Abstract
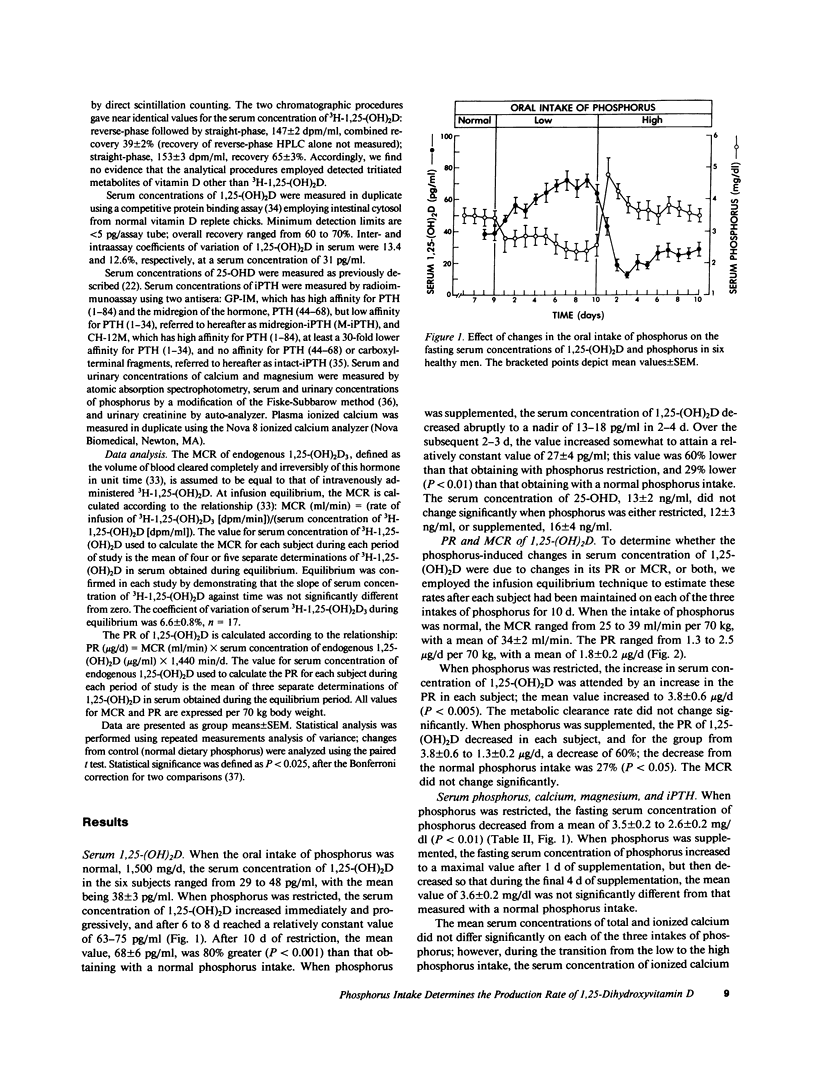
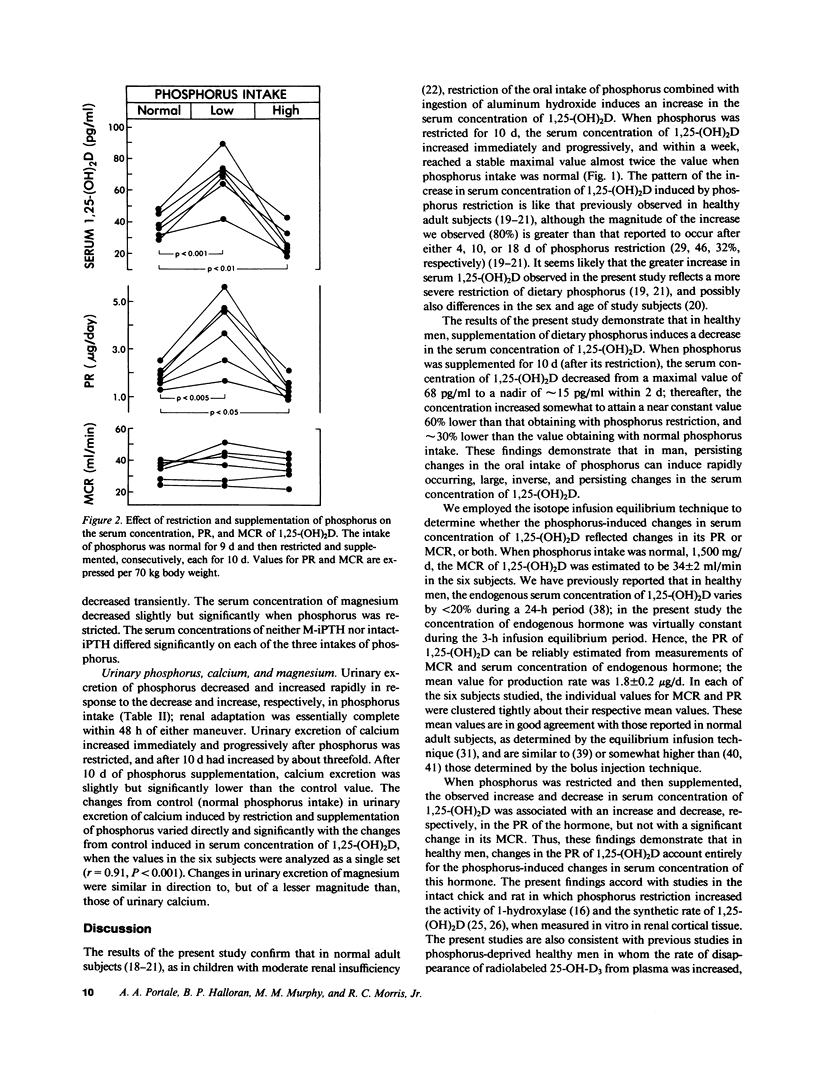
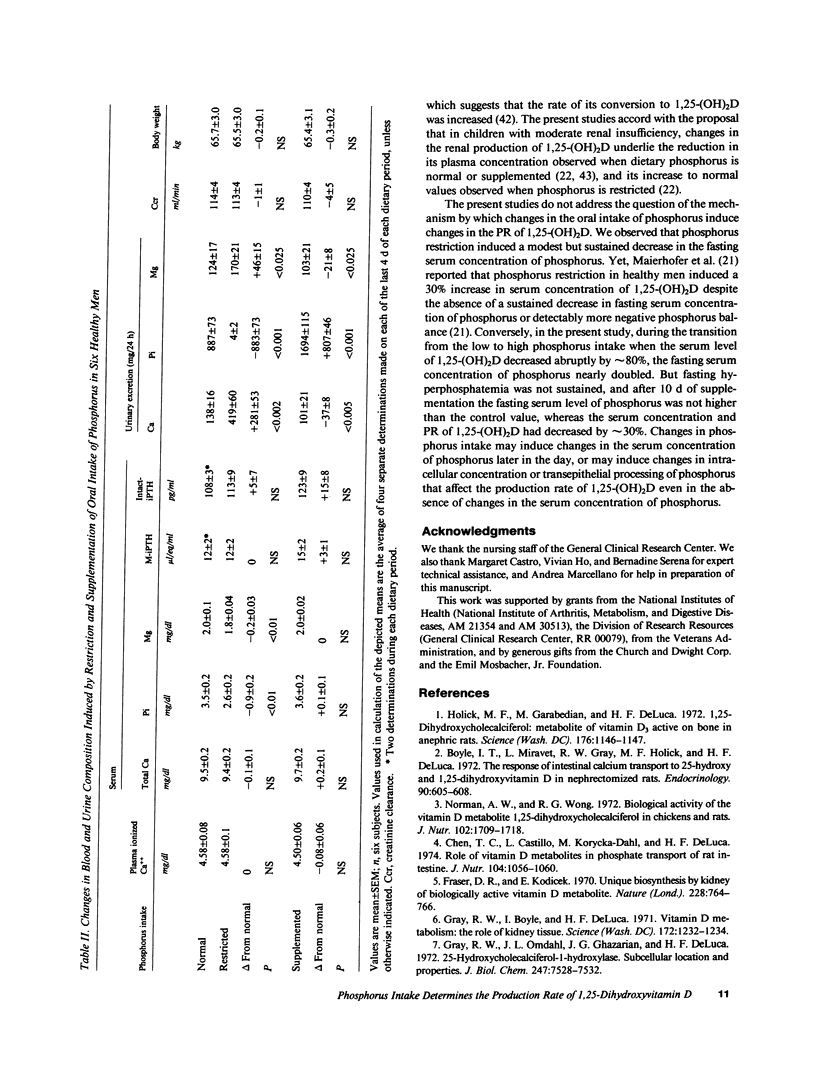
Changes in the oral intake of phosphorus could induce the reported changes in the serum concentration of 1,25-dihydroxyvitamin D (1,25-(OH)2D) by inducing changes in its production rate (PR) or metabolic clearance rate (MCR), or both. To investigate these possibilities, we employed the constant infusion equilibrium technique to measure the PR and MCR of 1,25-(OH)2D in six healthy men in whom the oral intake of phosphorus was initially maintained at 1,500 mg/70 kg body weight per d for 9 d, then restricted to 500 mg/d (coupled with oral administration of aluminum hydroxide) for 10 d, and then supplemented to 3,000 mg/d for 10 d. With phosphorus restriction, the serum concentration of 1,25-(OH)2D increased by 80% from a mean of 38 +/- 3 to 68 +/- 6 pg/ml, P less than 0.001; the PR increased from 1.8 +/- 0.2 to 3.8 +/- 0.6 micrograms/d, P less than 0.005; the MCR did not change significantly. The fasting serum concentration of phosphorus decreased from 3.5 +/- 0.2 to 2.6 +/- 0.2 mg/dl, P less than 0.01. With phosphorus supplementation, the serum concentration of 1,25-(OH)2D decreased abruptly, reaching a nadir within 2 to 4 d; after 10 d of supplementation, the mean concentration of 27 +/- 4 pg/ml was lower by 29%, P less than 0.01, than the value measured when phosphorus intake was normal. The PR decreased to 1.3 +/- 0.2 micrograms/d, P less than 0.05; the MCR did not change significantly. The fasting serum concentration of phosphorus increased significantly, but only initially. These data demonstrate that in healthy men, reductions and increases in the oral intake of phosphorus can induce rapidly occurring, large, inverse, and persisting changes in the serum concentration of 1,25-(OH)2D. Changes in the PR of 1,25-(OH)2D account entirely for the phosphorus-induced changes in serum concentration of this hormone.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter L. A., DeLuca H. F. Stimulation of 25-hydroxyvitamin D3-1alpha-hydroxylase by phosphate depletion. J Biol Chem. 1976 May 25;251(10):3158–3161. [PubMed] [Google Scholar]
- Booth B. E., Tsai H. C., Morris R. C., Jr Parathyroidectomy reduces 25-hydroxyvitamin D3-1 alpha-hydroxylase activity in the hypocalcemic vitamin D-deficient chick. J Clin Invest. 1977 Dec;60(6):1314–1320. doi: 10.1172/JCI108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. E., Tsai H. C., Morris R. C., Jr Vitamin D status regulates 25-hydroxyvitamin D3-1 alpha-hydroxylase and its responsiveness to parathyroid hormone in the chick. J Clin Invest. 1985 Jan;75(1):155–161. doi: 10.1172/JCI111668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle I. T., Miravet L., Gray R. W., Holick M. F., Deluca H. F. The response of intestinal calcium transport to 25-hydroxy and 1,25-dihydroxy vitamin D in nephrectomized rats. Endocrinology. 1972 Mar;90(3):605–608. doi: 10.1210/endo-90-3-605. [DOI] [PubMed] [Google Scholar]
- Broadus A. E., Magee J. S., Mallette L. E., Horst R. L., Lang R., Jensen P. S., Gertner J. M., Baron R. A detailed evaluation of oral phosphate therapy in selected patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 1983 May;56(5):953–961. doi: 10.1210/jcem-56-5-953. [DOI] [PubMed] [Google Scholar]
- Chen T. C., Castillo L., Korycka-Dahl M., DeLuca H. F. Role of vitamin D metabolites in phosphate transport of rat intestine. J Nutr. 1974 Aug;104(8):1056–1060. doi: 10.1093/jn/104.8.1056. [DOI] [PubMed] [Google Scholar]
- Dluhy R. G., Axelrod L., Underwood R. H., Williams G. H. Studies of the control of plasma aldosterone concentration in normal man. II. Effect of dietary potassium and acute potassium infusion. J Clin Invest. 1972 Aug;51(8):1950–1957. doi: 10.1172/JCI107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez J. H., Gray R. W., Lemann J., Jr Dietary phosphate deprivation in women and men: effects on mineral and acid balances, parathyroid hormone and the metabolism of 25-OH-vitamin D. J Clin Endocrinol Metab. 1976 Nov;43(5):1056–1068. doi: 10.1210/jcem-43-5-1056. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nat New Biol. 1973 Feb 7;241(110):163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Gallagher J. C., Riggs B. L., Jerpbak C. M., Arnaud C. D. The effect of age on serum immunoreactive parathyroid hormone in normal and osteoporotic women. J Lab Clin Med. 1980 Mar;95(3):373–385. [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon G. G., Southren A. L., Tochimoto S., Rand J. J., Olivo J. Effect of hyperthyroidism and hypothyroidism on the metabolism of testosterone and androstenedione in man. J Clin Endocrinol Metab. 1969 Feb;29(2):164–170. doi: 10.1210/jcem-29-2-164. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Caldas A. E., Wilz D. R., Lemann J., Jr, Smith G. A., DeLuca H. F. Metabolism and excretion of 3H-1,25-(OH)2-vitamin D3 in healthy adults. J Clin Endocrinol Metab. 1978 May;46(5):756–765. doi: 10.1210/jcem-46-5-756. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Napoli J. L. Dietary phosphate deprivation increases 1,25-dihyroxyvitamin D3 synthesis in rat kidney in vitro. J Biol Chem. 1983 Jan 25;258(2):1152–1155. [PubMed] [Google Scholar]
- Gray R. W., Omdahl J. L., Ghazarian J. G., DeLuca H. F. 25-Hydroxycholecalciferol-1-hydroxylase. Subcellular location and properties. J Biol Chem. 1972 Dec 10;247(23):7528–7532. [PubMed] [Google Scholar]
- Gray R. W., Wilz D. R., Caldas A. E., Lemann J., Jr The importance of phosphate in regulating plasma 1,25-(OH)2-vitamin D levels in humans: studies in healthy subjects in calcium-stone formers and in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 1977 Aug;45(2):299–306. doi: 10.1210/jcem-45-2-299. [DOI] [PubMed] [Google Scholar]
- Gray R., Boyle I., DeLuca H. F. Vitamin D metabolism: the role of kidney tissue. Science. 1971 Jun 18;172(3989):1232–1234. doi: 10.1126/science.172.3989.1232. [DOI] [PubMed] [Google Scholar]
- Halloran B. P., Portale A. A., Castro M., Morris R. C., Jr, Goldsmith R. S. Serum concentration of 1,25-dihydroxyvitamin D in the human: diurnal variation. J Clin Endocrinol Metab. 1985 Jun;60(6):1104–1110. doi: 10.1210/jcem-60-6-1104. [DOI] [PubMed] [Google Scholar]
- Henry H. L., Midgett R. J., Norman A. W. Regulation of 25-hydroxyvitamin D3-1-hydroxylase in vivo. J Biol Chem. 1974 Dec 10;249(23):7584–7592. [PubMed] [Google Scholar]
- Henry H. L. Regulation of the hydroxylation of 25-hydroxyvitamin D3 in vivo and in primary cultures of chick kidney cells. J Biol Chem. 1979 Apr 25;254(8):2722–2729. [PubMed] [Google Scholar]
- Holick M. F., Garabedian M., DeLuca H. F. 1,25-dihydroxycholecalciferol: metabolite of vitamin D3 active on bone in anephric rats. Science. 1972 Jun 9;176(4039):1146–1147. doi: 10.1126/science.176.4039.1146. [DOI] [PubMed] [Google Scholar]
- Hulter H. N., Sebastian A., Sigala J. F., Licht J. H., Glynn R. D., Schambelan M., Biglieri E. G. Pathogenesis of renal hyperchloremic acidosis resulting from dietary potassium restriction in the dog: role of aldosterone. Am J Physiol. 1980 Feb;238(2):F79–F91. doi: 10.1152/ajprenal.1980.238.2.F79. [DOI] [PubMed] [Google Scholar]
- Insogna K. L., Broadus A. E., Gertner J. M. Impaired phosphorus conservation and 1,25 dihydroxyvitamin D generation during phosphorus deprivation in familial hypophosphatemic rickets. J Clin Invest. 1983 Jun;71(6):1562–1569. doi: 10.1172/JCI110912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobaugh B., Drezner M. K. Abnormal regulation of renal 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the X-linked hypophosphatemic mouse. J Clin Invest. 1983 Feb;71(2):400–403. doi: 10.1172/JCI110783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lufkin E. G., Kumar R., Heath H., 3rd Hyperphosphatemic tumoral calcinosis: effects of phosphate depletion on vitamin D metabolism, and of acute hypocalcemia on parathyroid hormone secretion and action. J Clin Endocrinol Metab. 1983 Jun;56(6):1319–1322. doi: 10.1210/jcem-56-6-1319. [DOI] [PubMed] [Google Scholar]
- Maierhofer W. J., Gray R. W., Adams N. D., Smith G. A., Lemann J., Jr Synthesis and metabolic clearance of 1,25-dihydroxyvitamin D as determinants of serum concentrations: a comparison of two methods. J Clin Endocrinol Metab. 1981 Sep;53(3):472–475. doi: 10.1210/jcem-53-3-472. [DOI] [PubMed] [Google Scholar]
- Maierhofer W. J., Gray R. W., Lemann J., Jr Phosphate deprivation increases serum 1,25-(OH)2-vitamin D concentrations in healthy men. Kidney Int. 1984 Mar;25(3):571–575. doi: 10.1038/ki.1984.56. [DOI] [PubMed] [Google Scholar]
- Midgett R. J., Spielvogel A. M., Coburn J. W., Norman A. W. Studies on calciferol metabolism. VII. The renal production of the biologically active form of vitamin D, 1,25-dihydroxycholecalciferol; species, tissue and subcellular distribution. J Clin Endocrinol Metab. 1973 Jun;36(6):1153–1161. doi: 10.1210/jcem-36-6-1153. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Wong R. G. Biological activity of the vitamin D metabolite 1,25-dihydroxycholecalciferol in chickens and rats. J Nutr. 1972 Dec;102(12):1709–1718. doi: 10.1093/jn/102.12.1709. [DOI] [PubMed] [Google Scholar]
- Portale A. A., Booth B. E., Halloran B. P., Morris R. C., Jr Effect of dietary phosphorus on circulating concentrations of 1,25-dihydroxyvitamin D and immunoreactive parathyroid hormone in children with moderate renal insufficiency. J Clin Invest. 1984 Jun;73(6):1580–1589. doi: 10.1172/JCI111365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portale A. A., Booth B. E., Tsai H. C., Morris R. C., Jr Reduced plasma concentration of 1,25-dihydroxyvitamin D in children with moderate renal insufficiency. Kidney Int. 1982 Apr;21(4):627–632. doi: 10.1038/ki.1982.70. [DOI] [PubMed] [Google Scholar]
- Seeman E., Kumar R., Hunder G. G., Scott M., Heath H., 3rd, Riggs B. L. Production, degradation, and circulating levels of 1,25-dihydroxyvitamin D in health and in chronic glucocorticoid excess. J Clin Invest. 1980 Oct;66(4):664–669. doi: 10.1172/JCI109902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard R. M., Horst R. L., Hamstra A. J., DeLuca H. F. Determination of vitamin D and its metabolites in plasma from normal and anephric man. Biochem J. 1979 Jul 15;182(1):55–69. doi: 10.1042/bj1820055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAIT J. F., LITTLE B., TAIT S. A., FLOOD C. The metabolic clearance rate of aldosterone in pregnant and nonpregnant subjects estimated by both single-injection and constant-infusion methods. J Clin Invest. 1962 Dec;41:2093–2100. doi: 10.1172/JCI104667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAIT J. F. REVIEW: THE USE OF ISOTOPIC STEROIDS FOR THE MEASUREMENT OF PRODUCTION RATES IN VIVO. J Clin Endocrinol Metab. 1963 Dec;23:1285–1297. doi: 10.1210/jcem-23-12-1285. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F. Rat renal 25-hydroxyvitamin D3 1- and 24-hydroxylases: their in vivo regulation. Am J Physiol. 1984 Feb;246(2 Pt 1):E168–E173. doi: 10.1152/ajpendo.1984.246.2.E168. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973 Feb;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Van Den Berg C. J., Kumar R., Wilson D. M., Heath H., 3rd, Smith L. H. Orthophosphate therapy decreases urinary calcium excretion and serum 1,25-dihydroxyvitamin D concentrations in idiopathic hypercalciuria. J Clin Endocrinol Metab. 1980 Nov;51(5):998–1001. doi: 10.1210/jcem-51-5-998. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]


