Abstract
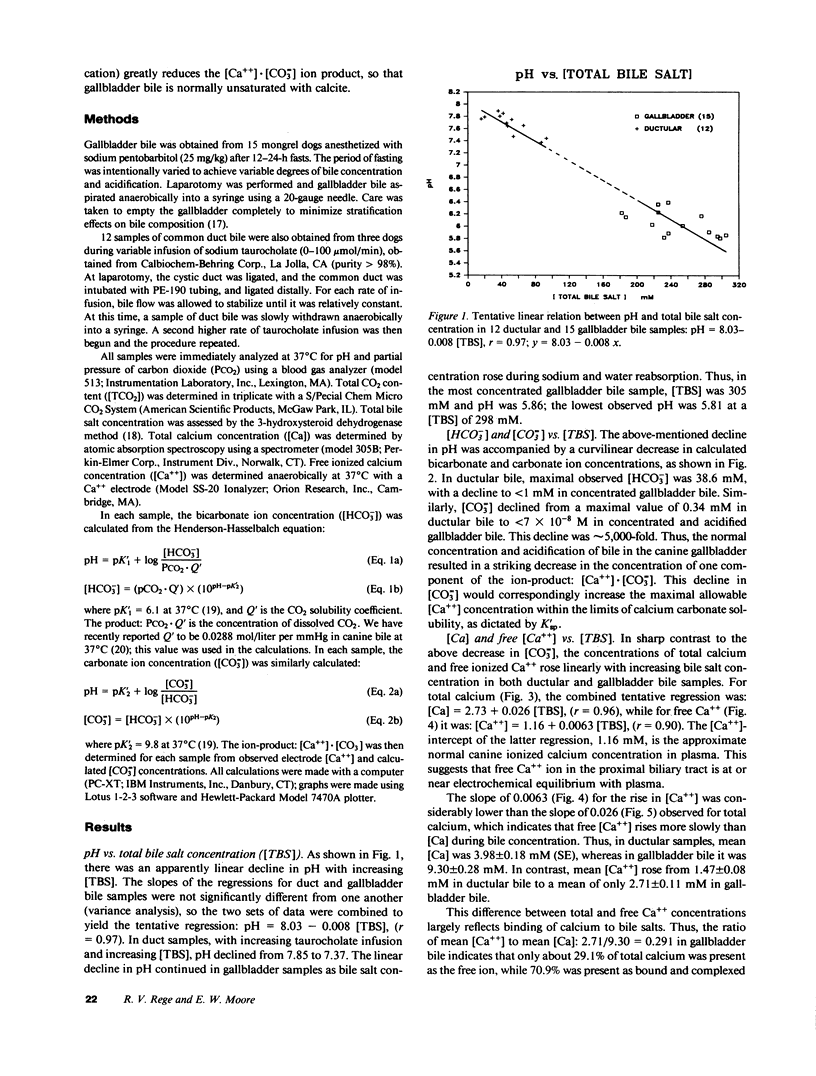
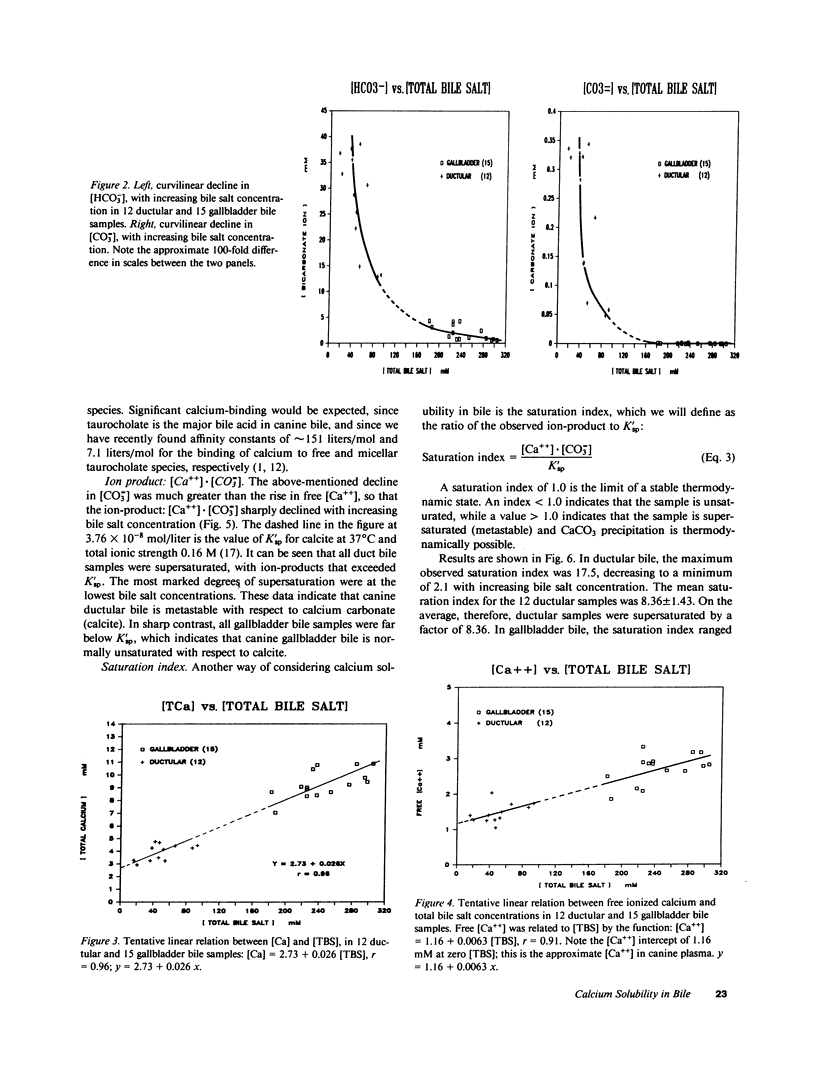
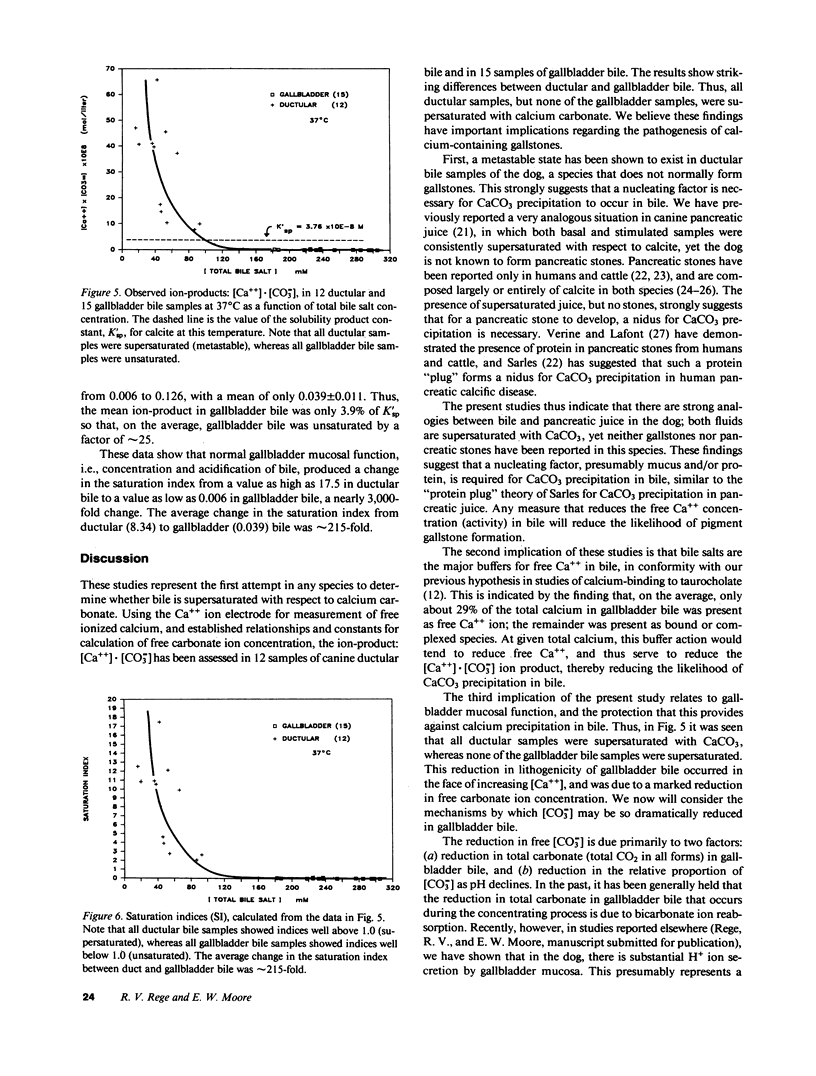
Calcium precipitation in bile is a requisite event in the initiation and growth of all pigment gallstones. Calcium solubility in bile is thus of great importance. This is the first attempt to define the ion-product of CaCO3 in bile in any species. If the ion-product: [Ca++] X [CO = 3] exceeds solubility product (K'sp), the sample is supersaturated and CaCO3 precipitation is thermodynamically possible. We have recently determined K'sp of calcite to be 3.76 X 10(-8) mol/liter at 37 degrees C and total ionic strength = 0.16 M. Gallbladder (GB) bile was obtained from 15 anesthetized dogs after 12-24-h fasts. Duct bile was obtained from three dogs (n = 12) during variable taurocholate infusion. Samples were assayed for pH, partial pressure of carbon dioxide (PCO2), total bile salt concentration ([TBS]), total calcium concentration ([Ca]), and free calcium ion concentration ([Ca++]). With increasing [TBS] in both GB and duct bile, there was a linear decline in pH, a curvilinear decline in [HCO-3] and [CO = 3], and linear increase in [Ca++] and [Ca]. All ductular samples were supersaturated with CaCO3, with saturation indices (SI) as high as 17.5 and a mean of 8.36 +/- 1.43 (SE). In sharp contrast, none of the GB samples were supersaturated, due to the marked decline in [CO = 3] upon concentration and acidification of bile. In GB bile, the SI ranged from 0.006 to 0.126, with a mean of 0.039 +/- 0.011. The gallbladder thus produced a change in the SI from a value as high as 17.5 to a value as low as 0.006 in concentrated GB bile, which is a nearly 3,000-fold change. The average change in the SI was approximately 215-fold. Since all duct samples were supersaturated, and since the dog does not normally form gallstones, the data support our previous hypotheses that: (a) in canine bile, as in canine pancreatic juice, a nucleating factor is necessary for CaCO3 precipitation; (b) bile salts are important buffers for Ca++ in bile; and (c) normal GB mucosal function (concentration and acidification of bile) plays an important role in reducing CaCO3 lithogenicity in GB bile.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGREN H., LARSSON K. CRYSTALLINE COMPONENTS OF BILIARY CALCULI. Scand J Clin Lab Invest. 1963;15:457–462. [PubMed] [Google Scholar]
- Bateson M. C., Bouchier I. A., Trash D. B., Maudgal D. P., Northfield T. C. Calcification of radiolucent gall stone during treatment with ursodeoxycholic acid. Br Med J (Clin Res Ed) 1981 Sep 5;283(6292):645–646. doi: 10.1136/bmj.283.6292.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been J. M., Bills P. M., Lewis D. Microstructure of gallstones. Gastroenterology. 1979 Mar;76(3):548–555. [PubMed] [Google Scholar]
- Burnstein M. J., Ilson R. G., Petrunka C. N., Taylor R. D., Strasberg S. M. Evidence for a potent nucleating factor in the gallbladder bile of patients with cholesterol gallstones. Gastroenterology. 1983 Oct;85(4):801–807. [PubMed] [Google Scholar]
- Carey M. C., Small D. M. The physical chemistry of cholesterol solubility in bile. Relationship to gallstone formation and dissolution in man. J Clin Invest. 1978 Apr;61(4):998–1026. doi: 10.1172/JCI109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A. F., Grundy S. M., Lachin J. M., Lan S. P., Baum R. A., Hanson R. F., Hersh T., Hightower N. C., Jr, Marks J. W., Mekhjian H. Pretreatment biliary lipid composition in white patients with radiolucent gallstones in the National Cooperative Gallstone Study. Gastroenterology. 1982 Oct;83(4):738–752. [PubMed] [Google Scholar]
- Holan K. R., Holzbach R. T., Hermann R. E., Cooperman A. M., Claffey W. J. Nucleation time: a key factor in the pathogenesis of cholesterol gallstone disease. Gastroenterology. 1979 Oct;77(4 Pt 1):611–617. [PubMed] [Google Scholar]
- Holzbach R. T., Marsh M., Olszewski M., Holan K. Cholesterol solubility in bile. Evidence that supersaturated bile is frequent in healthy man. J Clin Invest. 1973 Jun;52(6):1467–1479. doi: 10.1172/JCI107321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. W., Celic L., Ostrow J. D. Interactions between ionized calcium and sodium taurocholate: bile salts are important buffers for prevention of calcium-containing gallstones. Gastroenterology. 1982 Nov;83(5):1079–1089. [PubMed] [Google Scholar]
- Moore E. W. The role of calcium in the pathogenesis of gallstones: Ca++ electrode studies of model bile salt solutions and other biologic systems. With an hypothesis on structural requirements for Ca++ binding to proteins and bile acids. Hepatology. 1984 Sep-Oct;4(5 Suppl):228S–243S. [PubMed] [Google Scholar]
- Perrin M., Thozet A., Vérine H. Etude radiocristallographique de calculs pancréatiques d'origine bovine. C R Seances Soc Biol Fil. 1969;163(1):187–189. [PubMed] [Google Scholar]
- Périnet G., Vérine H., Lafont R., Haladjian J. Etude thermique de calculs pancréatiques humains et bovins. C R Acad Sci Hebd Seances Acad Sci D. 1972 Jun 12;274(24):3303–3305. [PubMed] [Google Scholar]
- Sarles H. Chronic calcifying pancreatitis--chronic alcoholic pancreatitis. Gastroenterology. 1974 Apr;66(4):604–616. [PubMed] [Google Scholar]
- Schoenfield L. J., Lachin J. M. Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: the National Cooperative Gallstone Study. A controlled trial of efficacy and safety. Ann Intern Med. 1981 Sep;95(3):257–282. doi: 10.7326/0003-4819-95-3-257. [DOI] [PubMed] [Google Scholar]
- Sutor D. J., Wilkie L. I. Calcium in bile and calcium salts in gallstones. Clin Chim Acta. 1977 Aug 15;79(1):119–127. doi: 10.1016/0009-8981(77)90469-7. [DOI] [PubMed] [Google Scholar]
- TALALAY P. Enzymic analysis of steroid hormones. Methods Biochem Anal. 1960;8:119–143. doi: 10.1002/9780470110249.ch3. [DOI] [PubMed] [Google Scholar]
- Weinman S. A., Reuss L. Na+-H+ exchange at the apical membrane of Necturus gallbladder. Extracellular and intracellular pH studies. J Gen Physiol. 1982 Aug;80(2):299–321. doi: 10.1085/jgp.80.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson B. W., Percy-Robb I. W. Contribution of biliary lipids to calcium binding in bile. Gastroenterology. 1980 Apr;78(4):696–702. [PubMed] [Google Scholar]
- Wosiewitz U. Limy bile and radiopaque, calcified gallstones: a combined analytical, radiographic, and micromorphologic investigation. Pathol Res Pract. 1980;167(2-4):273–286. doi: 10.1016/S0344-0338(80)80057-4. [DOI] [PubMed] [Google Scholar]


