Abstract
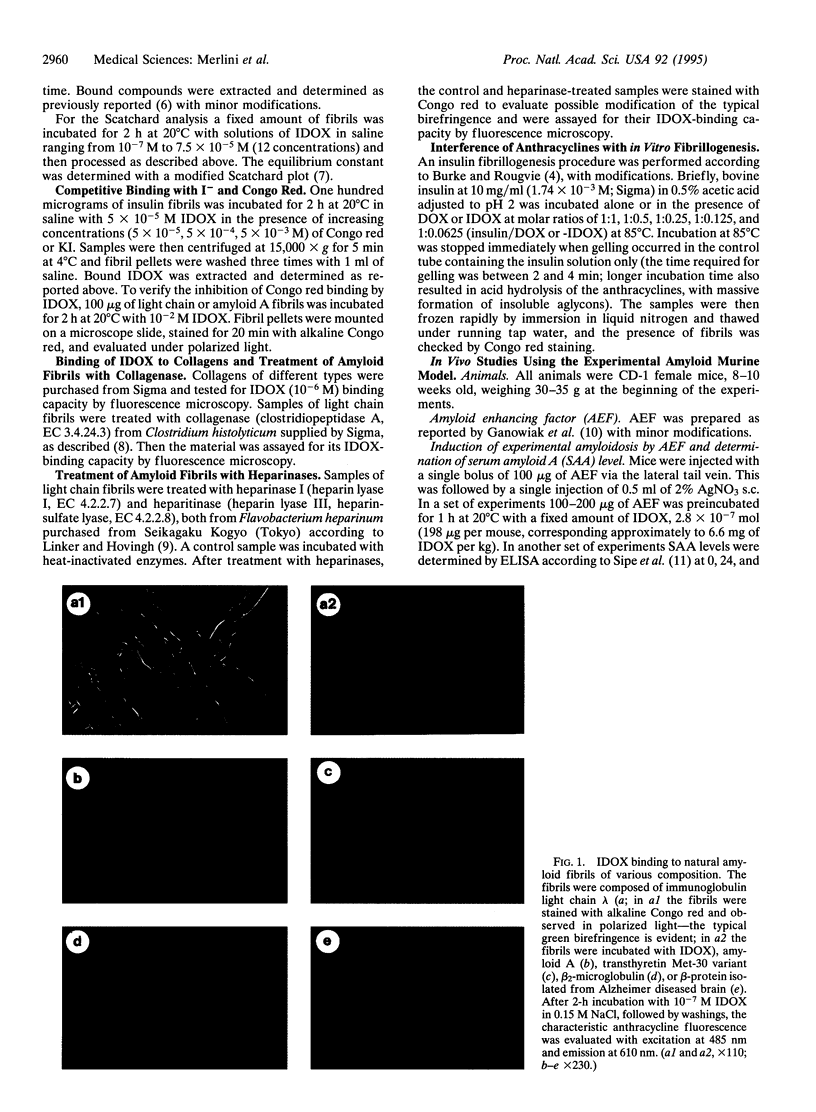
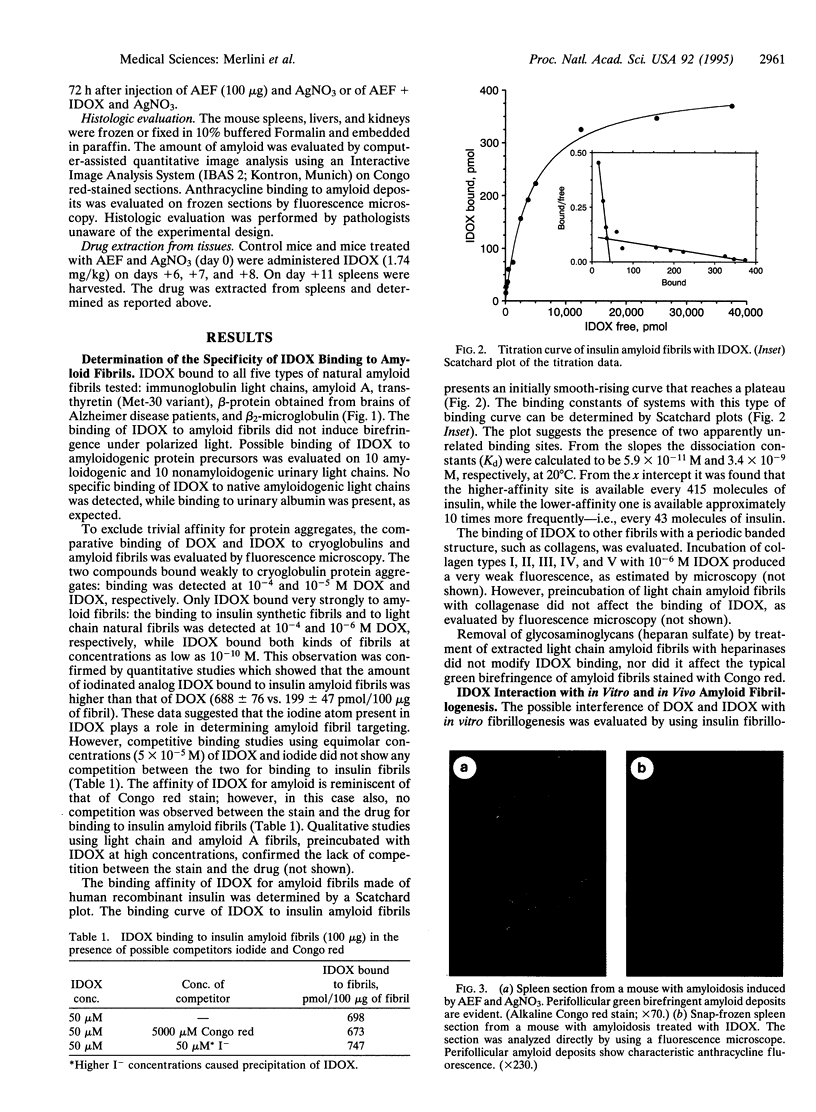
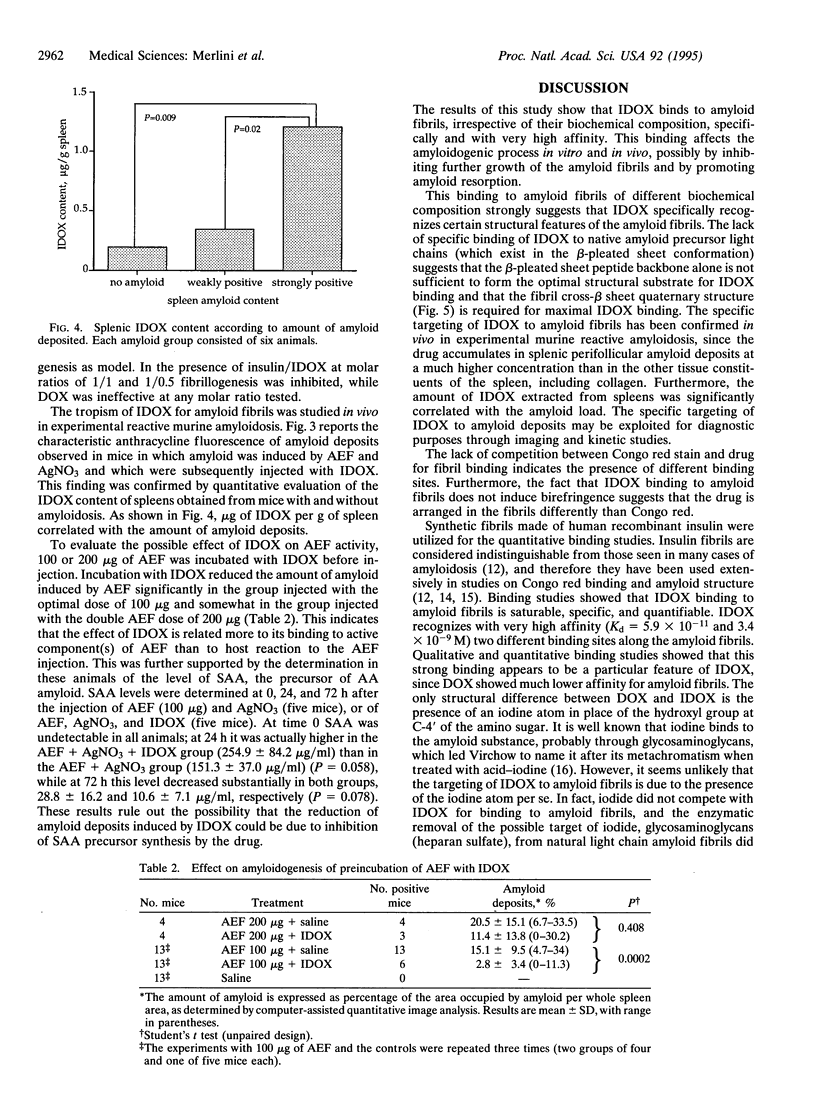
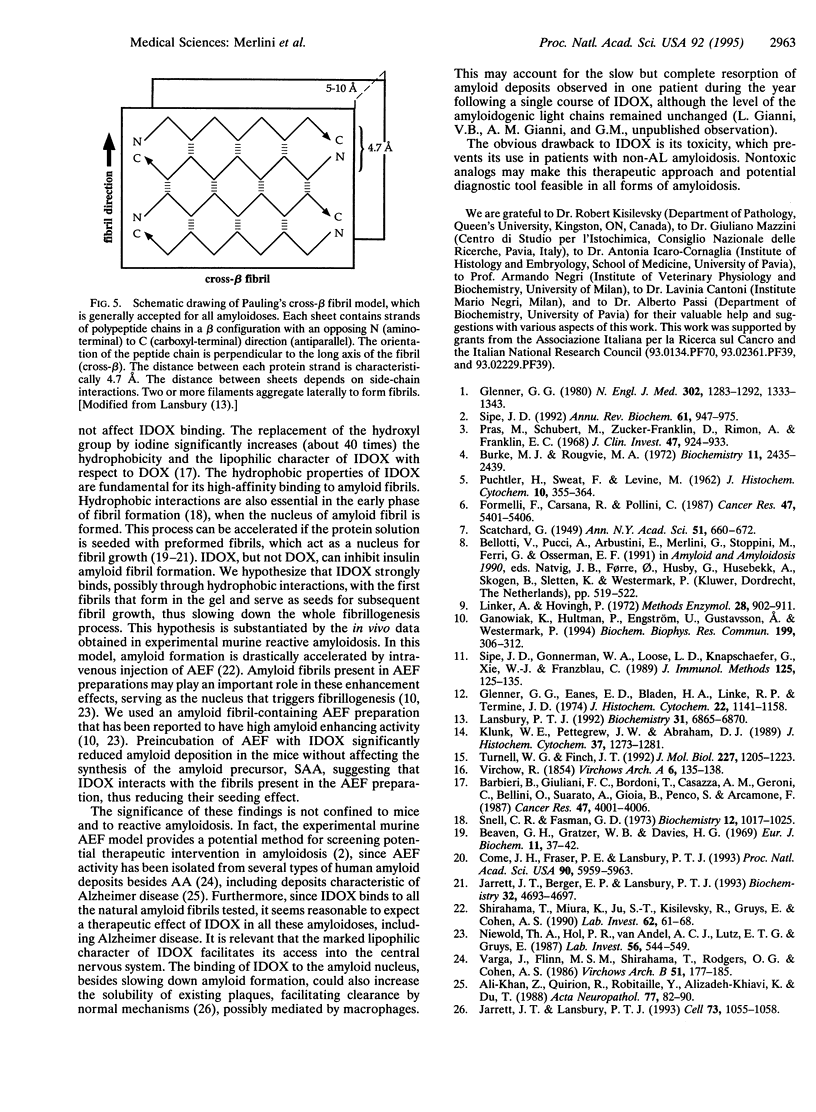
All types of amyloidosis are structurally characterized by the cross beta-pleated sheet conformation of the fibrils, irrespective of their biochemical composition. The clinical observation that the anthracycline 4'-iodo-4'-deoxy-doxorubicin (IDOX) can induce amyloid resorption in patients with immunoglobulin light chain amyloidosis was the starting point for this investigation of its possible mechanism of action. IDOX binds strongly to all five types of natural amyloid fibrils tested: immunoglobulin light chains, amyloid A, transthyretin (methionine-30 variant), beta-protein (Alzheimer), and beta 2-microglobulin. Quantitative binding studies showed that IDOX, but not doxorubicin, binds strongly to amyloid fibrils. This binding is saturable and involves two apparently distinct binding sites with Kd values of 5.9 x 10(-11) M and 3.4 x 10(-9) M. IDOX inhibited in vitro insulin amyloid fibrillogenesis. In vivo studies using the experimental amyloid murine model confirmed the specific targeting of IDOX to amyloid deposits. Preincubation of amyloid enhancing factor with IDOX significantly reduced the formation of amyloid deposits. It is hypothesized that IDOX exerts its beneficial effects through the inhibition of fibril growth, thus increasing the solubility of existing amyloid deposits and facilitating their clearance. IDOX may represent the progenitor of a class of amyloid-binding agents that could have both diagnostic and therapeutic potential in all types of amyloidoses.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali-Khan Z., Quirion R., Robitaille Y., Alizadeh-Khiavi K., Du T. Evidence for increased amyloid enhancing factor activity in Alzheimer brain extract. Acta Neuropathol. 1988;77(1):82–90. doi: 10.1007/BF00688246. [DOI] [PubMed] [Google Scholar]
- Barbieri B., Giuliani F. C., Bordoni T., Casazza A. M., Geroni C., Bellini O., Suarato A., Gioia B., Penco S., Arcamone F. Chemical and biological characterization of 4'-iodo-4'-deoxydoxorubicin. Cancer Res. 1987 Aug 1;47(15):4001–4006. [PubMed] [Google Scholar]
- Beaven G. H., Gratzer W. B., Davies H. G. Formation and structure of gels and fibrils from glucagon. Eur J Biochem. 1969 Nov;11(1):37–42. doi: 10.1111/j.1432-1033.1969.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Burke M. J., Rougvie M. A. Cross- protein structures. I. Insulin fibrils. Biochemistry. 1972 Jun 20;11(13):2435–2439. doi: 10.1021/bi00763a008. [DOI] [PubMed] [Google Scholar]
- Come J. H., Fraser P. E., Lansbury P. T., Jr A kinetic model for amyloid formation in the prion diseases: importance of seeding. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5959–5963. doi: 10.1073/pnas.90.13.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formelli F., Carsana R., Pollini C. Pharmacokinetics of 4'-deoxy-4'-iodo-doxorubicin in plasma and tissues of tumor-bearing mice compared with doxorubicin. Cancer Res. 1987 Oct 15;47(20):5401–5406. [PubMed] [Google Scholar]
- Ganowiak K., Hultman P., Engström U., Gustavsson A., Westermark P. Fibrils from synthetic amyloid-related peptides enhance development of experimental AA-amyloidosis in mice. Biochem Biophys Res Commun. 1994 Feb 28;199(1):306–312. doi: 10.1006/bbrc.1994.1229. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Eanes E. D., Bladen H. A., Linke R. P., Termine J. D. Beta-pleated sheet fibrils. A comparison of native amyloid with synthetic protein fibrils. J Histochem Cytochem. 1974 Dec;22(12):1141–1158. doi: 10.1177/22.12.1141. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Berger E. P., Lansbury P. T., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993 May 11;32(18):4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993 Jun 18;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Klunk W. E., Pettegrew J. W., Abraham D. J. Quantitative evaluation of congo red binding to amyloid-like proteins with a beta-pleated sheet conformation. J Histochem Cytochem. 1989 Aug;37(8):1273–1281. doi: 10.1177/37.8.2666510. [DOI] [PubMed] [Google Scholar]
- Lansbury P. T., Jr In pursuit of the molecular structure of amyloid plaque: new technology provides unexpected and critical information. Biochemistry. 1992 Aug 4;31(30):6865–6870. doi: 10.1021/bi00145a001. [DOI] [PubMed] [Google Scholar]
- Niewold T. A., Hol P. R., van Andel A. C., Lutz E. T., Gruys E. Enhancement of amyloid induction by amyloid fibril fragments in hamster. Lab Invest. 1987 May;56(5):544–549. [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahama T., Miura K., Ju S. T., Kisilevsky R., Gruys E., Cohen A. S. Amyloid enhancing factor-loaded macrophages in amyloid fibril formation. Lab Invest. 1990 Jan;62(1):61–68. [PubMed] [Google Scholar]
- Sipe J. D. Amyloidosis. Annu Rev Biochem. 1992;61:947–975. doi: 10.1146/annurev.bi.61.070192.004503. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., Gonnerman W. A., Loose L. D., Knapschaefer G., Xie W. J., Franzblau C. Direct binding enzyme-linked immunosorbent assay (ELISA) for serum amyloid A (SAA). J Immunol Methods. 1989 Dec 20;125(1-2):125–135. doi: 10.1016/0022-1759(89)90085-9. [DOI] [PubMed] [Google Scholar]
- Snell C. R., Fasman G. D. Kinetics and thermodynamics of the helix leads to transconformation of poly(L-lysine) and L-leucine copolymers. A compensation phenomenon. Biochemistry. 1973 Mar 13;12(6):1017–1025. doi: 10.1021/bi00730a001. [DOI] [PubMed] [Google Scholar]
- Turnell W. G., Finch J. T. Binding of the dye congo red to the amyloid protein pig insulin reveals a novel homology amongst amyloid-forming peptide sequences. J Mol Biol. 1992 Oct 20;227(4):1205–1223. doi: 10.1016/0022-2836(92)90532-o. [DOI] [PubMed] [Google Scholar]
- Varga J., Flinn M. S., Shirahama T., Rodgers O. G., Cohen A. S. The induction of accelerated murine amyloid with human splenic extract. Probable role of amyloid enhancing factor. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(3):177–185. doi: 10.1007/BF02899027. [DOI] [PubMed] [Google Scholar]




