Abstract
Alcohol (EtOH) decreases protein synthesis and mammalian target of rapamycin (mTOR)-mediated signaling and blunts the anabolic response to growth factors in skeletal muscle. The purpose of the current investigation was to determine whether acute EtOH intoxication antagonizes the contraction-induced increase in protein synthesis and mTOR signaling in skeletal muscle. Fasted male mice were injected intraperitoneally with 3 g/kg EtOH or saline (control), and the right hindlimb was electrically stimulated (10 sets of 6 contractions). The gastrocnemius muscle complex was collected 30 min, 4 h, or 12 h after stimulation. EtOH decreased in vivo basal protein synthesis (PS) in the nonstimulated muscle compared with time-matched Controls at 30 min, 4 h, and 12 h. In Control, but not EtOH, PS was decreased 15% after 30 min. In contrast, PS was increased in Control 4 h poststimulation but remained unchanged in EtOH. Last, stimulation increased PS 10% in Control and EtOH at 12 h, even though the absolute rate remained reduced by EtOH. The stimulation-induced increase in the phosphorylation of S6K1 Thr421/Ser424 (20–52%), S6K1 Thr389 (45–57%), and its substrate rpS6 Ser240/244 (37–72%) was blunted by EtOH at 30 min, 4 h, and 12 h. Phosphorylation of 4E-BP1 Ser65 was also attenuated by EtOH (61%) at 4 h. Conversely, phosphorylation of extracellular signal-regulated kinase Thr202/Tyr204 was increased by stimulation in Control and EtOH mice at 30 min but only in Control at 4 h. Our data indicate that acute EtOH intoxication suppresses muscle protein synthesis for at least 12 h and greatly impairs contraction-induced changes in synthesis and mTOR signaling.
Keywords: anabolic resistance, skeletal muscle metabolism, resistance exercise, p70S6K1, 4E-binding protein
acute and chronic alcohol intoxication decreases rates of skeletal muscle protein synthesis predominately in type II muscle fibers at least in part through impairment of mammalian target of rapamycin (mTOR)-dependent translation initiation (27, 29–31, 49). mTOR is a serine/threonine (Ser/Thr) kinase that exists in two distinct protein complexes, mTORC1 and mTORC2. Together these protein complexes represent a central metabolic integration point that regulates numerous cellular processes, including translation initiation and subsequently protein synthesis (20). Upon activation, mTORC1 phosphorylates its primary downstream substrates, eukaryotic initiation factor (eIF)-4E binding protein (4E-BP1) and p70S6K1 (S6K1). Multisite phosphorylation of 4E-BP1 is required for the release of eIF4E (from 4E-BP1), resulting in initiation of cap-dependent translation (17, 18, 39, 47). On the other hand, phosphorylation of S6K1 by mTORC1 is specific to Thr389 which, in the conventional model, is facilitated by the conformation change induced by phosphorylation of the COOH-terminal autoinhibitory domain containing Thr421 and Ser424 as well as Ser411 and Ser418 (43, 50). S6K1 activates several downstream substrates, including ribosomal protein S6 (rpS6), eukaryotic elongation factor-2 (eEF2), programmed cell death protein 4 (PDCD4), and eIF4B, which contribute to initiation of translation, elongation, and presumably protein synthesis (43, 52). Acute alcohol intoxication suppresses basal phosphorylation of rpS6 and 4E-BP1 and increases binding of the translational repressor protein, 4E-BP1, with eIF4E (32, 34, 35). Additionally, the mTORC1-mediated anabolic response of muscle to hormones [insulin and insulin-like growth factor-I (IGF-I)] and nutrients (leucine) is suppressed by prior administration of an acute bolus of alcohol in a dose- and time-dependent fashion (28, 32, 34, 53).
Muscle contraction stimulates protein synthesis and, when repetitive in nature, muscle hypertrophy develops in an mTOR-dependent manner; however, additional signaling pathways have also been implicated (7, 13, 19, 26, 38, 58). The precise mechanism(s) of mTORC1 activation following a mechanical stimulus like muscle contraction or stretch remains to be determined, since it is independent of exogenous nutrients, phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling, IGF-I responsive pathways, MEK/extracellular signal-regulated kinase (ERK) signaling, or changes in intracellular calcium levels (22–24, 38, 46, 48, 57–59). Most recently, it was hypothesized that contraction-induced mTORC1 activation is mediated, at least in part, by diacylglycerol kinase ζ-regulated synthesis of phosphatidic acid (14, 59). Muscle contraction induces phosphorylation of several substrates within the mTORC1 signaling pathway, including S6K1, 4E-BP1, mTOR, rpS6, eIF4B, and eEF2, in a time-dependent and stimulus-specific manner (11, 13, 38, 48, 58). Several different experimental paradigms result in increased mTOR signaling and/or protein synthesis, including weighted or resistance exercise, muscle overload induced by synergistic muscle ablation, and electrically stimulated muscle contraction (6, 12, 26, 46, 48, 58). This last model, which uses electrical stimulation to induce maximal muscle contractions, mimics the signaling and hypertrophic responses observed following resistance exercise and has a potential clinical advantage, since it can be performed on patients with physical limitations caused by illness or disease (6, 12).
The effects of alcohol on electrically stimulated muscle contraction-induced increases of skeletal muscle protein synthesis have not been investigated despite the direct clinical relevance; stimulated muscle contraction is a potential therapeutic modality for those with chronic alcohol-induced myopathy that is characterized by similar molecular changes as those occurring with acute intoxication (25, 32, 35). Therefore, the purpose of the present study was to determine whether acute alcohol intoxication impairs mTOR-mediated protein synthesis stimulated by in vivo muscle contraction. Because changes in mTOR signaling and protein synthesis are time-dependent following muscle contraction, and the effects of alcohol may subside as it is metabolized over time, analysis was performed at an early (30 min), intermediate (4 h), and late (12 h) time point following muscle contraction.
METHODS
Animals.
Viral antibody-free male C57BL/6 mice aged 11–12 wk old were purchased from Charles River Laboratories (Wilmington, MA) and acclimated to the animal facility at the College of Medicine at Penn State Hershey for at least 1 wk before experimental use. Mice were housed in shoe-box cages with corn cob bedding under controlled environmental conditions (12:12-h light-dark) and were provided Teklad Global 2019 (Harlan Teklad, Boston, MA) and water ad libitum until the start of the experiment. All experimental procedures were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals and were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine.
Experimental design and muscle contraction protocol.
Before each experiment, mice were fasted overnight with the muscle stimulation protocol initiated between 8:00 A.M. and 1:00 P.M. the next day. To minimize differences due to the length of fast, mice were randomly assigned to either the Alcohol (n = 7–10) or Control (n = 7–10) treatment and tested in an alternating fashion (i.e., one mouse from the Control group followed by one from the Alcohol group). Mice were administered alcohol at a dose of 3 g/kg via intraperitoneal injection or were given an equal volume of 0.9% sterile saline (Control). Administration of alcohol via intraperitoneal injection or oral gavage results in similar absorption and clearance rates as evidenced by analogous blood alcohol concentrations (BAC) (9). Alcohol was administered before muscle contraction based on prior work showing that pretreatment with alcohol antagonizes mTOR activation following other anabolic stimuli, including leucine, IGF-I, and insulin (27, 28, 53). Additionally, patients with chronic alcohol-induced myopathy might have detectable circulating levels of alcohol when stimulated muscle contraction would be performed as a therapeutic intervention, making this a relevant preclinical model.
Immediately after alcohol treatment, mice were anesthetized via isoflurane (2–3% in O2 with 1.5% maintenance). Hair was shaved from the right leg/hip, and a small incision through the skin and muscle was made for isolation of the sciatic nerve. Needle electrodes were placed over the nerve, and maximal muscular contractions were evoked using a current stimulator (model A365; World Precision Instruments, Sarasota, FL) interfaced with Powerlab 4/35 and LabChart software (ADI Instruments, Colorado Springs CO). The stimulation protocol occurred as previously described by Baar and Esser (6). Briefly, the protocol included 10 sets of 6 contractions (each lasting 3 s) with 10 s rest between contractions and 60 s rest after each set of 6 contractions (6). Pulse height was set to 6 volts and current to 1 mA to ensure maximal contractions were evoked. Each contraction produced a lengthening action of the tibialis anterior and extensor digitorum longus muscles, with a concomitant shortening of the triceps surae complex (i.e., gastrocnemius, plantaris, and soleus). The contralateral leg served as the nonstimulated control. At the cessation of the protocol, mice were given 1 ml of warm sterile saline subcutaneously and allowed to recover for the specified amount of time (30 min, 4 h, or 12 h). Mice remained in the fasted state throughout the recovery period but had free access to water. At the appropriate time point, the gastrocnemius and plantaris muscle complex (referred to as muscle from here on) was excised from the electrically stimulated and nonstimulated leg and immediately placed between aluminum blocks precooled to the temperature of liquid nitrogen. The gastrocnemius and plantaris muscles were used, since they are predominately composed of type II muscle fibers and respond to stimulated muscle contraction as well as experience the greatest decrement following treatment with alcohol (6, 36, 44). Blood was collected from the vena cava in heparinized syringes and centrifuged (10,000 g for 10 min) for isolation of plasma. Both frozen tissue and plasma were stored at −80°C until analyzed.
Experimental time points were specifically chosen based on: 1) preliminary investigations showing 30 min was sufficient time to allow for the induction of S6K1 Thr389 phosphorylation following contraction; 2) previous literature confirming a significant anabolic response by 30 min that is maximal between 3 and 12 h and maintained for at least 16 h (6, 26, 38); and 3) literature supporting a progressive time-dependent decrease in BAC following a peak at 60 min after intraperitoneal injection in mice, which corresponds to our 30-min time point (40).
Western blotting.
One-half of the frozen gastrocnemius complex (50–90 mg) was homogenized using a glass mortar and pestle in 10 volumes of ice-cold buffer consisting of (in mmol/l): 50 HEPES, 0.1% Triton X, 4 EGTA, 10 EDTA, 15 sodium pyrophosphate, 100 β-glycerophosphate, 25 sodium fluoride, 5 sodium orthovanadate, and 1 cOmplete, Mini, EDTA-free Protease Inhibitor Cocktail Tablet (Roche Applied Science, Madison, WI) per 10 ml of buffer. Protein concentration was quantified using Bio-Rad Protein Assay Dye reagent concentrate (Hercules, CA), and SDS-PAGE was carried out using equal amounts of total protein per sample. The membranes were incubated overnight at 4°C with primary antibody (Cell Signaling, Beverly, MA, unless otherwise noted). Antibodies against Akt, Akt (Ser473 and Thr308), PRAS40, PRAS40 (Thr246), S6K1, S6K1 (Thr389 and Thr421/Ser424), rpS6, rpS6 (Ser240/244 and Ser235/236), 4E-BP1 (Bethyl Laboratories, Montgomery, TX), 4E-BP1 (Ser65), PDCD4, PDCD4 (Ser67) (Abcam, Cambridge, MA), eIF4B, eIF4B (Ser422), eEF2, eEF2 (Thr56), ERK1/2 (Thr202/Tyr204), p42/44 MAPK, mTOR, and mTOR (Ser2481 and Ser2448) were used. The FluorChem M Multifluor System (ProteinSimple, San Jose, CA) was used for visualization following exposure to ECL reagent (Thermo Scientific, Waltham, MA). Images were analyzed using AlphaView (ProteinSimple) and Image J software (National Institutes of Health).
Protein synthesis.
A separate group of age-matched male C57BL/6 mice was used to determine the in vivo rate of muscle protein synthesis in the gastrocnemius complex 30 min, 4 h, or 12 h postelectrically stimulated muscle contraction. All experimental protocols were performed as described above except that at 30 min, 4 h, and 12 h after completion of electrically stimulated muscle contraction mice were injected with l-[2,3,4,5,6-3H]phenylalanine (Phe; 150 mM, 30 μCi/ml; 0.5 ml) for an additional 15 min before tissue collection. Mice were then anesthetized with isoflurane, and blood was collected in heparinized syringes from the vena cava for measurement of plasma Phe concentration and radioactivity. Muscles were excised and immediately clamped between aluminum blocks precooled in liquid nitrogen. High-performance liquid chromatography was used for the measurement of specific radioactivity of plasma Phe levels in the supernatant from trichloroacetic acid-treated plasma extracts. The global rate of [3H]Phe incorporation into protein within the muscle was assessed as previously described by our laboratory (29, 56).
Blood alcohol concentration.
The plasma alcohol concentration was determined in all samples using a rapid analyzer (Analox Instruments, Lunenburg, MA). Duplicate measurements were performed, and the average is reported.
Statistical analysis.
All data were analyzed using commercial statistic software (SigmaPlot; Systat, San Jose, CA). Within each time point (30 min, 4 h, 12 h) data were analyzed using a repeated-measures two-way ANOVA (contraction × alcohol) with Student-Neuman-Keuls post hoc tests when appropriate. Comparisons across time points were not performed. Data are presented as means ± SE and considered significant when P < 0.05.
RESULTS
Blood alcohol concentration.
The BAC was measured in plasma from all mice at each time point. Thirty minutes after muscle contraction (∼1 h after injection of alcohol), the BAC was 66 ± 3 mmol/l, which is equivalent to ∼300 mg/dl (0.3%). At the 4-h time point, the average BAC had dropped to 38 ± 3 mmol/l, which is equivalent to 175 mg/dl (0.17%). While these BACs are high, similar levels have been reported in trauma patients admitted to the emergency department (15, 21). By 12 h, the BAC was below detectable levels in alcohol-treated mice. Lastly, no alcohol was detected in the plasma from the saline-treated Control animals at any time point.
Muscle protein synthesis.
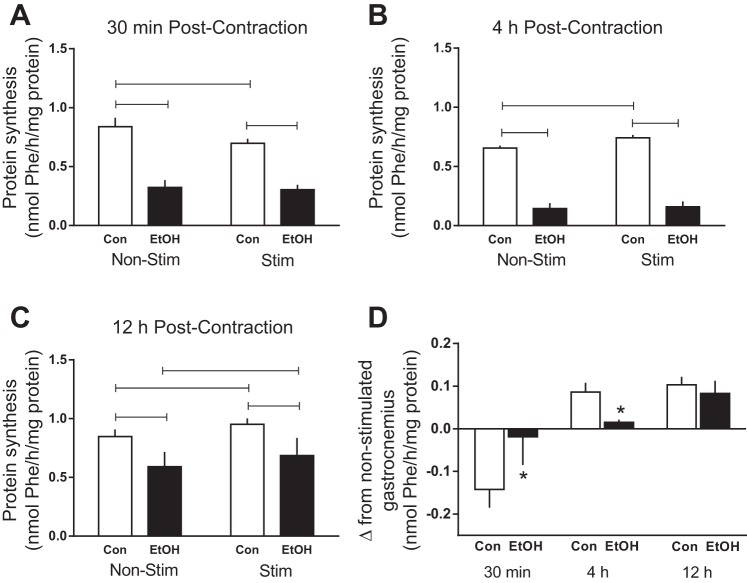
Alcohol suppressed protein synthesis in both nonstimulated and stimulated muscle at 30 min (∼60%), 4 h (∼75%), and 12 h (∼40%) compared with time-matched control values (Fig. 1, A, B, and C). Electrically stimulated muscle contraction decreased protein synthesis 17% at 30 min in Control mice, but this effect was not present in Alcohol mice (Fig. 1A). At 4 h, muscle contraction significantly increased protein synthesis by 11% in Control mice, but this enhancement was not observed in Alcohol mice (Fig. 1B). At the 12-h time point, muscle contraction increased protein synthesis 12% in the stimulated muscle of both the Control and alcohol-treated mice. However, the rate of synthesis in the stimulated muscle from alcohol-treated mice remained lower than the stimulated Control mice (Fig. 1C). To more clearly present the magnitude of change induced by electrically stimulated muscle contraction in Control and Alcohol mice, Fig. 1D shows the change in absolute synthetic rates (stimulated muscle minus nonstimulated muscle within an animal) for each time point.
Fig. 1.
Protein synthesis following alcohol and/or electrically stimulated muscle contraction. Rates of synthesis were assessed in the muscle at 30 min (A), 4 h (B), and 12 h (C) post-muscle contraction. The absolute delta change was calculated by subtracting the nonstimulated value from that of the stimulated muscle from each animal and are presented for both Control and Alcohol treatment groups at each time point (D). Shaded bars, alcohol (EtOH)-treated mice (n = 7–10); open bars, Control (Con) mice (n = 7–10). Non-Stim, control condition; Stim, muscle underwent electrically stimulated muscle contraction. Horizontal bars, statistical differences between groups (P < 0.05) (A, B, and C). *Statistical differences from time-matched Control group (P < 0.05) (D). Values are expressed as means ± SE.
mTOR signaling 30 min post-muscle contraction.
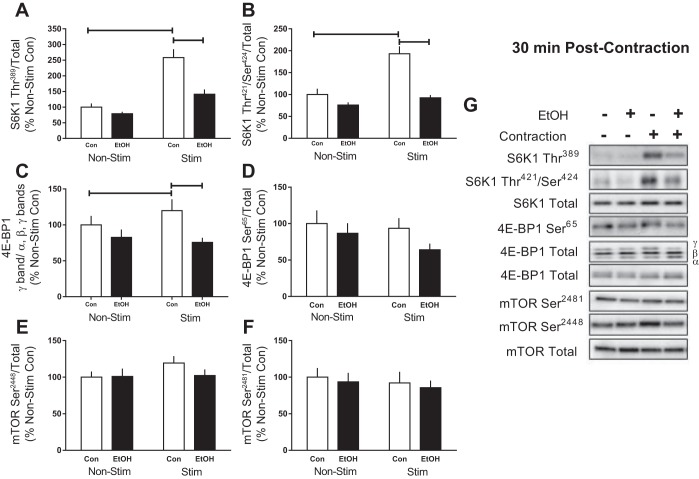
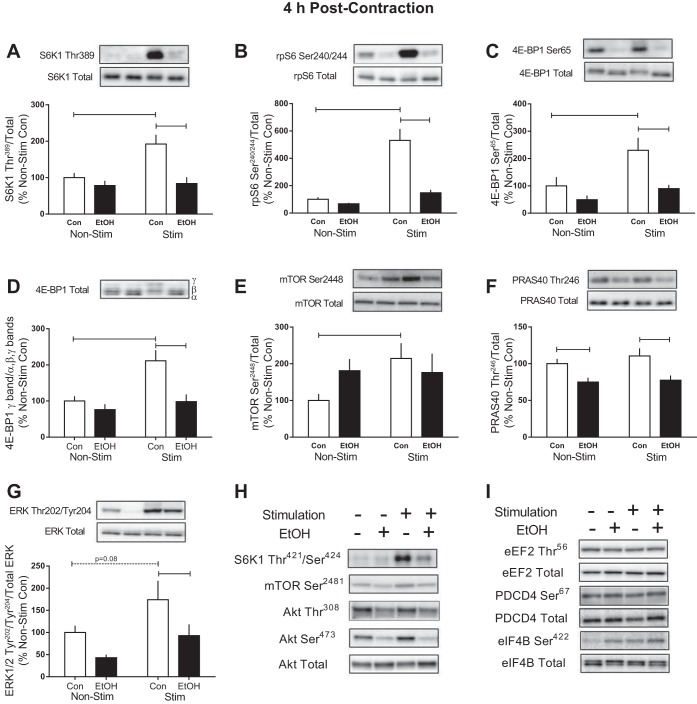
We next assessed the phosphorylation of the primary mTORC1 substrates, S6K1 and 4E-BP1. Muscle contraction increased the phosphorylation of Thr389 (158%) and Thr421/Ser424 (93%) on S6K1 after 30 min, whereas alcohol prevented the stimulation-induced increase and reduced the magnitude of change compared with that observed in the stimulated leg of Controls (Fig. 2, A and B). Electrically stimulated muscle contraction also increased the presence of the slower-migrating γ-isoform of 4E-BP1 (20%) in Controls, whereas alcohol reduced migration induced by stimulation compared with Controls (Fig. 2C). In contrast, phosphorylation of Ser65 on 4E-BP1 in muscle from Control was unchanged by stimulation, whereas the combination of alcohol and stimulated muscle contraction reduced its phosphorylation at this early 30-min time point (Fig. 2D). Phosphorylation of mTOR at the autocatalytic Ser2481 site and PI3K/AKT-mediated residue, Ser2448, was not changed by alcohol and/or muscle stimulation (Fig. 2, E and F).
Fig. 2.
Effect of alcohol and electrically stimulated muscle contraction on mammalian target of rapamycin (mTOR) complex 1 signaling. p70S6K1 (S6K1, A and B), 4E-binding protein (4E-BP1, C and D), and mTOR (E and F) were assessed 30 min post-muscle contraction in the muscle. Bar graphs represent quantification of Western blot images (G) normalized to the total amount of the respective protein with the control nonstimulated value set to 100%. Shaded bars, alcohol-treated mice (n = 9); open bars, control mice (n = 9). Horizontal bars indicate statistical differences between groups (P < 0.05). Values are expressed as means ± SE.
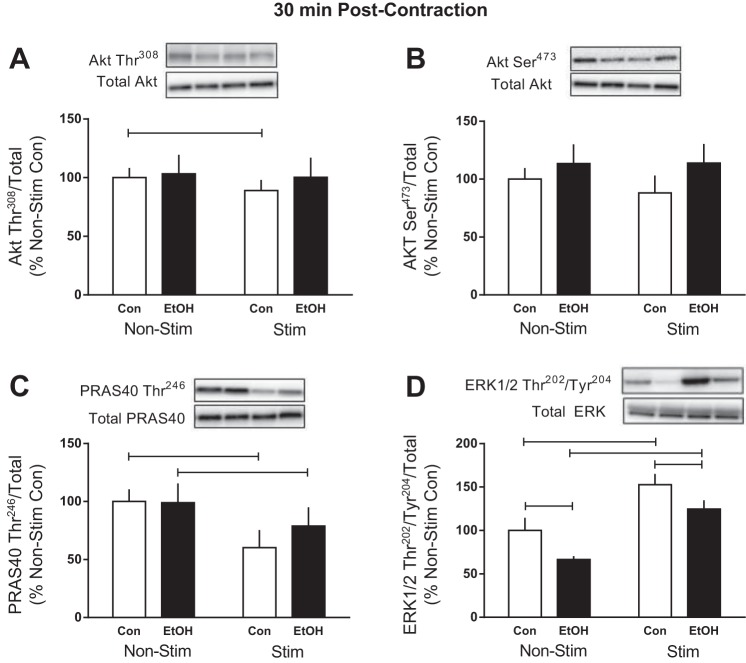
The precise signaling cascade connecting mechanical stimulation to changes in mTORC1 signaling and then eventually stimulating protein synthesis is still under investigation; however, signaling upstream and/or independent of mTOR activation may contribute (1, 38, 46). At 30 min post-electrical muscle stimulation, phosphorylation of Akt Thr308 (12%) and its downstream substrate in mTORC1, PRAS40 Thr246 (40%), was decreased by stimulation, whereas phosphorylation of Akt Ser473 was not altered (Fig. 3, A–C). Conversely, ERK Thr202/Tyr204 phosphorylation was increased 53% in the stimulated muscle of Control mice (Fig. 3D). Alcohol abrogated many of these effects, since there was no change in the phosphorylation of Akt Thr308 and Ser473 or PRAS40 Thr246 following stimulated muscle contraction in the presence of alcohol (Fig. 3, A–C). In contrast, ERK Thr202/Tyr204 was increased by muscle stimulation in alcohol-treated mice; however, the stimulation-induced increase was still blunted in Alcohol compared with Control mice (Fig. 3D).
Fig. 3.
Effect of alcohol and electrically stimulated muscle contraction on proteins upstream of mTORC1. After cessation of muscle contraction (30 min), protein kinase B (Akt) Thr308 (A), Akt Ser473 (B), PRAS40 Thr246 (C), and extracellular signal-regulated kinases (ERK)1/2 Thr202/Tyr204 (D) were assessed in the muscle. Bars graphs represent quantification of Western blot images normalized to the total amount of respective protein with the control nonstimulated value set to 100%. Shaded bars, alcohol-treated mice (n = 9); open bars, control mice (n = 9). Horizontal bars indicate statistical differences between groups (P < 0.05). Values are expressed as means ± SE.
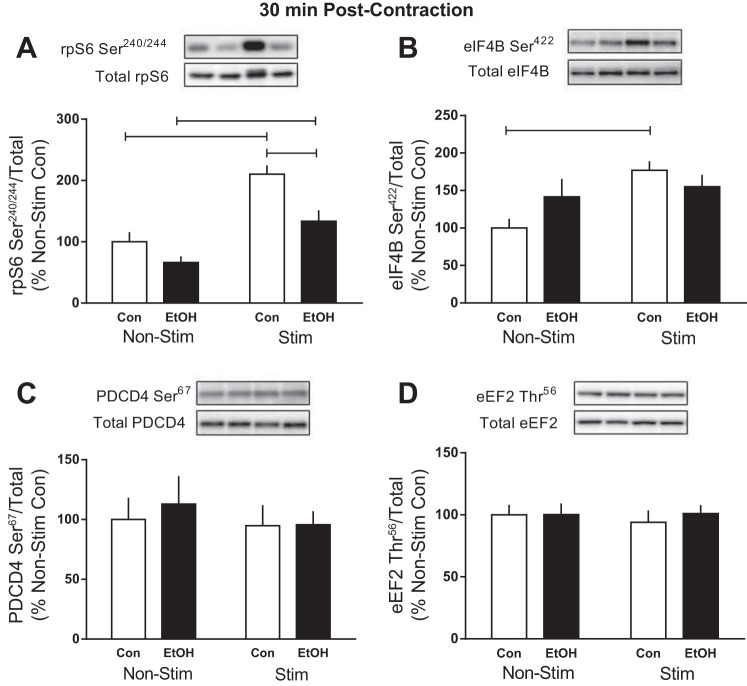
In response to phosphorylation by mTOR, S6K1 is activated and signals to numerous substrates downstream that contribute to different aspects of translation initiation and protein elongation (52). Electrically stimulated muscle contraction increased rpS6 Ser240/244 phosphorylation in gastrocnemius at 30 min in both control (110%) and alcohol-treated (100%) mice (Fig. 4A). However, the magnitude of response to contraction was reduced by alcohol (∼40%). The contraction-induced increase in the phosphorylation of eIF4B on Ser422 observed in control mice (∼80%) was not present in alcohol-treated mice (Fig. 4B). The phosphorylation of two additional downstream targets, PDCD4 and eEF2, remained unchanged by muscle stimulation and/or acute alcohol intoxication (Fig. 4, C and D).
Fig. 4.
Effect of alcohol and electrically stimulated muscle contraction on proteins downstream of S6K1. Ribosomal protein S6 (rpS6) Ser240/244 (A), eukaryotic initiation factor (eIF) 4B Ser422 (B), programmed cell death protein 4 (PDCD4) Ser67 (C), and eukaryotic elongation factor-2 (eEF2) Thr56 (D) were assessed 30 min post-muscle contraction in the muscle. Bars graphs represent quantification of Western blot images normalized to the total amount of respective protein with the control nonstimulated value set to 100%. Shaded bars, alcohol-treated mice (n = 9); open bars, control mice (n = 9). Horizontal bars indicate statistical differences between groups (P < 0.05). Values are expressed as means ± SE.
mTOR signaling 4 h post-muscle contraction.
Four hours after cessation of muscle contraction, the phosphorylation of S6K1Thr389 (92%) and Thr421/Ser424 (115%), rpS6 Ser240/244 (430%), phosphorylation of the γ-isoform of 4E-BP1 (111%), 4E-BP1 Ser65 (∼80%), and mTOR Ser2448 (114%) was increased by stimulated muscle contraction in Control mice (Fig. 5, A–E). At this time point, alcohol prevented the stimulation-induced increase in the phosphorylation of each of these proteins except for mTOR Ser2448 where no differences between the Control and Alcohol groups were detected (Fig. 5, A–E). Furthermore, Akt Ser473 and Thr308, as well as mTOR Ser2481, were unchanged by muscle contraction or alcohol at this time point (Fig. 5H and Table 1). PRAS40 phosphorylation on Thr246 was suppressed by alcohol but unchanged by muscle contraction in either group (Fig. 5F), while conversely, the phosphorylation of ERK Thr202/Tyr204 in the contracted muscle of alcohol-treated mice was decreased compared with control mice (Fig. 5G). Proteins important in translation initiation and downstream of S6K1 phosphorylation, including eEF2 Thr56, PDCD4 Ser67, and eIF4B Ser422, were unchanged by both alcohol and muscle contraction at this time point (Fig. 5I and Table 1).
Fig. 5.
mTORC1 signaling in response to alcohol and electrically stimulated muscle contraction. After cessation of muscle contraction (4 h), S6K1Thr389 (A), rpS6 Ser240/244 (B), 4E-BP1Ser65 (C), hyperphosphorylation of total 4E-BP1 (D), mTOR Ser2448 (E), PRAS40 Thr246 (F), and ERK1/2 Thr202/Tyr204 (G) were assessed in the muscle. Bars graphs represent quantification of Western blots normalized to the total amount of respective protein with the control nonstimulated value set to 100%. Representative images from Western blots for proteins listed in Table 1 and described in the text are shown in H and I. Shaded bars, alcohol-treated mice (n = 8–9); open bars, control mice (n = 9). Horizontal bars indicate statistical differences between groups (P < 0.05). Values are expressed as means ± SE.
Table 1.
Alcohol and/or electrically stimulated muscle contraction-induced changes in phosphorylation of selected proteins in muscle
| Non-Stim, % |
Stim, %, |
|||
|---|---|---|---|---|
| Protein | Control | EtOH | Control | EtOH |
| S6K1 Thr421/Ser424/total | 100 ± 4 | 103 ± 4 | 215 ± 20* | 128 ± 7^ |
| mTOR Ser2481/total | 100 ± 10 | 83 ± 5 | 105 ± 6 | 95 ± 9 |
| Akt Thr308/total | 100 ± 13 | 74 ± 7 | 94 ± 9 | 68 ± 5 |
| Akt Ser473/total | 100 ± 12 | 70 ± 14 | 95 ± 9 | 60 ± 15 |
| eEF2 Thr56/total | 100 ± 8 | 100 ± 14 | 95 ± 9 | 92 ± 10 |
| PDCD4 Ser67/total | 100 ± 15 | 94 ± 7 | 101 ± 7 | 104 ± 10 |
| eIF4B Ser422/total | 100 ± 13 | 147 ± 32 | 137 ± 10 | 146 ± 25 |
Values are means ± SE. EtOH, alcohol; S6K1, p70S6K1; mTOR, mammalian target of rapamycin; Akt, protein kinasse B; eEF2, eukaryotic elongation factor 2; PDCD4, programmed cell death protein 4; eIF4B, eukaryotic initiation factor 4B; Non-Stim, control condition; Stim, muscle underwent electrically stimulated muscle contraction.
P < 0.05 compared with Control Non-Stim value. ^P < 0.05 compared with value from the Control Stim group.
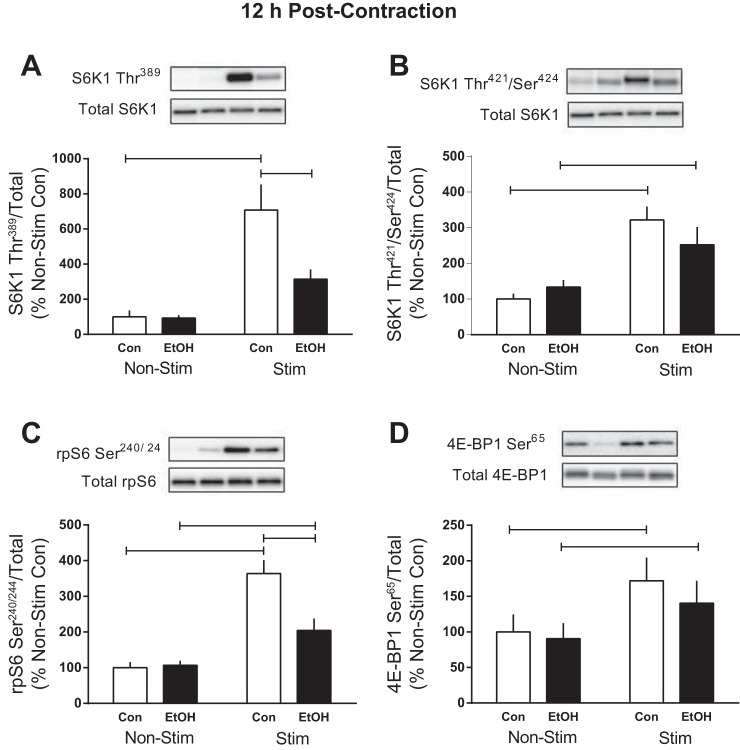
Signaling of primary mTOR substrates 12 h post-muscle contraction.
Measurement of selected mTORC1 substrates was also performed 12 h after cessation of muscle contraction, a time point at which no alcohol was present in the circulation. The contraction-induced increase in S6K1 Thr389 (∼600%) and Thr421/Ser424 (220%), as well as rpS6 Ser240/244 (265%) and 4E-BP1 Ser65 (∼70%), persisted in Control animals at this late time point (Fig. 6, A–D). Alcohol only suppressed the phosphorylation of S6K1 Thr389 and rpS6 Ser240/244 in contracted muscle compared with Controls, whereas phosphorylation of S6K1 Thr421/Ser424 and 4E-BP1 Ser65 exhibited restored responsiveness to muscle contraction (Fig. 6, A–D). Phosphorylation of ERK1/2 Thr202/Tyr204 was not altered by contraction and/or alcohol intoxication at this time point (data not shown).
Fig. 6.
Effect of alcohol and electrically stimulated muscle contraction on mTORC1 signaling. After muscle contraction (12 h), S6K1Thr389 (A), S6K1 Thr421/Ser424 (B), rpS6 Ser240/244 (C), and 4E-BP1Ser65 (D) were assessed in muscle. Bars graphs represent quantification of Western blot images normalized to the total amount of respective protein with the control nonstimulated value of 100%. Shaded bars, alcohol-treated mice (n = 8); open bars, control mice (n = 8). Horizontal bars indicate statistical differences between groups (P < 0.05). Values are expressed as means ± SE.
Magnitude of change across time in S6K1 and rpS6 phosphorylation.
While not a primary aim of this study, we selected four representative samples within each treatment group and submitted them to SDS-PAGE on the same gel for visualization of differences in the magnitude of response to muscle contraction and alcohol treatment over time (Fig. 7, A and B). Statistical analysis was not performed because of the small sample size (n = 4); however, muscle contraction evoked a similar level of activation of S6K1 Thr389 and rpS6 Ser240/244 at each time point in Control animals. As described above, alcohol impaired this contraction-mediated increase at each time point.
Fig. 7.
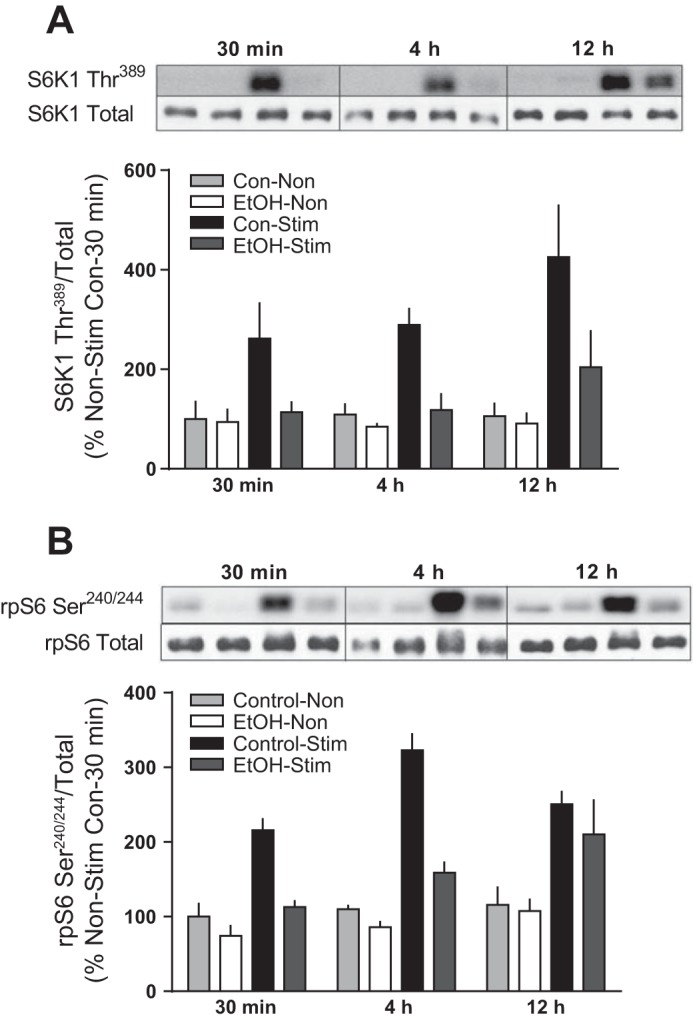
Comparison of the relative expression at each time point after alcohol and electrically stimulated muscle contraction. S6K1 Thr389 (A) and rpS6 Ser240/244 (B) were measured at 30 min, 4 h, and 12 h postmuscle contraction. Samples were resolved on the same gel, and Western blotting was performed. Bars graphs represent quantification of Western blot images normalized to the total amount of respective protein with all values set relative to the 30-min control nonstimulated value set to 100%. Shaded bars, alcohol-treated mice (n = 4); open bars, control mice (n = 4). Statistical analysis was not performed, since the graph is only representative of 4 mice/group. Values are expressed as means ± SE.
DISCUSSION
The present study investigated the time-dependent effects of acute alcohol intoxication on rates of protein synthesis and mTORC1 signaling in skeletal muscle following electrically stimulated muscle contraction. Alcohol decreased protein synthesis in the nonstimulated muscle at each time point (30 min, 4 h, and 12 h) compared with time-matched controls. Furthermore, alcohol prevented the muscle contraction-induced change in synthesis observed in Control mice at 30 min and 4 h. Although synthesis in the stimulated muscle was increased by a similar percent in Control and Alcohol mice at the 12-h time point, the absolute rate of protein synthesis remained suppressed in the alcohol-treated muscle because of the alcohol-induced reduction of basal protein synthesis. Alcohol also suppressed the phosphorylation of several proteins within the canonical mTORC1 signaling cascade that were increased by stimulated muscle contraction. However, activation of this pathway was often discordant with changes in rates of synthesis in response to muscle contraction and/or alcohol, highlighting the importance of measuring both responses.
Stimulated muscle contraction in control mice.
Although seemingly contradictory, the decrease in muscle protein synthesis observed at 30 min in Control mice is a well-recognized phenomenon (5, 8, 38, 45, 51). Definitive mechanistic explanations are still lacking, but contributing factors likely include the contraction-mediated decrease in Ca2+ release, enhanced ATP turnover, and increased eEF2 phosphorylation (4, 5, 8, 38, 45, 51). The magnitude of the early contraction-induced decrease in synthesis is directly related to the stimulus intensity, and as such we speculate that the maximal contractions produced by the current protocol resulted in a large decrease in synthesis that was still recovering at the early (30-min) time point (51). Similar to our findings, stretch of L6 myotubes decreased protein synthesis at early time points before rates returned to basal levels later in recovery (5).
Moreover, our data at 30 min postcontraction in control muscle show a discordant response between rates of synthesis and mTORC1 signaling, since protein synthesis was decreased concomitant with increased phosphorylation of S6K1 and its substrates eIF4B and rpS6. Despite similar findings being reported in humans and ex vivo rodent muscle preparations (2, 3, 5, 45), mechanistic explanations and the physiological relevance of this seemingly dissonant response between signaling and synthesis remains to be determined. Because the synthesis rate had recovered and was increased in accordance with mTORC1 activity at the 4- and 12-h time points (in Control mice), it is possible that the early induction of these factors is imperative for the later, long-lasting augmentation of protein synthesis. In support of this speculation, treatment with the mTOR inhibitor rapamycin before resistance exercise prevents the increase in protein synthesis in the post-exercise period (13, 26).
Further highlighting the need for measuring rates of synthesis directly were our findings at both 30 min and 4 h showing discordance in the phosphorylation of proteins within the mTORC1 signaling pathway. This included increased phosphorylation of S6K1, rpS6, eIF4B, and 4E-BP1 at 30 min in response to muscle stimulation while mTOR Ser2448 and Ser2481, previously thought to be markers of mTOR activity, remained unchanged at this time point. A similar relationship has been reported following stretch of L6 skeletal myoblasts, since mTOR Ser2448 was unchanged despite increased phosphorylation of S6K1 Thr389 and 4E-BP1 Thr37/46 (5). Currently, the importance of Ser2448 phosphorylation in the activation of mTOR is uncertain, since mutation of this residue to alanine does not alter the kinase activity of mTORC1 (10). However, there is also the possibility that phosphorylation of S6K1 and/or its substrates occurred by an mTOR-independent mechanism similar to that reported in rat epitrochlearis muscle undergoing ex vivo electrical stimulation in the presence of the mTOR inhibitor Torin1 (38). In summary, our findings from Control animals in response to electrically stimulated muscle contraction highlight the importance of assessing both signaling and protein synthesis to achieve a true representation of the physiological impact of the stimulus.
Alcohol-related changes independent of muscle contraction.
The exact mechanism of the alcohol-induced decrease in basal muscle protein synthesis has not been fully elucidated, although the concurrent impairment of mTORC1 signaling has previously been reported, including decreased phosphorylation of rpS6 Ser240/244 and 4E-BP1 Thr37/46 (28, 32, 34, 35). In contrast, our current findings did not reveal significant differences in several mTORC1 substrates in the nonstimulated leg following alcohol treatment, suggesting that mTOR-independent effects may be contributing to the observed reduction in rates of muscle protein synthesis. However, not all investigations link mTORC1 activity to the regulation of basal protein synthesis, since treatment with rapamycin decreases S6K1 activity but does not alter the basal rate of muscle protein synthesis or translational activity (16, 26). Furthermore, alcohol may affect other aspects of translation initiation, including reducing the formation of eIF4F complex (i.e., decrease eIF4E-eIF4G binding), which would limit binding of the mRNA to the 43S preinitiation complex (33). In chronic alcohol-fed rats, the activity of eIF2B has also been significantly suppressed, effectively limiting translation initiation and protein synthesis, and could have contributed to the present findings (36).
Additionally, differences in experimental design may have contributed to the observed discrepancies from our past data. Currently, mice were fasted overnight and remained fasted until death (∼16–24 h later), whereas previous studies used freely fed mice or overnight-fasted rats (28, 32, 34, 35). Such a prolonged fast in mice causes weight loss, depletes glycogen, and results in nearly undetectable levels of phosphorylated proteins (e.g., S6K1 Thr389) within the mTORC1 pathway as protein synthesis is suppressed to conserve energy (54). Thus, we may have failed to detect potential alcohol-induced changes in mTOR signaling in the basal condition, since protein detection was optimized to highlight changes induced by muscle stimulation within the same animal.
Effects of alcohol and muscle contraction.
The current findings extend previous work showing alcohol administered before other anabolic stimuli (refeeding, insulin, IGF-I, and leucine) suppresses the induction of protein synthesis and mTORC1 signaling (e.g., 4E-BP1 and S6K1 phosphorylation) (28, 32, 34, 53). We hypothesized that the contraction-induced increase in muscle protein synthesis would be maintained in the presence of alcohol, since muscle contraction activates mTORC1 independent of pathways previously identified to be inhibited by alcohol, including PI3K/Akt and/or hormonal stimulation (24, 48, 55). Contrary to expectations, our data show that alcohol impairs the mechanical activation of mTORC1 signaling similar to other previously reported anabolic stimuli (28, 32, 34, 53). Furthermore, protein synthesis and signaling through mTORC1 (assessed by measurement of the phosphorylation of S6K1 on Thr389 and rpS6 on Ser240/244) remained suppressed by alcohol even after the BAC had returned to undetectable levels at 12 h. Although not required, the MEK/ERK pathway may also contribute to contraction-induced changes in muscle protein synthesis and mTOR signaling (41, 42, 46). Presently though, alcohol decreased ERK1/2 phosphorylation in the nonstimulated and stimulated muscle compared with Controls, and the increase in phosphorylation caused by stimulated muscle contraction was not sufficient to overcome the alcohol-induced suppression of protein synthesis, arguing against a role for this pathway in the control of protein synthesis.
Results from the current investigation suggest that alcohol has a long-lasting effect on protein synthesis as well as mTOR signaling induced by electrically stimulated muscle contraction, albeit the effects are not always concordant, especially at earlier time points. Furthermore, we show that, even 12 h after acute alcohol treatment, the rate of protein synthesis is still suppressed compared with control animals, implying that an episode of heavy drinking the night before could limit muscular adaptations from exercise performed the following morning. These findings also suggest that if muscle contraction is to be administered in a therapeutic setting that chronic alcoholic individuals should be encouraged to abstain before the exercise session. Last, the impact of either a single incidence of acute alcohol intoxication (i.e., binge drinking) or chronic alcohol ingestion on muscle contraction-induced increases in muscle mass over a more prolonged time frame remains to be determined.
GRANTS
This work was supported in part by National Institutes of Health Grants R37-AA-011290 to C. H. Lang and F32-AA-23422 to J. L. Steiner.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.L.S. and C.H.L. conception and design of research; J.L.S. performed experiments; J.L.S. and C.H.L. analyzed data; J.L.S. and C.H.L. interpreted results of experiments; J.L.S. prepared figures; J.L.S. drafted manuscript; J.L.S. and C.H.L. edited and revised manuscript; J.L.S. and C.H.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Maithili Navaratnarajah, Anne Pruznak, and Gina Deiter for technical assistance. We also thank Dr. Sean Stocker for assistance with the electrical stimulation protocol and Dr. Brad Gordon for helpful discussions.
REFERENCES
- 1.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol 9: 747–758, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM, Hawley JA, Coffey VG. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol 591: 2319–2331, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92: 1080–1088, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Atherton PJ, Rennie MJ. It's no go for protein when it's all go. J Physiol 587: 1373–1374, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atherton PJ, Szewczyk NJ, Selby A, Rankin D, Hillier K, Smith K, Rennie MJ, Loughna PT. Cyclic stretch reduces myofibrillar protein synthesis despite increases in FAK and anabolic signalling in L6 cells. J Physiol 587: 3719–3727, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol 276: C120–C127, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Bylund-Fellenius AC, Ojamaa KM, Flaim KE, Li JB, Wassner SJ, Jefferson LS. Protein synthesis versus energy state in contracting muscles of perfused rat hindlimb. Am J Physiol Endocrinol Metab 246: E297–E305, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Chen MM, Palmer JL, Ippolito JA, Curtis BJ, Choudhry MA, Kovacs EJ. Intoxication by intraperitoneal injection or oral gavage equally potentiates postburn organ damage and inflammation (Abstract). Mediators Inflamm 2013: 971481, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res 69: 1821–1827, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M. Decrease in Akt/PKB signalling in human skeletal muscle by resistance exercise. Eur J Appl Physiol 104: 57–65, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 106: 1374–1384, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster DA. Regulation of mTOR by phosphatidic acid? Cancer Res 67: 1–4, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Friedman LS. Complications associated with blood alcohol concentration following injury. Alcohol 48: 391–400, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Garelick MG, Mackay VL, Yanagida A, Academia EC, Schreiber KH, Ladiges WC, Kennedy BK. Chronic rapamycin treatment or lack of S6K1 does not reduce ribosome activity in vivo. Cell Cycle 12: 2493–2504, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev 13: 1422–1437, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev 15: 2852–2864, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman CA. The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev Physiol Biochem Pharmacol 166: 43–95, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Holmes JF, Adams C, Rogers P, Vu P. Successful conviction of intoxicated drivers at a level I trauma center. West J Emerg Med 15: 480–485, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornberger TA, Chien S. Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle. J Cell Biochem 97: 1207–1216, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci USA 103: 4741–4746, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J 380: 795–804, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korzick DH, Sharda DR, Pruznak AM, Lang CH. Aging accentuates alcohol-induced decrease in protein synthesis in gastrocnemius. Am J Physiol Regul Integr Comp Physiol 304: R887–R898, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem 280: 7570–7580, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-I stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am J Physiol Endocrinol Metab 283: E917–E928, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Lang CH, Frost RA, Deshpande N, Kumar V, Vary TC, Jefferson LS, Kimball SR. Alcohol impairs leucine-mediated phosphorylation of 4E-BP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am J Physiol Endocrinol Metab 285: E1205–E1215, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Lang CH, Frost RA, Kumar V, Wu D, Vary TC. Impaired protein synthesis induced by acute alcohol intoxication is associated with changes in eIF4E in muscle and eIF2B in liver. Alcohol Clin Exp Res 24: 322–331, 2000. [PubMed] [Google Scholar]
- 30.Lang CH, Kimball SR, Frost RA, Vary TC. Alcohol myopathy: impairment of protein synthesis and translation initiation. Int J Biochem Cell Biol 33: 457–473, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Lang CH, Kumar V, Liu X, Frost RA, Vary TC. IGF-I induced phosphorylation of S6K1 and 4E-BP1 in heart is impaired by acute alcohol intoxication. Alcohol Clin Exp Res 27: 485–494, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Lang CH, Lynch CJ, Vary TC. Alcohol-induced IGF-I resistance is ameliorated in mice deficient for mitochondrial branched-chain aminotransferase. J Nutr 140: 932–938, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang CH, Lynch CJ, Vary TC. BCATm deficiency ameliorates endotoxin-induced decrease in muscle protein synthesis and improves survival in septic mice. Am J Physiol Regul Integr Comp Physiol 299: R935–R944, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang CH, Pruznak AM, Deshpande N, Palopoli MM, Frost RA, Vary TC. Alcohol intoxication impairs phosphorylation of S6K1 and S6 in skeletal muscle independently of ethanol metabolism. Alcohol Clin Exp Res 28: 1758–1767, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Lang CH, Pruznak AM, Nystrom GJ, Vary TC. Alcohol-induced decrease in muscle protein synthesis associated with increased binding of mTOR and raptor: Comparable effects in young and mature rats (Abstract). Nutr Metab 6: 4, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang CH, Wu D, Frost RA, Jefferson LS, Kimball SR, Vary TC. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol Endocrinol Metab 277: E268–E276, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Liu Q, Wang J, Kang SA, Thoreen CC, Hur W, Ahmed T, Sabatini DM, Gray NS. Discovery of 9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phenyl)benzo[h][1,6]naphthyridin-2(1H)-one (Torin2) as a potent, selective, and orally available mammalian target of rapamycin (mTOR) inhibitor for treatment of cancer. J Med Chem 54: 1473–1480, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Vertommen D, Rider MH, Lai YC. Mammalian target of rapamycin-independent S6K1 and 4E-BP1 phosphorylation during contraction in rat skeletal muscle. Cell Signal 25: 1877–1886, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Livingstone MB, M;. Rapamycin-insensitive mTORC1 activity controls eIF4E:4E-BP1 binding. F1000 Research 1, 2012. http://f1000r.es/NM6hpo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livy DJ, Parnell SE, West JR. Blood ethanol concentration profiles: a comparison between rats and mice. Alcohol 29: 165–171, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Ma L, Teruya-Feldstein J, Bonner P, Bernardi R, Franz DN, Witte D, Cordon-Cardo C, Pandolfi PP. Identification of S664 TSC2 phosphorylation as a marker for extracellular signal-regulated kinase mediated mTOR activation in tuberous sclerosis and human cancer. Cancer Res 67: 7106–7112, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J 441: 1–21, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Martin F, Ward K, Slavin G, Levi J, Peters TJ. Alcoholic skeletal myopathy, a clinical and pathological study. Q J Med 55: 233–251, 1985. [PubMed] [Google Scholar]
- 45.Miranda L, Horman S, De Potter I, Hue L, Jensen J, Rider MH. Effects of contraction and insulin on protein synthesis, AMP-activated protein kinase and phosphorylation state of translation factors in rat skeletal muscle. Pflugers Arch 455: 1129–1140, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki M, McCarthy JJ, Fedele MJ, Esser KA. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J Physiol 589: 1831–1846, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mothe-Satney I, Yang D, Fadden P, Haystead TA, Lawrence JC., Jr Multiple mechanisms control phosphorylation of PHAS-I in five (S/T)P sites that govern translational repression. Mol Cell Biol 20: 3558–3567, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Neil TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol 587: 3691–3701, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preedy VR, Keating JW, Peters TJ. The acute effects of ethanol and acetaldehyde on rates of protein synthesis in type I and type II fibre-rich skeletal muscles of the rat. Alcohol Alcohol 27: 241–251, 1992. [PubMed] [Google Scholar]
- 50.Price DJ, Mukhopadhyay NK, Avruch J. Insulin-activated protein kinases phosphorylate a pseudosubstrate synthetic peptide inhibitor of the p70 S6 kinase. J Biol Chem 266: 16281–16284, 1991. [PubMed] [Google Scholar]
- 51.Rose AJ, Alsted TJ, Jensen TE, Kobberø JB, Maarbjerg SJ, Jensen J, Richter EA. A Ca(2+)-calmodulin-eEF2K-eEF2 signalling cascade, but not AMPK, contributes to the suppression of skeletal muscle protein synthesis during contractions. J Physiol 587: 1547–1563, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 31: 342–348, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Sneddon AA, Koll M, Wallace MC, Jones J, Miell JP, Garlick PJ, Preedy VR. Acute alcohol administration inhibits the refeeding response after starvation in rat skeletal muscle. Am J Physiol Endocrinol Metab 284: E874–E882, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Sokolović M, Sokolović A, Wehkamp D, Ver Loren van Themaat E, de Waart DR, Gilhuijs-Pederson LA, Nikolsky Y, van Kampen AH, Hakvoort TB, Lamers WH. The transcriptomic signature of fasting murine liver (Abstract). BMC Genomics 9: 528, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol 586: 283–291, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vary TC, Jefferson LS, Kimball SR. Amino acid-induced stimulation of translation initiation in rat skeletal muscle. Am J Physiol Endocrinol Metab 277: E1077–E1086, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Witkowski S, Lovering RM, Spangenburg EE. High-frequency electrically stimulated skeletal muscle contractions increase p70s6k phosphorylation independent of known IGF-I sensitive signaling pathways. FEBS Lett 584: 2891–2895, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.You JS, Frey JW, Hornberger TA. Mechanical stimulation induces mTOR signaling via an ERK-independent mechanism: implications for a direct activation of mTOR by phosphatidic acid. PLoS One 7: e47258, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.You JS, Lincoln HC, Kim CR, Frey JW, Goodman CA, Zhong XP, Hornberger TA. The role of diacylglycerol kinase zeta and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J Biol Chem 289: 1551–1563, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]