Abstract
Progressive fibrosis involves accumulation of activated collagen producing mesenchymal cells. Fibrocytes are hematopoietic-derived cells with mesenchymal features that potentially have a unique and critical function during fibrosis. Fibrocytes have been proposed as an important direct contributor of type I collagen deposition during fibrosis based largely on fate-mapping studies. To determine the functional contribution of hematopoietic cell-derived type I collagen to fibrogenesis we utilize a double transgenic system to specifically delete the type I collagen gene across a broad population of hematopoietic cells. These mice develop a robust fibrotic response similar to littermate genotype control mice injured with bleomycin indicating that fibrocytes are not a necessary source of type I collagen. Using collagen-promoter GFP mice we find that fibrocytes express type I collagen. However, fibrocytes with confirmed deletion of the type I collagen gene have readily detectable intracellular type I collagen indicating that uptake of collagen from neighboring cells account for much of the fibrocyte collagen. Collectively these results clarify several seemingly conflicting reports regarding the direct contribution of fibrocytes to collagen deposition.
INTRODUCTION
Fibrosis is a common feature of many systemic inflammatory conditions and can also occur as a primary progressive disease. Fibrosis can lead to organ dysfunction and significant morbidity. Collectively, fibrosis is the leading cause of death in developed countries. Idiopathic Pulmonary Fibrosis (IPF) is the most common primary fibrotic lung disorder, affecting greater than 5 million people world-wide. The median survival of patients with this condition is 3–5 years from the time of diagnosis and current medical therapy is largely ineffective(1–5). Unfortunately, despite intense investigation, we still have a poor understanding of the pathogenesis of tissue fibrosis and fibrotic diseases are difficult to treat. Recent evidence suggests that fibrogenesis is more complex than originally thought and involves activation of and coordination of many cells types that contribute directly and indirectly to fibrogenesis. A more thorough delineation of the processes that underlie fibrogenesis requires the investigation of novel pathways and cell types.
Fibrosis is characterized by accumulation of activated fibroblasts and excessive deposition of fibrotic extracellular matrix proteins, especially type I collagen. Extensive research has focused on mechanisms of type I collagen synthesis by fibroblasts. However, the origin of type I collagen secreting cells remains unclear and controversial(6). Injury leads to sequential remodeling of the extracellular matrix with rapid replacement by plasma derived matrix proteins and eventual replacement with fibrillar collagens derived from activated fibrogenic cells. Initiation of these events can occur through recruitment of circulating cells with fibrogenic potential into the microenvironment, proliferation and activation of quiescent fibroblasts, and transdifferentiation of structural cells into fibroblast-like cells. The original paradigm assumed that the accumulated activated fibroblasts were derived from proliferation and activation of quiescent resident tissue fibroblasts. However, recently other possibilities for the origin of collagen producing cells have been proposed including epithelial cells, endothelial cells, pericytes, mesenchymal stem cells, and fibrocytes(7–14). Differentiating among these possibilities is important because pathways leading to their activation may be distinct. There is significant overlap in mechanisms of activation of fibroblasts, epithelial cells, endothelial cells and pericytes. For example, TGFβ is the most well established cytokine leading to fibroblast to myofibroblast transition and epithelial to mesenchymal transition(15, 16). Most, if not all, factors promoting mesenchymal transition of epithelial and endothelial cells have also been studied in models of fibroblast activation. Fibrocyte recruitment may be unique in their ability to respond to a number of cytokines and chemokines typically associated with activation of inflammatory cells(17).
Fibrocytes are hematopoietic bone marrow derived cells that express both fibroblast and leukocyte markers. They circulate in peripheral blood and can be isolated from many tissues including the lung. Cultured fibrocytes have been shown to express a number of fibrotic extracellular matrix proteins including collagen I, collagen III and fibronectin(17–21). In addition, fibrocytes maintain expression of common leukocyte markers including CD45, CD13 and CD34. Fibrocytes have been shown to secrete a number of profibrotic cytokines which could potentially help orchestrate fibrogenesis. Importantly, fibrocytes express a number of chemokine receptors including CXCR4, CCR7 and CCR2 which likely mediate recruitment and activation of fibrocytes to areas of tissue damage(22–24). Thus, recruitment of fibrocytes to sites of injury suggests an important transition from inflammation to fibrogenesis with the ability of fibrocytes to respond to inflammatory cytokines, produce fibrotic extracellular matrix proteins and foster further activation of fibroblasts and other cell types(17). While, several reports indicate that fibrocytes express type I collagen, others have suggested that uptake of secreted type I collagen by hematopoietic cells accounts for the fibrocyte population(25, 26). Thus, whether fibrocytes contribute to matrix accumulation via direct or indirect mechanisms remains unclear.
Prior studies on the origin of fibrogenic effector cells have relied primarily on generation of fate-mapping chimeric mice by either transplanting bone marrow from a transgenic reporter mouse into a wild-type mice or by crossing cell type specific Cre mice with transgenic lox-stop-lox reporter mice. Following induction of fibrosis, the genetically labeled cells are then co-stained for markers of activated fibroblasts, such as α-smooth muscle actin. However, fate-mapping studies have only led to further controversy. For example, there are contrasting reports on occurrence of the fibrocyte-myofibroblast transition, epithelial-mesenchymal and pericyte-myofibroblast transition in models of fibrosis(12–14, 27–35). Furthermore, myofibroblasts are unlikely to be the only collagen-producing cells during fibrogenesis(36). Finally while fate-mapping and co-staining for extracellular matrix protein markers demonstrates some capacity of the labeled cell type to express fibrotic matrix proteins, fatemapping studies have a limited ability to determine the quantitative and functional contribution of different cell types to accumulation of fibrotic matrix proteins. An alternative approach is to delete genes important for the function of fibrogenic cells within specific cell populations.
In this study we investigate the possibility that hematopoietic cells are a major contributor to type I collagen deposition during lung fibrogenesis utilizing mice in which the col1a1 gene has been specifically deleted within hematopoietic cells.
MATERIALS AND METHODS
Mice
The floxed col1a1 mice in a C57/Bl6 background are previously described(37). Vav-iCre mice are from Jackson Laboratories(38). Type I collagen-GFP (Col-GFP) reporter mice are previously described(39). Mice were genotyped by PCR. Six to eight week-old mice were given 50 µl intratracheal injections of saline or bleomycin (2 units/kg) dissolved in saline via surgical tracheotomy(37). At various time points after injection, mice were euthanized and samples collected for analysis. All mice were bred and maintained in a specific pathogen-free environment and all animal experiments were approved by the University Animal Care and Use Committee at University of Michigan.
Reagents
Bleomycin was purchased from Millipore (Billerica, MA). Biotin conjugated rat anti-mouse CD16/32 and CD45 antibodies, PE- and Cy7-conjugated anti-mouse CD45 antibody, FITC-conjugated anti-mouse CD11b antibody and Cytofix/Cytoperm and Perm wash were from BD Biosciences (San Jose, CA). Anti-mouse CD45 magnetic beads and MACS Cell Separation columns were purchased from Miltenyl Biotec (San Diego, CA). Collagen I antibody was purchased from Rockland (Gilbertsville, PA). HRP-conjugated secondary antibodies are from Santa Cruz (Santa Cruz, CA). Collagenase A was purchased from Roche (Indianapolis, IN). Alexa Fluorconjugated secondary antibodies and fluorescein-conjugated type I collagen are from Life Technologies (Grand Island, NY). Rabbit IgG isotype control antibody was purchased from Southern Biotech (Birmingham, AL). Collagen III antibody was from Abcam (Cambridge, MA). LAMP1 antibody was from the University of Iowa Developmental Studies Hybridoma Bank. Adenoviruses expressing Cre was from the University of Iowa Gene Transfer Vector Core Facility. All other reagents were purchased from Sigma-Aldrich (St Louis, MO).
Isolation of primary mouse lung mesenchymal cells
Primary lung mesenchymal cells were isolated as previously described(22). Briefly, six to ten week old uninjured or bleomycin injured mice were sacrificed and lungs were perfused with PBS. Lungs were removed and minced to approximately1mm cubed pieces. Minced lungs were maintained in DMEM supplemented with 10% FBS in a 37°C, 5% CO2 incubator allowing primary lung mesenchymal cell outgrowth over 2–4 weeks. Primary mesenchymal cells were analyzed between passages two to four and within four weeks of isolation. In some experiments, primary lung mesenchymal cells from col1a1fl/fl mice were treated with adenovirus encoding Cre (50 pfu/cell) (37).
Isolation of whole lung single cell suspension
Whole lung single cell suspensions were prepared as previously described (22, 40, 41). In brief, mice were euthanized at the time points indicated. Lungs were perfused with PBS, excised and minced as above. Minced lungs were incubated in digestion solution containing collagenase (1mg/mL) and DNase (25 U/mL) in RPMI at 37°C for 30 minutes. Samples were dispersed by passing up and down 20 times with 10mL syringe. After pelleting, supernatants were decanted and hypotonic shock was performed to remove RBCs. Samples were washed once with RPMI. Cells were isolated from the crude digest by passing through a 40 µm cell strainer. The cells were washed in 40% percoll, then resuspended in RPMI. Immediately after isolation, purified whole lung single cell suspensions were used for further analysis.
Flow cytometry and cell sorting
Primary lung mesenchymal cells and whole lung single cell suspensions were analyzed by flow cytometry as previously described (22, 23). Resuspended cells were incubated in blocking buffer containing 1% BSA, anti-CD16/32 antibody in PBS for 30 minutes. Cells were then incubated with fluorescent-conjugated CD45 and CD11b antibodies in blocking buffer for 30 minutes. In some experiments, cells were fixed and permeabilized with Cytofix/Cytoperm per manufacturer’s protocol. Cells were then stained with rabbit anti-collagen I antibody or rabbit IgG isotype control in Perm wash buffer for at least one hour. After washing cells were stained with fluorescent-conjugated anti-rabbit secondary antibody and analyzed by flow cytometry using an Attune Flow Cytometer (Life Technologies). Cells were sorted using a MoFlo Astrios (Beckman Coulter) or using anti-CD45 magnetic beads and the MACS Cell Separation system as previously described (22). Flow data was analyzed using FloJo (Treestar) software.
Collagen uptake assay
Equal numbers of wild-type primary murine lung mesenchymal cells were plated on 6-well plates (42). After 24 hours, cells were treated with FITC-conjugated type I collagen (50 µg/mL). After 0, 10, 30 or 90 minutes, cells were washed with PBS to remove free collagen-FITC. Resuspended cells were then stained with PE-conjugated anti-CD45 and analyzed for CD45 and FITC by flow cytometry as above. In some experiments, cell surface collagen-FITC was quenched with trypan blue (200 µg/mL) prior to flow cytometry analysis as previously described(42–44).
H&E staining and Masson’s trichrome assay
Lungs from sacrificed mice were inflated to 25 cm H2O pressure and fixed with formaldehyde. Lungs were embedded in paraffin, sectioned and stained with H&E and Masson’s trichrome by the McClinchey Histology Lab (Stockbridge, MI). Lung sections were visualized on an Olympus BX-51 microscope and images captured with an Olympus DP-70 camera(37).
Immunofluorescence Staining
Primary lung mesenchymal cells from wild-type mice were seeded on chamber slides(27, 37, 45, 46). After 24 hours, cells were fixed, permeabilized and stained for the proteins indicated as previously described. Stained slides were visualized as above.
Hydroxyproline assay and bronchoalveolar lavage (BAL)
BAL samples were obtained and quantified on a hemocytometer as previsously described.. Hydroxyproline was measured by methods previously described(37, 46, 47). Briefly, homogenates from both lungs were incubated in 12N HCl for at least 16 hours at 120°C. After addition of citrate buffer and chloramine T solutions, samples were incubated for 20 minutes. Then, Erlich’s solution was added to the mixture and incubated for an additional 15 minutes at 65°C. The absorbance at 540 nm was measured and the hydroxyproline concentration was determined against a hydroxyproline standard curve.
Gene expression analysis
mRNA was isolated from cells and tissue with TRIzol (Invitrogen) according to manufacturer’s protocol. Reverse transcription was performed with SuperScript III First-strand synthesis kit (Invitrogen) and RT-PCR was performed to using the POWER SYBR Green PCR MasterMix Kit (Applied Biosystems) and Applied Biosystems 7000 sequence detection system. The relative expression levels of col1a1 in fold changes were calculated against β-actin or GAPDH as previously described (37, 46).
DNA analysis
DNA was isolated from cells by proteinase K digestion. PCR primers within the floxed portion of the floxed col1a1 gene (exon2 2–5) were: forward 5’-AGTCAGCTGCATACACAATGGCCT-3’ and reverse 5’-ATACGATGCTTACCCTTGGGCCTT -3’. Equal loading of DNA for PCR was confirmed using primers from an adjacent region outside the floxed portions (exon 6): forward 5’-AGAGACCGAGGAGCAGAATA-3’ and reverse 5’-CAGTCCAGTGTTTCTCCCTAAA-3’. PCR products were separated on agarose gels and visualized using a UVP transilluminator (UVP, LLC). Quantitative PCR analysis of the floxed col1a1 exons 2–5 was determined using the POWER SYBR Green PCR MasterMix Kit (Applied Biosystems) and Applied Biosystems 7000 sequence detection system. Relative amount of col1a1 exons 2–5 were calculated against col1a1 exon 6.
Immunoblot
Immunoblot of protein lysate was performed as previously described(27, 37, 45, 46). Scanned immunoblots are representative of at least three separate experiments. In some experiments densitometry was quantified by ImageJ as previously described (37, 46).
Statistical analysis
Data are expressed as mean ± SEM. The 2-tailed Student’s t test was used to determine differences between groups. A P value of less than 0.05 was accepted as significant.
RESULTS
Primary lung mesenchymal cells contain CD45-positive, collagen I expressing cells
We and others have shown that primary fibroblast-like mesenchymal cells derived from outgrowth of minced lung tissue contain a subpopulation of fibrocytes which have a mixed hematopoietic and mesenchymal phenotype and protein expression(17, 22). To verify these prior observations, primary murine lung mesenchymal cells were isolated and cultured for two weeks. Cells were stained for CD45, a leukocyte common antigen present on hematopoietic cells, and analyzed by flow cytometry. The cultured primary lung mesenchymal cells were found to contain approximately 75% (50–90%) CD45-negative cells (fibroblasts) and approximately 25% (10–50%) CD45-positive fibrocytes. We confirmed expression of type I collagen, a mesenchymal marker, within both populations by several techniques. The CD45-positive and negative populations were sorted and analyzed by immunoblot and compared to alveolar macrophages as a negative control. As expected, both CD45-positive and negative cells had type I collagen protein compared to undetectable levels of collagen I in alveolar macrophages (Figure 1). There were greater levels of type I collagen in the CD45-negative population compared to the CD45-positive population consistent with prior observations(22). Type I collagen protein in fibroblasts and fibrocytes was further verified by co-immunostaining for CD45 and type I collagen. Again, both populations stained for type I collagen but there was greater type I collagen within CD45-negative fibroblasts. Finally, to ensure expression of type I collagen within CD45-positive fibrocytes we utilized a previously described type I collagen promoter-GFP (Col-GFP) reporter mouse(39). Activation of the type I collagen promoter within fibroblasts and fibrocytes was confirmed by isolating primary lung mesenchymal cells from wild-type and Col-GFP mice. After two weeks in culture cells were stained for CD45-expression and analyzed by flow cytometry. CD45-positive and CD45-negative lung mesenchymal cells from Col-GFP mice had significantly greater FL1 (GFP) signal compared to wild-type cells indicating type I collagen promoter activity within cultured fibroblasts and fibrocytes (Figure 2). Consistent with the above results, CD45-negative cells had greater FL1 signal compared to CD45-positive population indicating greater activation of the collagen I promoter within fibroblasts. Collectively, these results indicate that primary lung mesenchymal cells contain a mixture of CD45-negative / collagen I-positive fibroblasts and CD45-positive / collagen I-positive fibrocytes.
Figure 1. In vitro-derived lung mesenchymal cells contain a CD45-positive / collagen I-positive population.
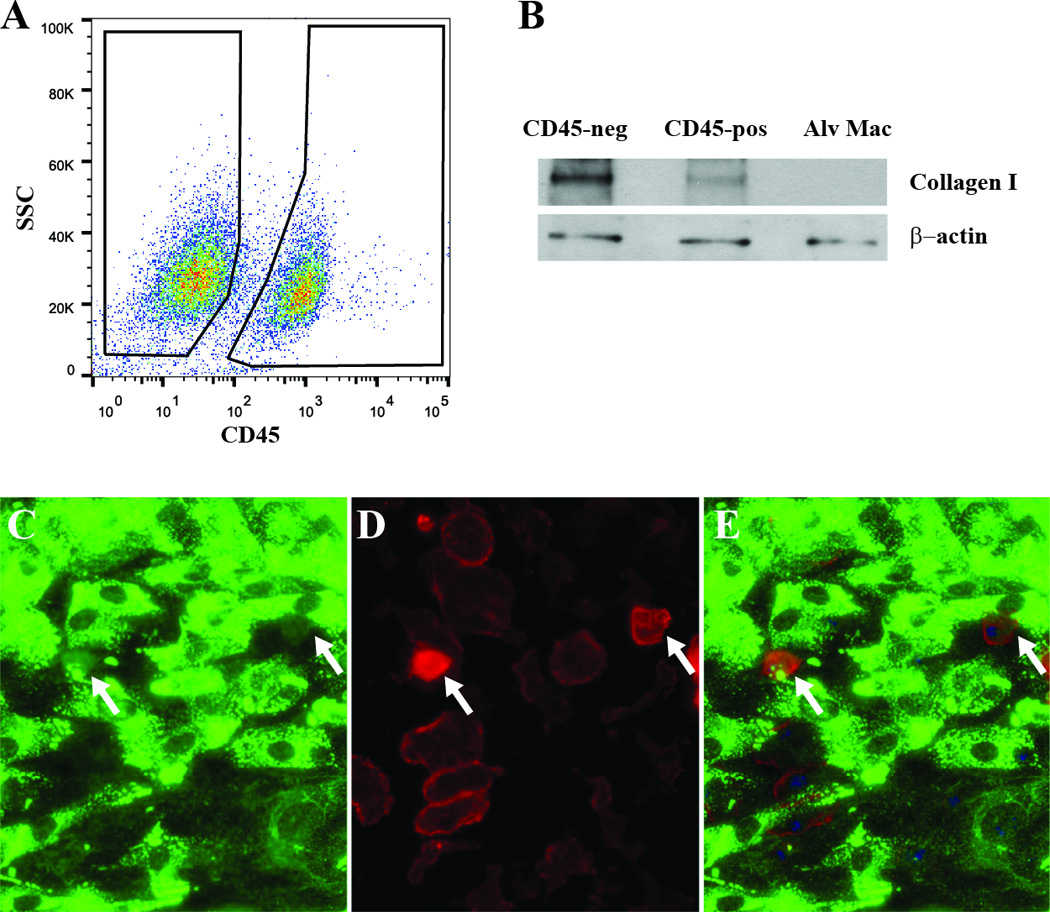
A) Primary lung mesenchymal cells from wild-type mice were stained for CD45 and analyzed by flow cytometry. The majority of cells are CD45-negative but a substantial subset are CD45-positive. B) The CD45-negative and positive populations were FACS sorted and analyzed by immunoblot and compared to alveolar macrophages as a negative control. Both CD45-positive and negative cells contained type I collagen protein but CD45-negative cells contained significantly more. Wild-type cells were co-stained for type I collagen (C), CD45 (D), and merged (E) demonstrating weak type I collagen staining within CD45-positive cells (200×).
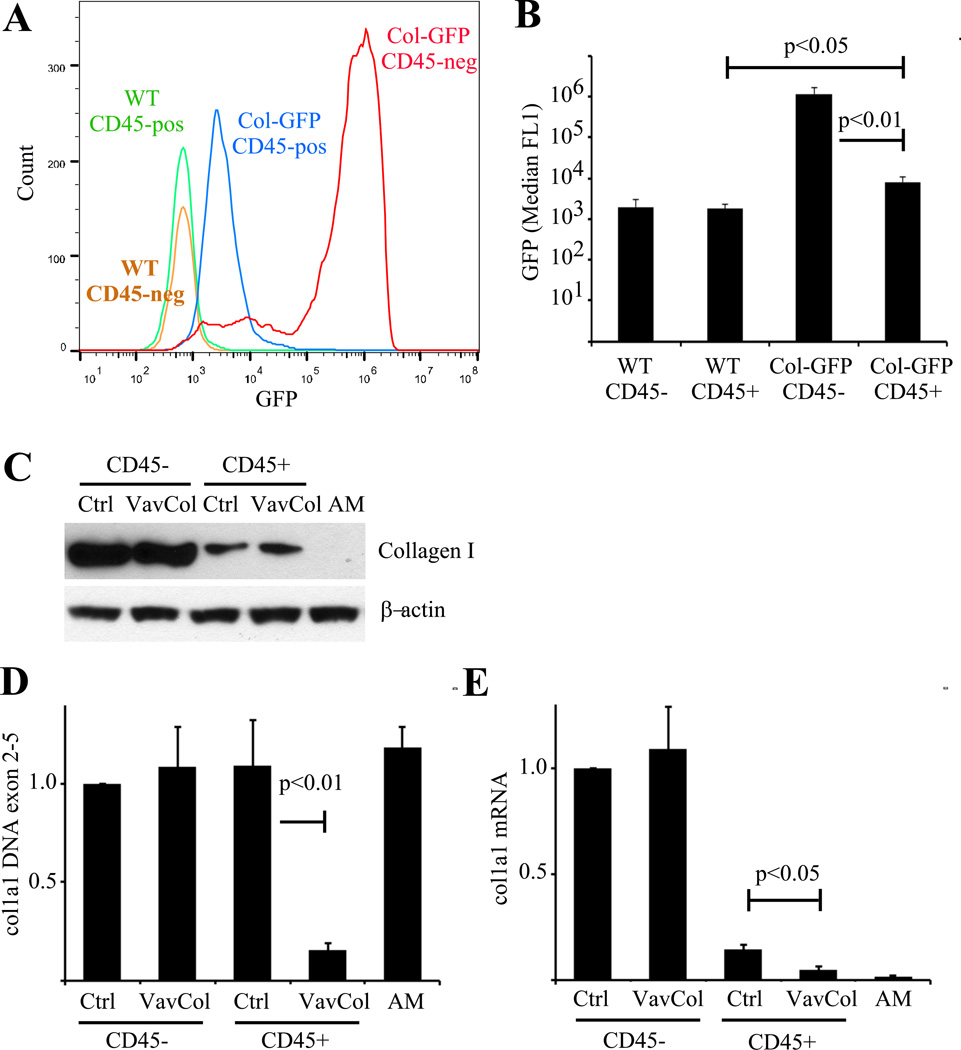
Figure 2. In vitro-derived lung fibrocytes express type I collagen.
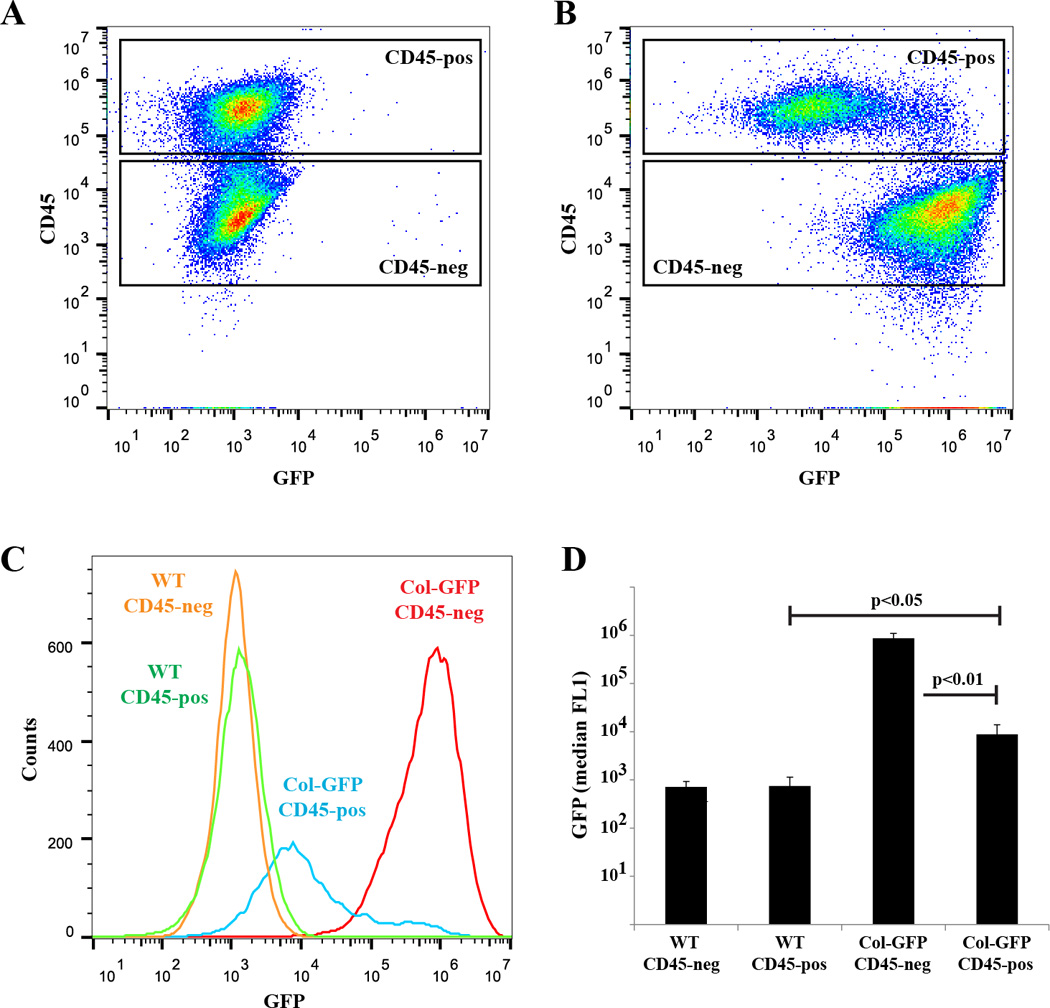
A&B) Primary lung mesenchymal cells from wild-type (A) and Col-GFP (B) mice were isolated and analyzed for expression of GFP and CD45 by flow cytometry. C) Histogram overlay of GFP expression by CD45-positive and negative lung mesenchymal cells. D) Quantification of GFP expression demonstrating greater GFP expression in CD45-negative fibroblasts (p<0.01), but significant GFP within CD45-positive fibrocytes from Col-GFP mice compared to wild-type cells (p<0.05), n=3.
Generation of mice with hematopoietic deletion of type I collagen
To determine the direct contribution of hematopoietic cells to collagen production during fibrogenesis, we utilized the previously described floxed col1a1 (col1a1fl/fl) mouse in which loxP sites flank exons 2–5 enabling permanent deletion of type I collagen within cells expressing Cre recombinase(37). Primary lung mesenchymal cells from col1a1fl/fl mice were treated in vitro with an adenovirus expressing Cre. After two weeks there was near complete deletion of type I collagen protein within CD45-positive and negative populations. Floxed collagen mice were then crossed with transgeneic Vav-cre mice which have been previously reported to delete floxed genes specifically within all hematopoietic cells(38). Double transgenic Vav-cre / col1a1fl/fl (Vav-Col) mice have normal lung histology at baseline compared to littermate control mice lacking either the Vav-cre or the col1a1fl genes (Figure 3). Primary lung mesenchymal cells were isolated from Vav-Col and littermate control mice. After two weeks in culture, cells were analyzed by flow cytometry. Vav-Col lung mesenchymal cells had similar proportions of CD45-positive and –negative cells compared to lung mesenchymal cells from littermate control mice or wild-type mice. Cells were then FACS sorted for CD45-positive and CD45-negative populations and lysed for DNA and protein isolation. DNA was analyzed by PCR using primers within the floxed portion of the floxed col1a1 allele. CD45-positive cells from the Vav-Col mice demonstrated significant loss of the floxed col1a1 exons and loss of col1a1 mRNA expression indicating robust recombination/deletion of type I collagen within fibrocytes. In contrast, CD45-negative cells from Vav-Col mice demonstrated persistent levels of the floxed col1a1 exons compared to CD45-positive and –negative cells from control mice (Figure 4). Thus, Vav-Col mice have deletion of the col1a1 gene specifically within fibrocytes. Next, protein isolated from the CD45-sorted cells were analyzed by immunoblot for type I collagen. As before, CD45-negative fibroblasts had more type I collagen than CD45-positive fibrocytes. Surprisingly, fibrocytes from Vav-Col mice had similar amounts of type I collagen compared to fibrocytes from littermate control mice (Figure 4) suggesting that much of the type I collagen protein within these cells is not through transcription/translation of type I collagen but perhaps through uptake of type I collagen secreted by the co-cultured fibroblasts.
Figure 3. Generation and of Mice with Deletion of Col1a1 within Hematopoietic Cells.
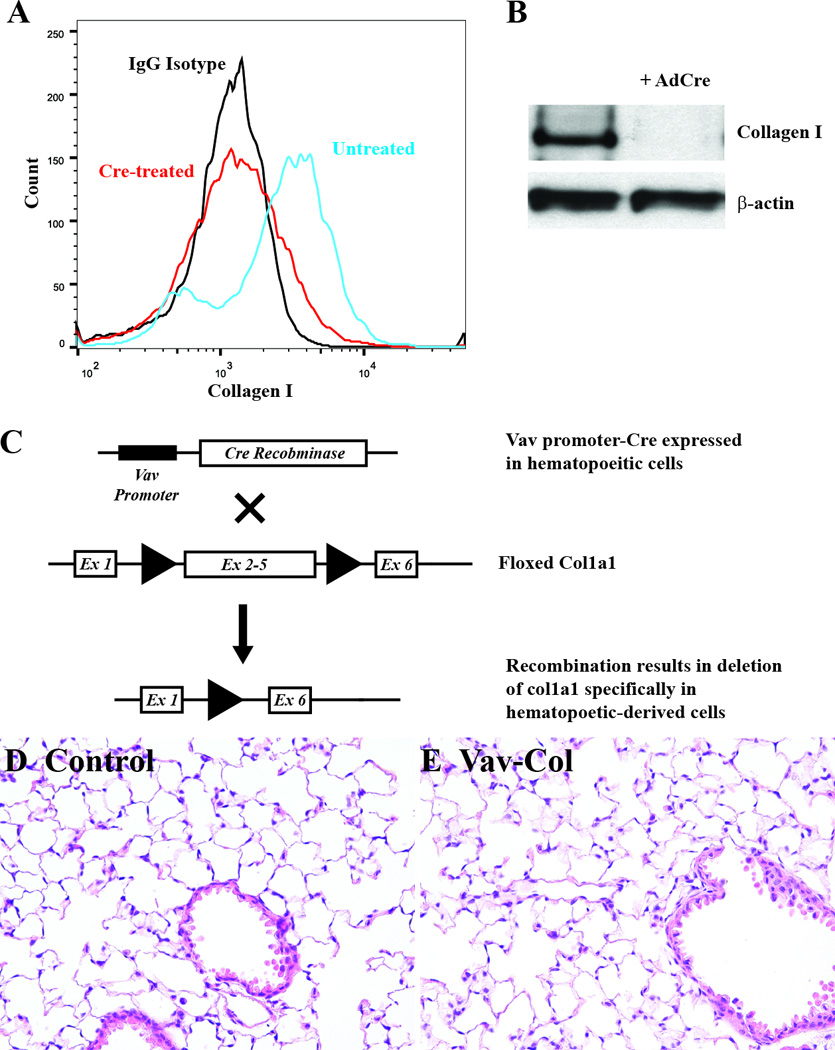
A&B) Primary lung mesenchymal cells from floxed col1a1 (col1a1fl/fl) mice were treated with adenovirus expressing Cre recombinase in vitro leading to loss of type I collagen by flow cytometry (A) and immmunoblot (B). C) Hematopoietic cell-specific and permanent deletion of col1a1 is achieved using double transgenic mice carrying the Vav-Cre transgene crossed to col1a1fl/fl mice (Vav-Col). The Vav promoter yields Cre expression specifically within hematopoietic cells resulting in permanent deletion col1a1 within hematopoietic cells and their derivatives. D&E) Uninjured lungs from Vav-Col mice (E) have normal histology compared to littermate genotype control mice lacking either the Vav-Cre or floxed col1a1 allele (D), H&E (200×).
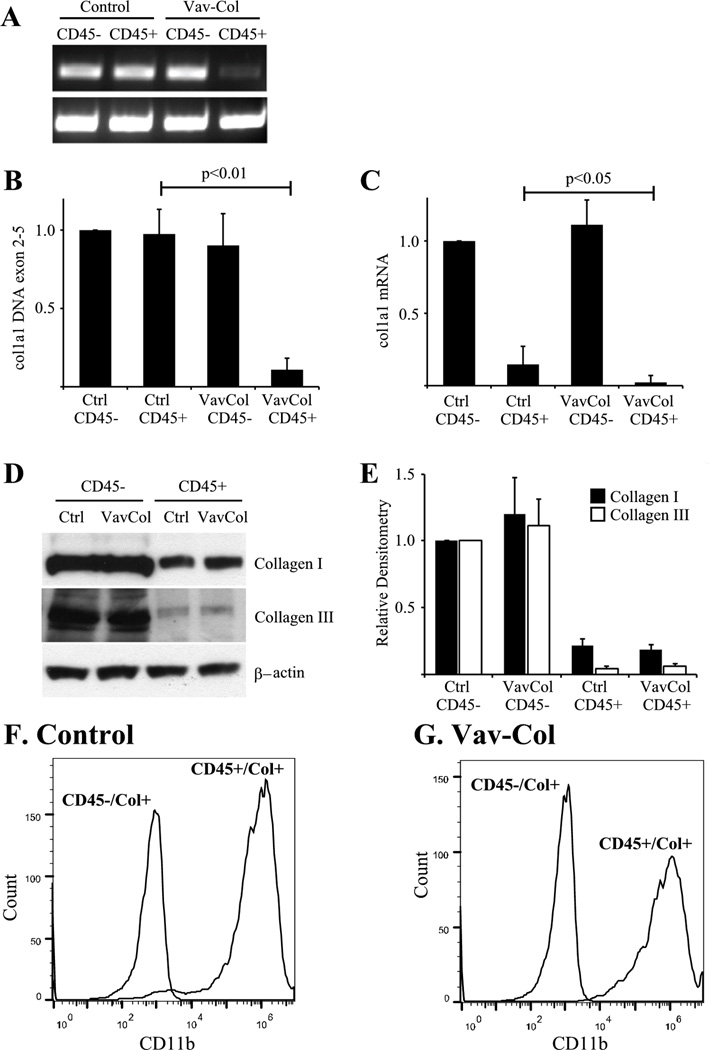
Figure 4. Fibrocytes from Vav-Col mice contain type I collagen protein even in the absence of type I collagen gene.
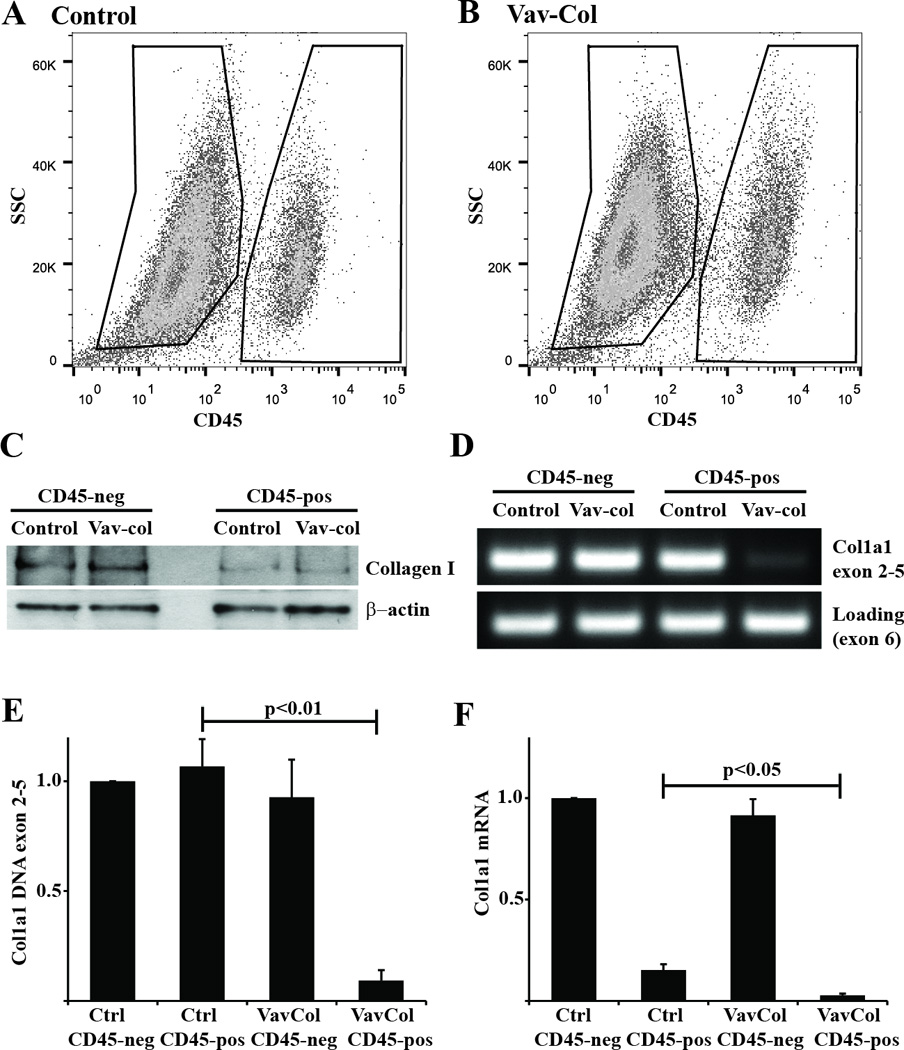
A&B) Primary lung mesenchymal cells from uninjured control (A) and Vav-Col (B) mice were FACS sorted for CD45-positive (pos) and negative (neg) populations. The Vav-Col and littermate control mice had similar proportions of CD45-pos and -neg cells. C) Immunoblot for type I collagen. CD45-neg cells have more type I collagen than CD45-pos cells. CD45-pos cells from control and Vav-Col mice have similar levels of type I collagen protein. D) PCR of DNA isolated from control and Vav-Col CD45-pos and -neg cells. Primers within the floxed region of col1a1 demonstrate absence of exons 2–5 in Vav-Col CD45-pos cells and intact col1a1 in CD45-neg cells and cells from control mice. Primers outside of the floxed region (in exon 6) were used as a loading control. E&F) CD45-pos cells from Vav-Col mice have less col1a1 exons 2–5 DNA (p<0.01) by qPCR (E) and less col1a1 mRNA by qPCR (F) (p<0.05) compared to CD45-pos cells from control mice, n=4.
Primary mesenchymal cells derived from outgrowth of minced lung tissue two weeks after intratracheal bleomycin administration demonstate a similar pattern. CD45-postive fibrocytes from bleomycin injured Col-GFP mice demonstate expression of GFP that is significantly less than GFP expression by CD45-negative fibroblasts (Figure 5A&B). CD45-positive fibrocytes from Vav-Col mice contain type I collagen protein despite absense of col1a1 exons 2–5 DNA and col1a1 mRNA (Figure 5C–E).
Figure 5. In vitro-derived fibrocytes from bleomycin injured mice express.
A&B) Two weeks after intratracheal bleomycin injury, minced lung outgrowth mesenchymal cells from wild-type and Col-GFP mice were isolated and analyzed by flow cytometry. A) Histogram overlay of GFP expression by CD45-postive (CD45+) and CD45-negative (CD45−) cells. B) Quantification of GFP expression demonstrating greater GFP expression in CD45- fibroblasts (p<0.01), but significant GFP within CD45+ fibrocytes from Col-GFP mice compared to wild-type cells (p<0.05), n=4. C–E) Two weeks after bleomycin minced lung outgrowth mesenchymal cells from control and Vav-Col mice were sorted for CD45 and compared to alveolar macrophage (AM) control. C) Immunoblot for type I collagen demonstrating less type I collagen within CD45+ cells but similar amounts of type I collagen between CD45+ cells from control mice and Vav-Col mice. D) DNA qCR for floxed col1a1 exons 2–5 DNA demonstrates CD45+ cells from Vav-Col mice have robust recombination compared to other samples (p<0.01), n=4. E) Vav-Col mice have loss of col1a1 mRNA compared to CD45+ cells from control mice by qPCR (p<0.05), n=4.
Hematopoietic-deletion of collagen does not attenuate fibrosis
Next, Vav-Col mice and littermate genotype control mice were injured with intratracheal bleomycin or saline. After two weeks, single cell preparations from the injured lungs were analyzed by flow cytometry for CD45 and intracellular type I collagen. As expected, bleomycin leads to increased numbers of collagen positive cells. Surprisingly, Vav-Col mice and genotype control mice injured with bleomycin exhibit similar numbers of CD45/collagen I double-positive cells (Figure 6). There was a trend toward more CD45-negative/collagen I-positive cells in Vav-Col mice suggesting that other cell types might be compensating for loss of functional fibrocytes, but these differences were not statistically significant (p=0.3). To ensure deletion of col1a1 within the CD45/collagen double-positive population, single cell suspensions of bleomycin injured mice were FACS sorted. DNA was immediately isolated from the sorted cells and analyzed for recombination using primers within the floxed region. As with the in vitro experiments, CD45-positive/collagen I-positive cells from Vav-Col mice had robust recombination/deletion of the col1a1gene compared to CD45-negative/collagen I positive cells from Vav-Col mice and cells from genotype control mice. Furthermore, protein and mRNA from CD45-postive and CD45-negative populations demonstrate loss of col1a1 mRNA within Vav-Col fibrocytes but similar levels of collagen I and collagen III protein by immunoblot (Figure 7). Sorted cells were further characterized for CD11b, another common fibrocyte marker, indicating high expression of CD11b within CD45-positive/collagen I-positive fibrocytes from Vav-Col and littermate control mice but not within CD45-negative/collagen I-positve fibroblasts (Figure 7F&G). Three weeks after bleomycin injury the extent of fibrosis was determined by hydroxyproline assay and trichrome staining and the extent of injury was determined by BAL cell count. Bleomycin injury led to increased total collagen content in both Vav-Col mice and littermate genotype control mice. The extent of fibrosis and injury was similar in Vav-Col and genotype control mice after bleomycin injury (Figure 8 and Supplemental Figure 1).
Figure 6. Vav-Col mice have similar numbers of fibrocytes compared to genotype control mice after bleomycin.
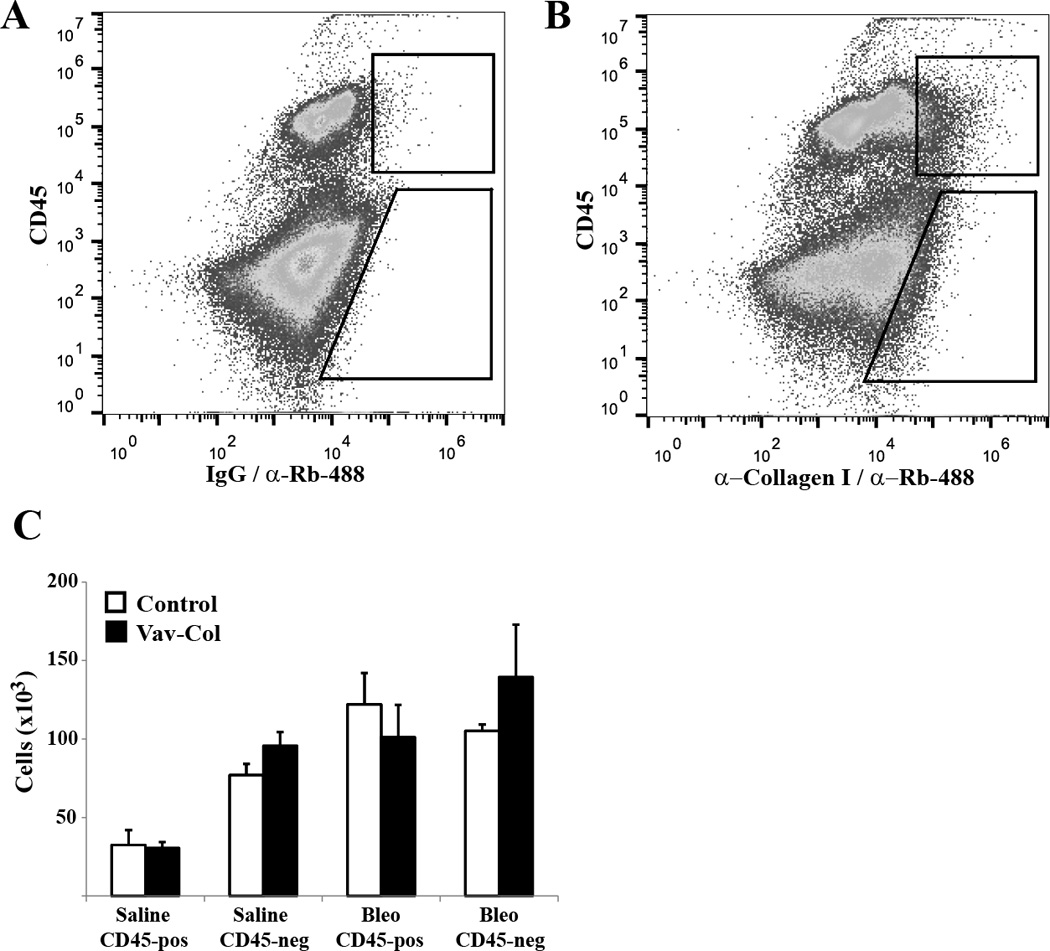
A&B) Single cell whole lung preparation from control and Vav-Col mice were analyzed by flow cytometry for CD45 and type I collagen (B) or isotype control for collagen staining (A). C) Bleomycin injury leads to increased numbers of collagen-positive cells. Bleomycin-injured Vav-Col mice have similar numbers of collagen-positive fibrocytes compared to littermate control mice (n=4).
Figure 7. Hematopoietic cell deletion of col1a1 does not affect fibrocyte type I collagen protein levels in vivo.
A) DNA was isolated from FACS sorted CD45-positive (CD45+)/collagen I-positive and CD45-negative (CD45−)/collagen I-positive cells from Vav-Col and control mice two weeks after bleomycin injury. PCR demonstrates loss of col1a1 exons 2–5 in CD45+/collagen I-positive fibrocytes from Vav-Col mice compared to intact col1a1 in cells from genotype control mice and CD45− cells from Vav-Col mice. Primers outside the floxed region (exon 6) are used as a loading control. B) qPCR DNA analysis for col1a1 exons 2–5 demonstate significant recomination within CD45+ cells from Vav-Col mice, n=4. C) qPCR analysis for col1a1 mRNA. CD45+ cells have less col1a1 mRNA than CD45− cells. CD45+ cells from Vav-Col mice have near complete absense of col1a1 mRNA compared to CD45+ cells from genotype control mice (p<0.05, n=4). D&E) Immunoblot (D) demonstrates similar levels of type I and type III collagen within CD45+ cells from littermate control and Vav-Col mice quantified by densitometry (E), n=4. F&G) CD45+/collagen I-positive (CD45+/Col+) fibrocytes from littermate control (F) and Vav-Col (G) mice also co-express CD11b compared to CD45−/collagen I-positive (CD45−/Col+) fibroblasts.
Figure 8. Mice with hematopoietic cell deletion of col1a1 are not protected from bleomycin-induced lung fibrosis.
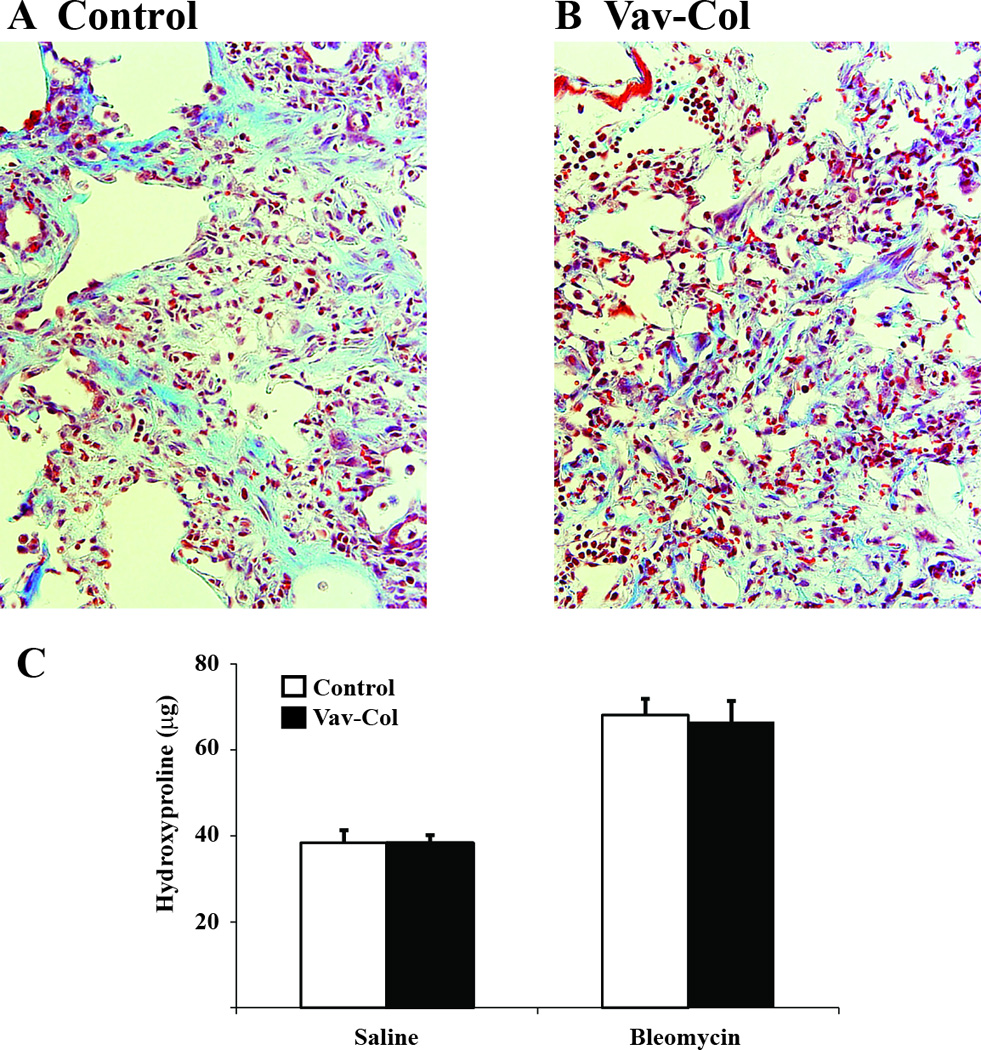
A&B) Trichrome stained lung sections from littermate control (A) and Vav-Col (B) mice three weeks after bleomycin injury demonstrate robust fibrosis in both (400×). C) Hydroxyproline assay from lungs of control and Vav-Col mice three weeks after intratracheal saline or bleomycin demonstrate similar induction of fibrosis (n=4–10).
Fibrocyte endocytosis of type I collagen
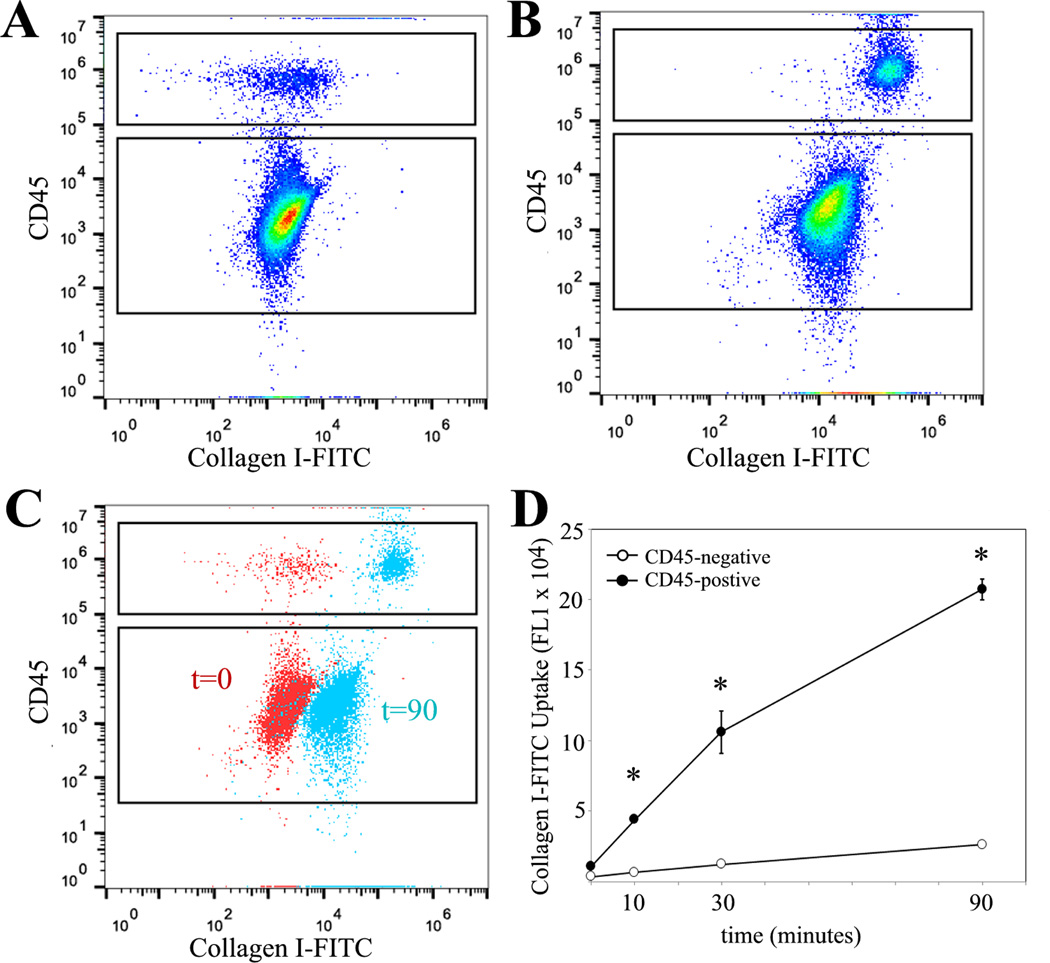
Our studies suggest that while hematopoietic cells have some capacity for activation of collagen genes, much of the type I collagen within these cells is not through production of type I collagen but rather through uptake of collagen produced by neighboring cells. Many hematopoietic cells have a significant capacity for uptake of particles and proteins through phagocytosis and pinocytosis (25, 26). To determine the capacity of primary lung mesenchymal cells to endocytose type I collagen in the culture media we utilized a purified type I collagen directly conjugated with FITC (Col-FITC) (42). Primary lung mesenchymal cells were isolated from wild-type mice. After two weeks in culture, cells were incubated with Col-FITC for 0, 10, 30 and 90 minutes. After the indicated times, cells were thoroughly washed to remove free Col-FITC and then cells were stained for CD45 and analyzed by flow cytometry. Both the CD45-positive and –negative populations demonstrated increased FL1 (FITC) signaling that increased over the 90 minutes indicating some capacity for Col-FITC uptake, consistent with prior reports of collagen uptake by different cell types (42, 48–51). There was significantly greater Col-FITC within the CD45-positive fibrocyte population compared to the CD45-negative fibroblast population. Internalization of Col-FITC was confirmed by co-localization with LAMP1, a lysozomal marker, and by cell surface fluorescence quenching with trypan blue. This indicates that fibrocytes have a robust ability to endocytose extracellular collagen (Figure 9 and Supplemental Figure 2).
Figure 9. Lung fibrocytes readily take up FITC-conjugated collagen in vitro.
A&B) Flow cytometry of primary mesenchymal cells for CD45 and FITC without addition of FITC-conjugated collagen I (t=0) (A) or 90 minutes after addition of FITC-conjugated (50 ug/mL) collagen I to the culture media (t=90) (B). C) Overlay of t=0 and t=90 plots demonstrating greater uptake of FITC-conjugated collagen I in CD45-positive cells. D) Timecourse of FITC-conjugated collagen I uptake demonstrating greater uptake by CD45-positive cells compared to CD45-negative cells (*p<0.01).
DISCUSSION
These results demonstrate that cells of hematopoietic origin produce type I collagen but are not a necessary source of type I collagen during experimental lung fibrosis. Furthermore, utilizing Vav-Cre transgenic mice to specifically delete type I collagen within hematopoietic cells we surprisingly find substantial intracellular type I collagen in fibrocytes despite confirming absence of the col1a1 gene. As our studies clarify, identification of intracellular type I collagen does not necessarily indicate collagen I production by that cell. Furthermore, since procollagen I is secreted and matured into collagen I by proteolytic cleavage in the extracellular space, identification of intracellular procollagen I also does not necessarily indicate collagen I production by that cell(52). Notably, we find activation of the type I collagen promoter by fibrocytes using GFP-reporter mice. Collectively our results suggest that fibrocytes express some type I collagen but a large portion of its intracellular collagen is through uptake from neighboring collagen-secreting cells.
We confirm prior observations that a commonly used minced lung cell outgrowth protocol to generate primary lung fibroblasts produces a mixed population of collagen-positive lung mesenchymal cells made up of CD45-negative fibroblasts and C45-positive fibrocytes(22). The resulting co-culture system may crudely model the close approximation of activated fibroblasts and fibrocytes that occurs in vivo during fibrogenesis. Both in vivo and in vitro we readily identify intracellular collagen within CD45-positive cells despite deletion of the collagen I gene indicating uptake of fibroblast derived type I collagen.
The contribution of fibrocytes to fibrogenesis remains controversial (17). Studies from fibrotic human tissue indicate that fibrocytes express some type I collagen protein and mRNA and that fibrocytes likely contribute to fibrogenesis (34, 53–56) Given the importance of type I collagen to fibrosis and the evidence that fibrocytes express type I collagen, it compelling to infer that fibrocytes a major function of fibrocytes is collagen deposition. However, the importance of fibrocyte-derived type I collagen to fibrogenesis has not been directly investigated. Several prior studies investigating the contribution of fibrocytes to fibrogenesis have utilized reporter gene chimera mice to quantify the fraction of collagen-positive cells of hematopoietic origin (14, 34, 35, 57). These prior studies are limited in their ability to assess the functional contribution of hematopoietic cells to collagen deposition which involves multiple regulated steps after collagen promoter activation (translation, secretion, maturation, fibrillogenesis, etc) (52). While our studies suggest that fibrocytes (and other hematopoietic cells) are not a necessary source of type I collagen during fibrosis, it remains possible that hematopoietic cells do contribute to some of the collagen accumulation. Pro-fibrotic cytokines released during injury may lead to recruitment of diverse populations of potential fibrotic effector cells. In this model, loss of one source of type I collagen may lead to augmented compensatory contribution of other collagen producing cells. Indeed, there is a suggestion of increased numbers of collagen-positive/CD45-negative cells in bleomycin injured Vav-Col mice compared to genotype control mice (Figure 6).
The cellular origin of type I collagen during fibrosis remains controversial. We recently reported that mice with deletion of type I collagen within alveolar epithelial cells developed less fibrosis but have more lung injury and death compared to genotype control mice, implying type I collagen from different cellular sources could have unique functions during fibrogenesis perhaps due to the unique spatial niche or timing of the collagen deposition (37). Indeed, we found that epithelial cell derived type I collagen activated collagen receptor-mediated signaling on neighbor fibroblasts, potentially leading to augmented fibroblast activation. Type I collagen itself has been well studied as an initiator of cell signaling through a number of cell surface receptors and could coordinate mesenchymal crosstalk. The importance of type I collagen from other cell types such as pericytes and mesenchymal stem cells is currently being pursued but we believe that the contribution of type I collagen by these different cell types will be more complicated than simply quantifying the fractional contribution to the overall collagen accumulation during fibrosis given the likely importance of collagen signaling and collagen-mediated cell migration.
Fibrocytes do not encompass all bone marrow derived mesenchymal cells which include mesenchymal stem/stromal cells (MSCs) which are defined, in part, as CD45-negative(58). There has been controversy on whether bone marrow derived MSCs are derived from the hematopoietic lineage or from a separate lineage. Thus, the direct contribution of bone marrow-derived MSCs to fibrosis remains unclear. However, one recent report using Vav-Cre mice in a fate-mapping strategy found evidence of labeled CD45-negative cells during fibrogenesis suggesting a common origin with hematopoietic cells (59).
We found that fibrocytes have a robust capacity for type I collagen uptake. Whether uptake of collagen occurs via collagen receptor-mediated process or by a less specific pinocytosis mechanism is unclear. Inhibition of several type I collagen receptors did not affect type I collagen uptake in our system (data not shown). Uptake of collagen by fibrocytes may result in transcriptional/phenotypic changes within fibrocytes with potential consequences for fibrosis. Finally, fibrocyte uptake of type I collagen may be an important mechanism for turnover of degraded collagen I or for clearance of abundantly secreted procollagen I (26).
Although deletion of type I collagen within fibrocytes did not attenuate fibrosis, we did find expression of type I collagen within cultured fibrocytes. Several groups have found co-expression of traditional hematopoietic and mesenchymal proteins within fibrocytes including cell surface receptors and secreted proteins. These studies suggest that fibrocytes may have unique and necessary functions during fibrogenesis through paracrine activation of other collagen-producing cells. Indeed, adoptive transfer of fibrocytes leads to augmented fibrosis in mice and several studies have found a correlation between increased numbers of fibrocytes and worse disease progression (23). Future studies into the function of fibrocytes during fibrogenesis could apply a similar strategy to the one employed in this report with deletion of mesenchymal genes within a hematopoietic population.
Supplementary Material
Acknowledgements
The authors thank Kelly McDonough and Zhen Geng for technical assistance.
Footnotes
This work was supported by National Institute of Health (NIH) grants R01 HL108904 (KKK) and R01 HL115618 (BBM).
The authors have no conflicting financial interests.
REFERENCES
- 1.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 3.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 4.Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis. 2008;3:8. doi: 10.1186/1750-1172-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 10.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, Shimokata K, Hasegawa Y. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43:161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of Lung Pericytes and Resident Fibroblasts in the Pathogenesis of Pulmonary Fibrosis. Am J Respir Crit Care Med. 2013 doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeBleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 17.Kleaveland KR, Moore BB, Kim KK. Paracrine functions of fibrocytes to promote lung fibrosis. Expert Rev Respir Med. 2014;8:163–172. doi: 10.1586/17476348.2014.862154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 19.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997;94:6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- 21.Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol. 2007;82:449–456. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- 22.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madsen DH, Ingvarsen S, Jurgensen HJ, Melander MC, Kjoller L, Moyer A, Honore C, Madsen CA, Garred P, Burgdorf S, Bugge TH, Behrendt N, Engelholm LH. The non-phagocytic route of collagen uptake: a distinct degradation pathway. J Biol Chem. 2011;286:26996–27010. doi: 10.1074/jbc.M110.208033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianchetti L, Barczyk M, Cardoso J, Schmidt M, Bellini A, Mattoli S. Extracellular matrix remodelling properties of human fibrocytes. Journal of cellular and molecular medicine. 2012;16:483–495. doi: 10.1111/j.1582-4934.2011.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J. Mol Med. 2004;82:175. doi: 10.1007/s00109-003-0517-9. [DOI] [PubMed] [Google Scholar]
- 29.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeMaio L, Buckley ST, Krishnaveni MS, Flodby P, Dubourd M, Banfalvi A, Xing Y, Ehrhardt C, Minoo P, Zhou B, Crandall ED, Borok Z. Ligand-independent transforming growth factor-beta type I receptor signalling mediates type I collagen-induced epithelial-mesenchymal transition. J Pathol. 2012;226:633–644. doi: 10.1002/path.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, Lawson WE. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180:657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5:334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Wheeler SE, Velikoff M, Kleaveland KR, LaFemina MJ, Frank JA, Chapman HA, Christensen PJ, Kim KK. Activated alveolar epithelial cells initiate fibrosis through secretion of mesenchymal proteins. Am J Pathol. 2013;183:1559–1570. doi: 10.1016/j.ajpath.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, Kioussis D. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 39.Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M, Brenner DA, Rippe RA. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology. 2003;37:267–276. doi: 10.1053/jhep.2003.50067. [DOI] [PubMed] [Google Scholar]
- 40.Bhan U, Trujillo G, Lyn-Kew K, Newstead MW, Zeng X, Hogaboam CM, Krieg AM, Standiford TJ. Toll-like receptor 9 regulates the lung macrophage phenotype and host immunity in murine pneumonia caused by Legionella pneumophila. Infect Immun. 2008;76:2895–2904. doi: 10.1128/IAI.01489-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballinger MN, Paine R, 3rd, Serezani CH, Aronoff DM, Choi ES, Standiford TJ, Toews GB, Moore BB. Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2006;34:766–774. doi: 10.1165/rcmb.2005-0246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atabai K, Jame S, Azhar N, Kuo A, Lam M, McKleroy W, Dehart G, Rahman S, Xia DD, Melton AC, Wolters P, Emson CL, Turner SM, Werb Z, Sheppard D. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119:3713–3722. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domingo-Gonzalez R, Katz S, Serezani CH, Moore TA, Levine AM, Moore BB. Prostaglandin E2-induced changes in alveolar macrophage scavenger receptor profiles differentially alter phagocytosis of Pseudomonas aeruginosa and Staphylococcus aureus post-bone marrow transplant. J Immunol. 2013;190:5809–5817. doi: 10.4049/jimmunol.1203274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee TH, McKleroy W, Khalifeh-Soltani A, Sakuma S, Lazarev S, Riento K, Nishimura SL, Nichols BJ, Atabai K. Functional genomic screen identifies novel mediators of collagen uptake. Mol Biol Cell. 2014;25:583–593. doi: 10.1091/mbc.E13-07-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, Chapman HA. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J, Velikoff M, Canalis E, Horowitz JC, Kim KK. Activated Alveolar Epithelial Cells Initiate Fibrosis Through Autocrine and Paracrine Secretion of Connective Tissue Growth Factor. Am J Physiol Lung Cell Mol Physiol. 2014 doi: 10.1152/ajplung.00243.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauman KA, Wettlaufer SH, Okunishi K, Vannella KM, Stoolman JS, Huang SK, Courey AJ, White ES, Hogaboam CM, Simon RH, Toews GB, Sisson TH, Moore BB, Peters-Golden M. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. J Clin Invest. 2010;120:1950–1960. doi: 10.1172/JCI38369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bundesmann MM, Wagner TE, Chow YH, Altemeier WA, Steinbach T, Schnapp LM. Role of urokinase plasminogen activator receptor-associated protein in mouse lung. Am J Respir Cell Mol Biol. 2012;46:233–239. doi: 10.1165/rcmb.2010-0485OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26:146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Kjoller L, Engelholm LH, Hoyer-Hansen M, Dano K, Bugge TH, Behrendt N. uPARAP/endo180 directs lysosomal delivery and degradation of collagen IV. Exp Cell Res. 2004;293:106–116. doi: 10.1016/j.yexcr.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 51.McKleroy W, Lee TH, Atabai K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L709–L721. doi: 10.1152/ajplung.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Cowper SE, Bucala R. Nephrogenic fibrosing dermopathy: suspect identified, motive unclear. The American Journal of dermatopathology. 2003;25:358. doi: 10.1097/00000372-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 54.Nihlberg K, Larsen K, Hultgardh-Nilsson A, Malmstrom A, Bjermer L, Westergren-Thorsson G. Tissue fibrocytes in patients with mild asthma: a possible link to thickness of reticular basement membrane? Respir Res. 2006;7:50. doi: 10.1186/1465-9921-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellini A, Marini MA, Bianchetti L, Barczyk M, Schmidt M, Mattoli S. Interleukin (IL)-4, IL-13, and IL-17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients. Mucosal Immunol. 2012;5:140–149. doi: 10.1038/mi.2011.60. [DOI] [PubMed] [Google Scholar]
- 56.Isgro M, Bianchetti L, Marini MA, Mattoli S. Involvement of fibrocytes in allergen-induced T cell responses and rhinovirus infections in asthma. Biochem Biophys Res Commun. 2013;437:446–451. doi: 10.1016/j.bbrc.2013.06.099. [DOI] [PubMed] [Google Scholar]
- 57.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. Journal of hepatology. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 58.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 59.Suga H, Rennert RC, Rodrigues M, Sorkin M, Glotzbach JP, Januszyk M, Fujiwara T, Longaker MT, Gurtner GC. Tracking the elusive fibrocyte: Identification and characterization of collagen producing hematopoietic lineage cells during murine wound healing. Stem cells. 2014 doi: 10.1002/stem.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.