces expression of oxidation-sensitive genes in areas of disturbed shear stress (46) in hypercholesterolemic mice. The lack of endogenous NO is an important progression factor of atherosclerosis (47) and l-arginine (48) may reduce atherogenesis in hypercholesterolemic mice. Thus, the synergistic long-term effects of moderate physical exercise, vitamin E, vitamin C, and l-arginine may reduce atherogenesis. The present study provides the evidence for the reduction in atherosclerotic lesions by the combined effects of graduated swim training together with antioxidants (vitamins E and C) and l-arginine cotreatment.
Methods
Mice, Exercise Protocol, and Metabolic Treatment. The experiments conformed to the National Institutes of Health guidelines for the care of laboratory animals and were carried out on six groups of 8-week-old male low-density lipoprotein (LDL) receptor-deficient hypercholesterolemic mice (mean weight 42.4 ± 13.2 g). We selected only male mice to avoid gender-related differences (49, 50). The mice were randomized to one of three dietary interventions: (i) High-cholesterol diet alone (containing 21% by weight fat, 0.15% by weight cholesterol, and 19.5% by weight casein; Harlan Teklad, Madison, WI), as described (49–51); (ii) high-cholesterol diet plus antioxidant supplementation (1.0% vitamin E added to the chow and 0.05% vitamin C added to the drinking water); and (iii) high-cholesterol diet plus both antioxidants and l-arginine (6% in drinking water). We studied six groups of 12 mice each. After 2 weeks of hypercholesterolemic diet and metabolic intervention, we stabilized the mouse metabolic homeostasis by a 2-week warmup before starting graduated physical exercise. Then, in each dietary regimen, mice were assigned randomly to either a physical exercise protocol or to have no lifestyle modifications. Graduated physical exercise consisted of a progressive swimming program in a 1-m2 surface area tank containing tap water 50 cm in depth and maintained at 23°C according to a protocol originally proposed by Orenstein et al. (52). After preliminary pilot experiments (data not shown), to induce a graduated training, on the first day, the swimming time was 10 min in duration, and this time was then increased by 10 min each subsequent day (first week) until the maximum swim duration of 60 min was reached at the end of the second week. Mice were towel-dried after each training session. On the third week, mice swam for 60 min twice a day, 5 days/week. Swimming sessions were supervised and the number of mice per session (n = 12) was kept low to avoid “gang swimming.” Body weight and water and food intake were measured during the entire study. The study protocol lasted 18 weeks (15 of which were on the full training regimen, i.e., 60 min twice a day, 5 days/week).
Preparation of Arterial Samples, Western Blot Analysis, and Immunohistochemistry. Mice were killed by CO2 asphyxia, and then the blood was drawn into heparinized tubes from the inferior vena cava. The dissection of the aorta was performed under a stereomicroscope, as described in detail (46, 49–51). A 2-mm cross section was saved for immunohistochemical analysis, as described (46, 49–51). Tissue sections (5 μm) were incubated with the E06 antibody (a generous gift of W. Palinski and J. L. Witztum, University of California at San Diego, La Jolla) against oxidation specific epitopes (46, 50), or with F4/80, an antibody against mouse macrophage-derived foam cells (46, 50). The aorta was then cut longitudinally; one half was placed in 250 μl of protein extraction buffer, and the other half was saved in 1 ml of KH2PO4 buffer (46, 49–51) for further determination of eNOS protein expression (Western blot with rabbit polyclonal anti-human eNOS antibody) and the signal was detected by using chemiluminescence (Amersham Pharmacia ECL) as described (46, 49–51).
Evaluation of Oxidative Stress and Nitrite and Nitrate (NOx) Levels. Cholesterolemia was determined enzymatically (50). LDL particles (d = 1.006–1.063 g/ml) were isolated from pooled plasma from two animals from each group by sequential-density ultracentrifugation, as described (50, 53). LDL protein was measured by Lowry's method (54). Oxidation of LDL was induced by 1 μM copper sulfate and lag-time was determined spectrophotometrically, as described (50). The formation of thiobarbituric acid reactive substances was determined by the thiobarbituric acid method (50). Lipid peroxides, measured by the LPO colorimetric kit (Kamiya Biomedical, Thousand Oaks, CA) (50), were assayed in cell sonicates of mouse peritoneal macrophages isolated from the peritoneal fluid, as described (55, 56). Isoprostane 8-epi-PGF2α purified from plasma samples was measured by using an immunoassay (Cayman Chemical, Ann Arbor, MI) (50, 57). Tissue concentrations of glutathione peroxidase, catalase, and Mn-superoxide dismutase were determined spectrophotometrically, as described (58). Finally, NOx levels in the plasma were measured with Griess reagent according to the manufacturer's instructions (Calbiochem).
Statistical Analysis. Results are expressed as mean ± SD. The difference among groups was evaluated by a 1- or 2-factor ANOVA and Student's t test by two independent investigators in a blinded fashion regarding treatment of mice. Statistical significance was accepted at P < 0.05.
Results
General Effects Afforded by Graduated Exercise in Hypercholesterolemic Mice. The body weight of exercised animals was significantly decreased in all three groups (Table 1). Furthermore, exercise lowered the total plasma cholesterol in all groups (Table 1).
Table 1. Characteristics of hypercholesterolemic mice and evaluation of oxidative stress and NOx production in the various study groups.
| Physical exercise
|
||
|---|---|---|
| Parameter | No | Yes |
| Body weight, g | ||
| Hypercholesterolemic diet | 43.6 ± 1.87 | 34.5 ± 1.63* |
| Hypercholesterolemic diet plus antioxidants | 48.5 ± 1.94 | 37.8 ± 1.45* |
| Hypercholesterolemic diet plus antioxidants plus l-arginine | 49.2 ± 2.02 | 38.5 ± 1.88* |
| Total blood cholesterol, mg/dl | ||
| Hypercholesterolemic diet | 871 ± 83 | 782 ± 65* |
| Hypercholesterolemic diet plus antioxidants | 936 ± 72 | 771 ± 58* |
| Hypercholesterolemic diet plus antioxidants plus l-arginine | 921 ± 81 | 795 ± 84* |
| Plasma isoprostanes, 8-epi-PGF2α, pg/ml | ||
| Hypercholesterolemic diet | 153 ± 39 | 162 ± 46 |
| Hypercholesterolemic diet plus antioxidants | 118 ± 24† | 119 ± 30† |
| Hypercholesterolemic diet plus antioxidants plus l-arginine | 110 ± 20† | 106 ± 18† |
| Macrophage lipid peroxidation, nmol peroxides/mg cell protein | ||
| Hypercholesterolemic diet | 77 ± 10 | 85 ± 15 |
| Hypercholesterolemic diet plus antioxidants | 49 ± 8† | 53 ± 9† |
| Hypercholesterolemic diet plus antioxidants plus l-arginine | 44 ± 6† | 43 ± 10† |
| LDL Lag-time, min | ||
| Hypercholesterolemic diet | 112 ± 26 | 108 ± 25 |
| Hypercholesterolemic diet plus antioxidants | 132 ± 18† | 127 ± 19† |
| Hypercholesterolemic diet plus antioxidants plus l-arginine | 144 ± 30† | 135 ± 28† |
| LDL-MDA, nmol/mg protein | ||
| Hypercholesterolemic diet | 24.6 ± 4.8 | 28.7 ± 5.8 |
| Hypercholesterolemic diet plus antioxidants | 14.9 ± 2.6‡ | 18.2 ± 3.2‡ |
| Hypercholesterolemic diet plus antioxidants plus l-arginine | 13.8 ± 3.7‡ | 16.4 ± 4.1‡ |
| Plasma NOx, μM | ||
| Hypercholesterolemic diet | 16.2 ± 1.5 | 26.4 ± 3.0§ |
| Hypercholesterolemic diet plus antioxidants | 20.4 ± 1.8¶ | 29.6 ± 2.8§ |
| Hypercholesterolemic diet plus antioxidants plus l-arginine | 27.5 ± 2.2∥ | 42.3 ± 4.1**†† |
Data are expressed as mean ± SD. MDA, malondialdehyde generated at 12 h after exposure to 1 μM copper sulfate. Lag-time represents an index of LDL oxidizability; increased values of lag-time reflect increased resistance of LDL to oxidative modification (see also Methods).
P < 0.05 vs. respective group with no exercise.
P < 0.05 vs. respective hypercholesterolemic diet.
P < 0.04 vs. respective hypercholesterolemic diet.
P < 0.01 vs. hypercholesterolemic diet with no exercise.
P < 0.05 vs. hypercholesterolemic diet with no exercise.
P < 0.01 vs. hypercholesterolemic diet with no exercise.
P < 0.001 vs. hypercholesterolemic diet with no exercise.
P < 0.01 vs. hypercholesterolemic diet plus antioxidants plus l-arginine with no exercise.
Effects of Exercise and Metabolic Intervention on Plasma Oxidative Stress. To test the hypothesis that metabolic intervention and graduated exercise can exert beneficial effects on oxidative stress as well as the prevention of atherosclerosis, we supplemented exercising hypercholesterolemic mice with antioxidants and l-arginine. As expected, indicators of oxidative stress decreased in treated mice. Indeed, plasma isoprostanes and parameters of LDL oxidizability (lag time and LDL-malondialdehyde) were significantly decreased after metabolic intervention (Table 1). Moreover, macrophage lipid peroxidation was significantly reduced in mice receiving metabolic intervention (Table 1). Graduated exercise did not modify significantly the degree of systemic oxidative stress in mice (Table 1). Nevertheless, plasma NOx levels were significantly increased by graduated exercise and metabolic intervention, achieving maximal and synergistic levels in the exercised group treated with antioxidants and l-arginine.
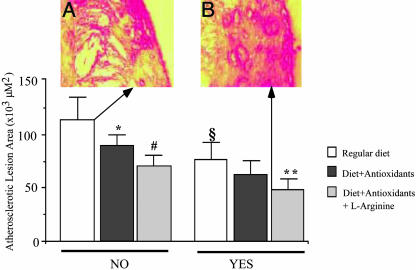
Effects of Exercise and Metabolic Intervention on Atherosclerosis Lesion Area. As usual in this model (43, 46, 51), atherosclerotic lesions were localized in the aortic arch and thoracic aorta. Under the present experimental conditions, determination of atherosclerotic lesion areas showed that graduated exercise significantly decreased the progression of atherosclerotic lesions (Fig. 1). This effect was further improved by cotreatment with metabolic intervention (Fig. 1). The significant combined protective effect was not seen in preliminary studies using vigorous treadmill exercise (data not shown). The decrease in plasma isoprostane levels correlated with the reduction in atherosclerotic lesion area in the group of exercised mice receiving diet and antioxidants (r = 0.51, P < 0.01) as well as in the group receiving diet, antioxidant, and l-arginine supplementation (r = 0.76, P < 0.001). Moreover, the increase in plasma NOx levels correlated with the reduction in atherosclerotic lesion area in the group of exercised mice receiving diet and antioxidants (r = 0.42, P < 0.05) as well as in the group receiving diet, antioxidant, and l-arginine supplementation (r = 0.68, P < 0.01). The number of foam cells (F4/80 antibody) decreased significantly in exercised mice, especially in mice receiving metabolic intervention (Fig. 2). Control immunohistochemistry without the primary antibody yielded no staining. The decrease of F4/80 immunostaining correlated with the reduction in atherosclerotic lesion area in the group of exercised mice receiving diet and antioxidants (r = 0.40, P < 0.05) as well as in the group receiving diet, antioxidant, and l-arginine supplementation (r = 0.54, P < 0.01). The decrease in total plasma cholesterol in groups of exercised mice was only poorly correlated with atherosclerotic lesion size (r = 0.26, P = NS).
Fig. 1.
Effect of graduated physical exercise on atherosclerotic lesion area in male hypercholesterolemic mice subjected to regular hypercholesterolemic diet, or diet containing antioxidants alone, or antioxidants plus l-arginine. Computerized quantification of lesion area was performed on aortas of atherogenic-fed animals. (A) Illustration of an extensive atherosclerotic lesion containing cholesterol crystals in a mouse receiving hypercholesterolemic diet and a sedentary lifestyle (no exercise). (B) Illustration of a typical intermediate atherosclerotic lesion in a mouse subjected to physical exercise and metabolic intervention with antioxidants and l-arginine. Error bars represent mean ± SD. *, P < 0.01 vs. regular diet no exercise; #, P < 0.001 vs. regular diet no exercise; §, P < 0.01 vs. no exercise; **, P < 0.01 vs. both hypercholesterolemic diet plus antioxidants and hypercholesterolemic diet plus antioxidants plus l-arginine no exercise.
Fig. 2.
Percentages of positive sections stained with E06 or F4/80 antibodies in the various experimental groups. (Upper) Immunohistochemical illustrations of representative atherosclerotic lesions in mice from different groups are given. (A–C) Examples of immunostaining with E06 in the respective group of exercised mice. (A1–C1) Examples of immunostaining with F4/80 in the respective group of exercised mice. As shown in both bars and figures, both early and intermediate lesions from the hypercholesterolemic group showed extensive staining for both antibodies, but the degree of staining was progressively decreased with graduated physical exercise and metabolic intervention. Error bars represent mean ± SD. ^, P < 0.05 vs. regular diet no exercise; *, P < 0.01 vs. regular diet no exercise; §, P < 0.01 vs. no exercise; **, P < 0.05 vs. both hypercholesterolemic diet plus antioxidants and hypercholesterolemic diet plus antioxidants plus l-arginine no exercise; §, P < 0.05 vs. both hypercholesterolemic diet plus antioxidants and hypercholesterolemic diet plus antioxidants plus l-arginine no exercise; #, P < 0.05 vs. regular diet and physical exercise; @, P < 0.01 vs. regular diet no exercise; §§, P < 0.001 vs. regular diet and physical exercise; £, P < 0.05 vs. diet plus antioxidants and physical exercise
Effects of Exercise and Metabolic Intervention on Arterial Oxidative Stress and eNOS/Inducible NOS (iNOS) Protein Levels. Immunostaining for arterial oxidation-specific epitopes (E06 antibody) was decreased in exercised mice receiving metabolic intervention (Fig. 3). The reduction in the expression of oxidation-specific epitopes correlated with the reduction in atherosclerotic lesion area in the group of exercised mice receiving diet and antioxidants (r = 0.48, P < 0.03) as well as in the group receiving diet, antioxidant, and l-arginine supplementation (r = 0.59, P < 0.01). Graduated exercise stimulated arterial activities of catalase, glutathione peroxidase, and Mn-superoxide dismutase (Table 2). There was a nonsignificant trend of attenuation of this exercise-induced increase in enzymatic activities by cotreatment with metabolic intervention. (Table 2). Graduated exercise increased arterial eNOS expression, especially in the group receiving cotreatment with antioxidants and l-arginine (Fig. 3). This result was also true for arterial iNOS expression (Table 2). The increase in eNOS expression correlated with the reduction in atherosclerotic lesion area in the group of exercised mice receiving diet and antioxidants (r = 0.35, P < 0.05) as well as in the group receiving diet, antioxidant, and l-arginine supplementation (r = 0.65, P < 0.002). Antioxidants alone slightly inhibited the induction of eNOS and iNOS proteins induced by exercise.
Fig. 3.
Determination of eNOS protein expression by Western blot of aortic protein extracts of hypercholesterolemic mice with the use of eNOS and γ-tubulin antibodies. Films were quantified by densitometry and results were expressed as mean ± SD of arbitrary units. (Insets) Representative blots of each group. *, P < 0.05 vs. regular diet; §, P < 0.01 vs. respective lane in no exercise; **, P < 0.01 vs. diet plus antioxidants plus l-arginine no exercise and diet plus antioxidants and exercise.
Table 2. Radical scavenger enzyme activities and iNOS expression in the various study groups.
| Physical exercise
|
||
|---|---|---|
| Parameter | No | Yes |
| Arterial catalase activity, units/mg of protein | ||
| Hypercholesterolemic diet | 266 ± 46 | 323 ± 40* |
| Hypercholesterolemic diet plus antioxidants | 281 ± 48 | 305 ± 51* |
| Hypercholesterolemic diet plus antioxidants plus l-arginine | 273 ± 46 | 310 ± 42* |
| Arterial glutathione peroxidase activity, milliunits/mg of protein | ||
| Hypercholesterolemic diet | 86 ± 16 | 105 ± 20† |
| Hypercholesterolemic diet plus antioxidants | 80 ± 18 | 95 ± 19† |
| Hypercholesterolemic diet plus antioxidants plus l-arginine | 83 ± 16 | 98 ± 23† |
| Arterial manganese-superoxide dismutase activity, units/mg of protein | ||
| Hypercholesterolemic diet | 3.6 ± 0.6 | 4.3 ± 0.5† |
| Hypercholesterolemic diet plus antioxidants | 3.1 ± 0.8 | 4.0 ± 0.7† |
| Hypercholesterolemic diet plus antioxidants plus l-arginine | 3.3 ± 0.6 | 4.1 ± 0.6† |
| Arterial iNOS by quantitative densitometric analysis of Western blots, arbitrary units | ||
| Hypercholesterolemic diet | 0.09 ± 0.03 | 0.20 ± 0.07§ |
| Hypercholesterolemic diet plus antioxidants | 0.08 ± 0.02 | 0.19 ± 0.04§ |
| Hypercholesterolemic diet plus antioxidants plus l-arginine | 0.12 ± 0.04‡ | 0.23 ± 0.08¶ |
Data are expressed as mean ± SD.
P < 0.05 vs. no exercise.
P < 0.01 vs. no exercise.
P < 0.05 vs. LDL receptor-/- no exercise.
P < 0.01 vs. no exercise.
P < LDL receptor-/- antioxidants plus l-arginine no exercise.
Discussion
By using male hypercholesterolemic mice on a high-fat diet, we have shown that graduated exercise reduced atherosclerotic lesions and this vasculoprotective effect was significantly enhanced by concomitant metabolic intervention with antioxidants and l-arginine. In general, graduated exercise minimized the degree of arterial oxidative stress. Moreover, both plasma and tissue oxidative stress were reduced and eNOS expression was increased in the aorta of these animals. The induction of eNOS by exercise has been reported (32, 36, 59, 60). In addition, we observed an exercise-induced increase in aortic iNOS protein expression that was also observed in another study (61). Overall, exercise-related induction of scavenger enzymes observed in the present study was consistent with previous observations (28–33). The mild oxidative stress induced by graduated exercise could be responsible for its beneficial effects on atherosclerosis, perhaps by inducing vascular antioxidant defenses. Moreover, in the present study, oxidative stress did not increase greatly in the exercised hypercholesterolemic group vs. the hypercholesterolemic group alone, although scavengers were up-regulated. That is, exercise actually decreased the magnitude of oxidative tress characteristic of the sedentary hypercholesterolemic group.
However, the benefits of graduated exercise on atherosclerosis cannot exclusively be attributed to an induction of aortic scavenger defenses alone because several other protective factors, such as the increased eNOS expression and the reduction in foam cell formation were favorably modified by exercise. Here, metabolic intervention with antioxidants and l-arginine induced beneficial effects in graduated exercised mice by further inhibiting atherogenesis. In another study (32), vigorous exercise together with vitamin E alone did not protect mice from atherosclerosis, and vitamin E administration partially inhibited exercise-induced increase in catalase. Thus, to achieve truly beneficial effects, the focus of attention needs to be on the right degree of exercise (graduated and moderate) and the ideal mixture of supplements (vitamin E together with vitamin C and l-arginine). More molecular studies at the transcriptional level combining antioxidant supplementation and exercise are necessary to further elucidate these protective mechanisms. Indeed, NO and eNOS bioactivity are important in the context of atherosclerosis and endothelial function (22, 23, 62). Antioxidants that reach the artery may protect NO against oxidative destruction and thereby increase NO bioactivity, and this may be required for organisms who have genetic deficiencies in scavengers. Notably, another study (63) showed that trained rats have reduced LDL oxidizability, lower urinary excretion of isoprostanes, lower platelet adhesiveness and aggregability, and higher platelet-derived NO metabolites and cGMP productions than the control sedentary group; amounts of preformed lipid peroxides decreased, whereas NO production increased after chronic moderate exercise. Moreover, exercise decreased oxidized LDL-potentiated platelet activation, most likely by enhancing platelet-derived NO release and bioactivity. Exercise also prevented impairment of vascular dysfunction in type 2 diabetic rats, presumably due to improvement of hyperglycemia and insulin resistance and increased production of NO (64). Thus, progressive adaptation to graduated exercise may represent an economical therapy to preserve health and diminish the rate of decline of the physiological processes associated with aging. Along these lines, there is a growing need to establish whether dynamic muscle performance can attenuate the clinical development of solid tumors (65).
Moderate exercise ameliorates endothelial function and reduces cardiovascular risk (6–14). Several highly plausible protective mechanisms have been postulated, including decreased myocardial oxygen demand, increased myocardial oxygen supply, reduced propensity toward ventricular arrhythmias, reduced platelet aggregation, improved lipid profile, and increased plasma fibrinolytic activity (4–8). Despite the heavy body of literature, pathogenic molecular mechanisms where exercise might benefit vascular diseases are poorly understood. However, it is conceivable that arterial cells can be affected by multiple signal transduction events promoted by physical exercise.
A metaanalysis demonstrated that physical activity is also an effective symptomatic treatment for patients with peripheral arterial atherosclerotic disease and claudication (26). The greatest improvement with exercise training occurred when training lasted at least 6 months, and when walking was the primary mode of moderate exercise (26). Exercise training may also be superior to peripheral angioplasty in improving exercise tolerance in such patients (27). Importantly, oral administration of l-arginine enhanced myocardial perfusion in coronary heart disease patients (44, 66–69). In addition, l-arginine enhanced the beneficial effect of exercise on endothelial dysfunction in patients with heart failure (70). Finally, regular exercise improves endothelial function in heart transplant recipients (71) and contributes to improved autonomic regulation in patients with coronary heart disease (72). All of these conditions are linked to prominent atherosclerosis and, therefore, can be further improved by cotreatment with metabolic intervention and graduated physical exercise training as proposed in the present study.
Acknowledgments
We thank our colleague Joseph Loscalzo for helpful discussions in the field, Arlene Lising for technical and secretarial assistance, and Francesco P. D'Armiento for his insightful pathological observations.
This work was supported by National Institutes of Health Grants HL-58433 and HL-66999, the Mayo Foundation, and National Research Funds from the University of Naples.
Abbreviations: eNOS, endothelial NO synthase; iNOS, inducible NOS; LDL, low-density lipoprotein; NOx, nitrite and nitrate.
References
- 1.Lakatta, E. G. & Spurgeon, H. A. (1987) Fed. Proc. 46, 1844–1849. [PubMed] [Google Scholar]
- 2.Blumenthal, J. A., Emery, C. F., Madden, D. J., George, L. K., Coleman, R. E., Riddle, M. W., McKee, D. C., Reasoner, J. & Williams, R. S. (1989) J. Gerontol. 44, M147–M157. [DOI] [PubMed] [Google Scholar]
- 3.De Angelis, K. L., Oliveira, A. R., Werner, A., Bock, P., Bello-Klein, A., Fernandes, T. G., Bello, A. A. & Irigoyen, M. C. (1997) Hypertension 30, 767–771. [DOI] [PubMed] [Google Scholar]
- 4.Kramsch, D. M., Aspen, A. J., Abramowitz, B. M., Kreimendahl, T. & Hood, W. B., Jr. (1981) N. Engl. J. Med. 305, 1483–1489. [DOI] [PubMed] [Google Scholar]
- 5.Lowe, D. A. & Always, S. E. (2002) J. Orthop. Sports Phys. Ther. 32, 36–43. [DOI] [PubMed] [Google Scholar]
- 6.Shephard, R. J. & Balady, G. J. (1999) Circulation 99, 963–972. [DOI] [PubMed] [Google Scholar]
- 7.Thompson, P. D., Buchner, D., Pina, I. L., Balady, G. J., Williams, M. A., Marcus, B. H., Berra, K., Blair, S. N., Costa, F., Gordon, N. F., et al. (2003) Arterioscler. Thromb. Vasc. Biol. 23, E42–E49. [Google Scholar]
- 8.Thompson, P. D., Buchner, D., Pina, I. L., Balady, G. J., Williams, M. A., Marcus, B. H., Berra, K., Blair, S. N., Costa, F., Gordon, N. F., et al. (2003) Circulation 107, 3109–3116. [DOI] [PubMed] [Google Scholar]
- 9.Chevion, S., Moran, D. S., Heled, Y., Shani, Y., Regev, G., Abbou, B., Berenshtein, E., Stadtman, E. R. & Epstein, Y. (2003) Proc. Natl. Acad. Sci. USA 100, 5119–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto, C., Higashi, Y., Kimura, M., Noma, K., Hara, K., Nakagawa, K., Kawamura, M., Chayama, K., Yoshizumi, M. & Nara, I. (2003) Circulation 108, 530–535. [DOI] [PubMed] [Google Scholar]
- 11.Vincent, K. R., Vincent, H. K., Braith, R. W., Lennon, S. L. & Lowenthal, D. T. (2002) Eur. J. Appl. Physiol. 87, 416–423. [DOI] [PubMed] [Google Scholar]
- 12.Keller, C., Fleury, J. & Mujezinovic-Womack, M. (2003) J. Gerontol. Nurs. 29, 18–23. [DOI] [PubMed] [Google Scholar]
- 13.Lee, I. M., Sesso, H. D., Oguma, Y. & Paffenbarger, R. S., Jr. (2003) Circulation 107, 1110–1116. [DOI] [PubMed] [Google Scholar]
- 14.O'Connor, G. T., Buring, J. E., Yusuf, S., Goldhaber, S. Z., Olmstead, E. M., Paffenbarger, R. S., Jr., & Hennekens, C. H. (1989) Circulation 80, 234–244. [DOI] [PubMed] [Google Scholar]
- 15.Piña, I. L., Apstein, C. S., Balady, G. J., Belardinelli, R., Chaitman, B. R., Duscha, B. D., Fletcher, B. J., Fleg, J. L., Myers, J. N. & Sullivan, M. J. (2003) Circulation 107, 1210–1225. [DOI] [PubMed] [Google Scholar]
- 16.Witztum, J. L. & Steinberg, D. (1991) J. Clin. Invest. 88, 1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinberg, D. & Witztum, J. L. (2002) Circulation 105, 2107–2111. [DOI] [PubMed] [Google Scholar]
- 18.Napoli, C., D'Armiento, F. P., Mancini, F. P., Witztum, J. L., Palumbo, G. & Palinski W. (1997) J. Clin. Invest. 100, 2680–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Napoli, C., Glass, C. K., Witztum, J. L., Deutch, R., D'Armiento, F. P. & Palinski, W. (1999) Lancet 354, 1234–1241. [DOI] [PubMed] [Google Scholar]
- 20.Pryor, W. A. (2000) Free Radical Biol. Med. 28, 141–164. [DOI] [PubMed] [Google Scholar]
- 21.Napoli, C. & Lerman L. O. (2001) Mayo Clin. Proc. 76, 619–631. [DOI] [PubMed] [Google Scholar]
- 22.de Nigris, F., Lerman, A., Ignarro, L. J., Williams-Ignarro, S., Sica, V., Baker, A. H., Lerman, L. O., Geng, Y. J. & Napoli, C. (2003) Trends Mol. Med. 9, 351–359. [DOI] [PubMed] [Google Scholar]
- 23.Heinecke, J. W. (2003) Am. J. Cardiol. 91, 12A–16A. [DOI] [PubMed] [Google Scholar]
- 24.Leaf, D. A., Kleinman, M. T., Hamilton, M. & Deitrick, R. W. (1999) Am. J. Med. Sci. 317, 295–300. [DOI] [PubMed] [Google Scholar]
- 25.Leeuwenburgh, C. & Heinecke, J. W. (2001) Curr. Med. Chem. 8, 829–838. [DOI] [PubMed] [Google Scholar]
- 26.Gardner, A. W. & Poehlman, E. T. (1995) J. Am. Med. Assoc. 274, 975–980. [PubMed] [Google Scholar]
- 27.Whyman, M. R. & Ruckley C. V. (1998) Cardiovasc. Surg. 6, 226–231. [DOI] [PubMed] [Google Scholar]
- 28.Reddy Avula, C. P. & Fernandes, G. (1999) Aging 11, 246–252. [DOI] [PubMed] [Google Scholar]
- 29.Avula, C. P. & Fernandes, G. (1999) J. Clin. Immunol. 19, 35–44. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita, N., Hoshida, S., Otsu, K., Asahi, M., Kuzuya, T. & Hori, M. (1999) J. Exp. Med. 189, 1699–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shern-Brewer, R., Santanam, N., Wetzstein, C., White-Welkley, J., Price, L. & Parthasarathy, S. (2000) in Handbook of Oxidants and Antioxidants in Exercise, eds. Sen, C. K., Packer, L. & Hanninen, O. (Elsevier, New York), pp. 1053–1067.
- 32.Meilhac, O., Ramachandran, S., Chiang, K., Santanam, N. & Parthasarathy, S. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1681–1687. [DOI] [PubMed] [Google Scholar]
- 33.Ji, L. L. (2002) Ann. N.Y. Acad. Sci. 959, 82–92. [DOI] [PubMed] [Google Scholar]
- 34.Gielen, S., Schuler, G. & Hambrecht, R. (2001) Circulation 103, E1–E6. [DOI] [PubMed] [Google Scholar]
- 35.Kingwell, B. A. (2000) FASEB. J. 14, 1685–1696. [DOI] [PubMed] [Google Scholar]
- 36.Stefano, G. B., Prevot, V., Cadet, P. & Dardik, I. (2001) Int. J. Mol. Med. 7, 119–129. [DOI] [PubMed] [Google Scholar]
- 37.Crawford, R. S., Kirk, E. A., Rosenfeld, M. E., LeBoeuf, R. C. & Chait, A. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 1506–1513. [DOI] [PubMed] [Google Scholar]
- 38.Thomas, S. R., Leichtweis, S. B., Pettersson, K., Croft, K. D., Mori, T. A. Brown, A. J. & Stocker R. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 585–593. [DOI] [PubMed] [Google Scholar]
- 39.Pratico, D., Tangirala, R. K., Rader, D. J., Rokach, J. & FitzGerald, G. A. (1998) Nat. Med. 4, 1189–1192. [DOI] [PubMed] [Google Scholar]
- 40.Pratico, D., Tangirala, R. K., Horkko, S., Witztum, J. L., Palinski, W. & FitzGerald, G. A. (2001) Blood 97, 459–464. [DOI] [PubMed] [Google Scholar]
- 41.Terasawa, Y., Ladha, Z., Leonard, S. W., Morrow, J. D., Newland, D., Sanan, D., Packer, L., Traber, M. G. & Farese, R. V., Jr. (2000) Proc. Natl. Acad. Sci. USA 97, 13830–13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakata, Y. & Maeda, N. (2002) Circulation 105, 1485–1490. [DOI] [PubMed] [Google Scholar]
- 43.Tsimikas, S., Shortal, B. P., Witztum, J. L. & Palinski, W. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 689–697. [DOI] [PubMed] [Google Scholar]
- 44.Boger, R. H. & Bode-Boger, S. M. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 79–99. [DOI] [PubMed] [Google Scholar]
- 45.Maxwell, A. J., Ho, H. V., Le, C. Q., Lin, P. S., Bernstein, D. & Cooke, J. P. (2001) J. Appl. Physiol. 90, 933–938. [DOI] [PubMed] [Google Scholar]
- 46.de Nigris, F., Lerman, L. O., Williams-Ignarro, S., Sica, G., Lerman, A., Palinski, W., Ignarro, L. J. & Napoli, C. (2003) Proc. Natl. Acad. Sci. USA 100, 1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kauser, K., da Cunha, V., Fitch, R., Mallari, C. & Rubanyi, G. M. (2000) Am. J. Physiol. 278, H1679–H1685. [DOI] [PubMed] [Google Scholar]
- 48.Aji, W., Ravalli, S., Szabolcs, M., Jiang, X. C., Sciacca, R. R., Michler, R. E. & Cannon, P. J. (1997) Circulation 95, 430–437. [DOI] [PubMed] [Google Scholar]
- 49.Napoli, C., de Nigris, F., Welch, J. S., Calara, F. B., Stuart, R., Glass, C. K. & Palinski, W. (2002) Circulation 105, 1360–1367. [DOI] [PubMed] [Google Scholar]
- 50.Napoli, C., Ackah, E., de Nigris, F., Del Soldato, P., D'Armiento, F. P., Crimi, E., Condorelli, M. & Sessa, W. C. (2002) Proc. Natl. Acad. Sci. USA 99, 12467–12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palinski, W., Napoli, C. & Reaven, P. D. (2000) in Vascular Disease and Injury: Preclinical Research. Contemporary Cardiology Series, eds. Simons, D. I. & Rogers, C. (Humana, Totowa, NJ), pp. 149–174.
- 52.Orenstein, T. L., Parker, T. G., Butany, J. W., Goodman, J. M., Dawood, F., Wen, W. H., Wee, L., Martino, T., McLaughlin, P. R. & Liu, P. P. (1995) J. Clin. Invest. 96, 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Napoli, C., Mancini, F. P., Corso, G., Malorni, A., Crescenzi, E., Postiglione, A. & Palumbo, G. (1997) J. Biochem. (Tokyo) 121, 1096–1101. [DOI] [PubMed] [Google Scholar]
- 54.Lowry, O. H., Rosebrough, N. J., Farr, J. L. & Randall, R. I. (1951) J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- 55.Napoli, C., Ambrosio, G., Scarpato, N., Corso, G., Palumbo, G., D'Armiento, F. P., Mancini, F. P., Malorni, A., Formisano, S., Rocco, A., et al. (1997) Am. Heart. J. 133, 585–595. [DOI] [PubMed] [Google Scholar]
- 56.Aviram, M., Dornfeld, L., Rosenblat, M., Volkova, N., Kaplan, M., Coleman, R., Hayek, T., Presser, D. & Fuhrman, B. (2000) Am. J. Clin. Nutr. 71, 1062–1076. [DOI] [PubMed] [Google Scholar]
- 57.Napoli, C., Martin-Padura, I., de Nigris, F., Giorgio, M., Mansueto, G., Somma, P., Condorelli, M., Sica, G., De Rosa, G. & Pelicci, P. G. (2003) Proc. Natl. Acad. Sci. USA 100, 2112–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Napoli, C., Witztum, J. L., de Nigris, F., Palumbo, G., D'Armiento, F. P. & Palinski, W. (1999) Circulation 99, 2003–2010. [DOI] [PubMed] [Google Scholar]
- 59.Sessa, W. C., Pritchard, K., Seyedi, N., Wang, J. & Hintze, T. H. (1994) Circ. Res. 74, 349–353. [DOI] [PubMed] [Google Scholar]
- 60.Niebauer, J., Maxwell, A. J., Lin, P. S., Wang, D., Tsao, P. S. & Cooke, J. P. (2003) Am. J. Physiol. 285, H535–H540. [DOI] [PubMed] [Google Scholar]
- 61.Yang, A. L., Tsai, S. J., Jiang, M. J., Jen, C. J. & Chen, H. (2002) J. Biomed. Sci. 9, 149–155. [DOI] [PubMed] [Google Scholar]
- 62.Napoli, C. & Ignarro, L. J. (2001) Nitric Oxide 5, 88–97. [DOI] [PubMed] [Google Scholar]
- 63.Wang, J. S., Lin, C. C., Chen, J. K. & Wong, M. K. (2000) Life Sci. 66, 1937–1948. [DOI] [PubMed] [Google Scholar]
- 64.Minami, A., Ishimura, N., Harada, N., Sakamoto, S., Niwa, Y. & Nakaya Y. (2002) Arterioscler. Thromb. 162, 85–92. [DOI] [PubMed] [Google Scholar]
- 65.Lu, Q., Ceddia, M. A., Price, E. A., Ye, S. M. & Woods, J. A. (1999) Am. J. Physiol. 276, R482–R489. [DOI] [PubMed] [Google Scholar]
- 66.Fujita, H., Yamabe, H. & Yokoyama, M. (2000) J. Nucl. Cardiol. 7, 97–102. [DOI] [PubMed] [Google Scholar]
- 67.Napoli, C. & Ignarro, L. J. (2003) Annu. Rev. Pharmacol. Toxicol. 43, 97–123. [DOI] [PubMed] [Google Scholar]
- 68.Piatti, P., Fracasso, G., Monti, L. D., Setola, E., Lucotti, P., Fermo, I., Paroni, R., Galluccio, E., Pozza, G., Chierchia, S. & Marginato, A. (2003) Circulation 107, 429–436. [DOI] [PubMed] [Google Scholar]
- 69.Maxwell, A. J., Zapien, M. P., Pearce, G. L., MacCallum, G. & Stone, P. H. (2002) J. Am. Coll. Cardiol. 39, 37–45. [DOI] [PubMed] [Google Scholar]
- 70.Hambrecht, R., Hilbrich, L., Erbs, S., Gielen, S., Fiehn, E., Schoene, N. & Schuler, G. (2000) J. Am. Coll. Cardiol. 35, 706–713. [DOI] [PubMed] [Google Scholar]
- 71.Schmidt, A., Pleiner, J., Bayerle-Eder, M., Wiesinger, G. F., Rodler, S., Quittan, M., Mayer, G. & Wolzt, M. (2002) Clin. Transplant. 16, 137–143. [DOI] [PubMed] [Google Scholar]
- 72.Lucini, D., Milani, R. V., Costantino, G., Lavie, C. J., Porta, A. & Pagani, M. (2002) Am. Heart. J. 143, 977–983. [DOI] [PubMed] [Google Scholar]