Abstract
Fossils show that coniferous forests extended into polar regions during the Mesozoic, a time when models and independent palaeo‐CO2 indicators suggest that the atmospheric CO2 concentration was at least double that of the present day. Consequently, such polar forests would have experienced high CO2 interacting with an extreme variation in light. Here we describe an experiment investigating this plant–environment interaction for extant tree species that were important components of polar forests, and give results from the first year of treatment. Specifically, we tested the hypotheses that growth in elevated CO2 (1) stimulates photosynthesis; (2) reduces photoinhibition during the polar summer; and (3) reduces respiration of above‐ and below‐ground plant organs. Our results indicate that CO2 fertilization generally does not affect photosynthesis under continuous daylight characteristic of the polar summer but does increase it when the period of illumination is shorter. Growth in elevated CO2 did not alter the potential for photoinhibition. CO2 enrichment significantly reduced leaf and root respiration rates by 50 and 25 %, respectively, in a range of evergreen taxa. Incorporating these observed CO2 effects into numerical simulations using a process‐based model of coniferous forest growth indicates that a high palaeo‐CO2 concentration would have increased the productivity of Cretaceous conifer forests in northern Alaska. This results from decreased respiratory costs that more than compensate for the absence of high CO2–high temperature interactions during the polar summer. The longer‐term effects of CO2 enrichment on seasonal changes in the above‐ and below‐ground carbon balance of trees are discussed.
Key words: Atmospheric CO2, carbohydrates, fossil plants, photosynthesis, photoinhibition
INTRODUCTION
Marine oxygen isotope data indicate that the Earth has been in a ‘greenhouse’ mode for approx. 80 % of the past 500 million years (Spicer and Chapman, 1990; Frakes et al., 1992). Between the Mesozoic and early Tertiary [250 to 50 million years ago (Ma)], the plant fossil record shows the presence of tall, dense coniferous forests on the high latitude landmasses (Spicer and Chapman, 1990). Such forests extended to 65–85°N during the Jurassic and Cretaceous periods in Alaska and northern Russia (Spicer and Herman, 2001), and to the high latitudes (75 to 85°S) of the southern hemisphere, including Antarctica (Jefferson, 1982; Francis, 1986; Falcon‐Lang et al., 2001), South America (Archangelsky, 1963), New Zealand (Pole, 1999; Thorn, 2001) and Australia (Douglas and Williams, 1982). Amongst the best‐studied high latitude fossil forests are those from Antarctica and the Canadian High Arctic. Analyses of fossil woods and in situ stumps of canopy‐forming trees indicate that adaptation to the polar environment allowed high primary productivity, as shown by wide growth rings in fossil woods and large fossil stumps, indicative of growth to over 30 m in height (Jefferson, 1982; Francis, 1986; Falcon‐Lang and Cantrill, 2000, 2001). Palaeobotanical investigations further suggest a geographical difference in the dominant leaf habit between hemispheres, with mid‐Cretaceous polar forests in Alaska and northern Russia being a mixture of deciduous and evergreen vegetation, and those in Australia and Antarctica being predominantly evergreen (Falcon‐Lang, 2000; Falcon‐Lang and Cantrill, 2000, 2001).
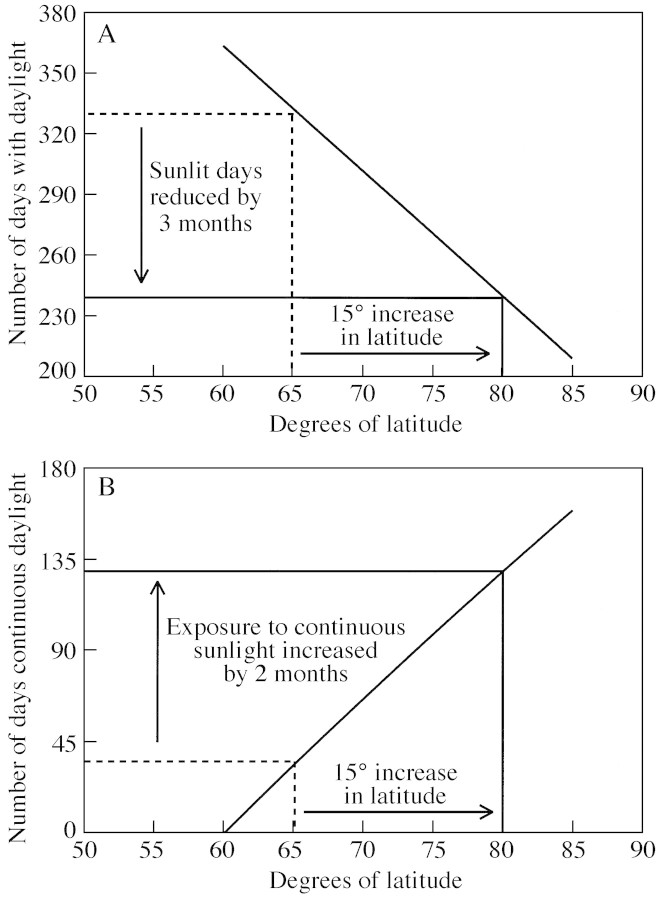
The polar environment, with its highly seasonal light regime, provides unusual conditions for tree and forest growth. Ancient forests located at 80°N, for example, experienced a growing season some 3 months shorter than those at 65°N (Fig. 1A), a severe constraint on photosynthetic carbon acquisition, and thus to survival during the long polar winter (Creber and Chaloner, 1985). To survive the extended darkness of the polar winter trees must store sufficient energy reserves, in the form of carbohydrates, to fuel their metabolism (Penning de Vries, 1975; Read and Francis, 1992). Furthermore, those same high latitude forests must endure 2 months more continuous polar sunlight compared with their counterparts at 65°N (Fig. 1B), with an associated increased risk of photoinhibition, i.e. reduced CO2 uptake caused by a decline in photosynthetic efficiency in high light conditions (Long et al., 1994). This risk is compounded by the negative feedback operating on CO2‐uptake if leaf carbohydrates accumulate (Sheen, 1994; Hymus et al., 1999).
Fig. 1. Changes in length of the growing season (A) and the polar summer (B) as a function of latitude, calculated from equations describing sun–earth geometry (Monteith and Unsworth, 1990). Broken lines indicate the growing season and polar summer lengths at 65°N, with solid lines indicating the situation 15° closer to the pole at 80°N.
One crucial difference between the environment of today’s polar vegetation and that of Mesozoic polar forests is the concentration of atmospheric CO2. Geochemical modelling of the long‐term carbon cycle and independent CO2 indicators suggest that for most of the past 250 Ma the atmospheric CO2 concentration was several times higher than at present (reviewed in Royer et al., 2001). It is well established that many key physiological processes of trees are strongly influenced by a CO2‐enriched atmosphere (Curtis and Wang, 1998; DeLucia et al., 1999; Norby et al., 1999; LaDeau and Clark, 2001). Leaf photosynthesis is typically stimulated by 40–80 % during growth with double the present concentration of CO2 (Drake et al., 1997; Curtis and Wang, 1998; DeLucia et al., 1999; Norby et al., 1999). Therefore, increased CO2 concentration has the potential to counteract the limitation on the growth of polar forests imposed by the short, high‐latitude growing season. Experimental evidence also suggests that the higher atmospheric CO2 may decrease photoinhibition (Hymus et al., 1999; Roden et al., 1999; Terry et al., 2000), suggesting an important interaction for ancient polar forests experiencing continuous irradiance, with the potential to cause photoinhibition during the summer. In addition, the carbon balance of polar forests will be determined not only by photosynthesis but also by respiration, which itself can be influenced directly and indirectly by elevated CO2 (Wullschleger et al., 1994; Bunce, 2001). For example, atmospheric CO2 enrichment reduces the mitochondrial respiration rates of leaves, roots and stems, through direct inhibition of enzyme activity (Drake et al., 1999).
These potential interactions are important for understanding polar forest ecology. It has been suggested that the deciduous habit of polar forests reduced respiratory costs allowing plants to tolerate winter darkness and mild temperatures (Axelrod, 1984; Creber and Chaloner, 1985). But if respiration is decreased by elevated CO2, the requirement for a deciduous habit is diminished. Alternatively, if elevated CO2 affects respiration indirectly, for example by altering substrate availability and the demand for respiratory products, the respiration rates of leaves grown at elevated CO2 may increase (Drake et al., 1999).
These physiological considerations show that the effects of palaeo‐CO2 concentrations may be especially important in understanding polar forest physiology and should be assessed (Beerling, 1998, 2000). However, the responses of trees to CO2 enrichment have not yet been assessed experimentally under a light regime relevant to polar forests. Here we describe an experimental approach investigating the effects of a high atmosphere CO2 concentration on the physiology of some extant genera of plants that were important components of northern and southern hemisphere polar forests (Table 1). We recognize that, whilst the morphology of extant and fossil taxa are similar, the species may not be the same, but we assume that their physiological characteristics are similar. The responses of young trees to atmospheric CO2 enrichment during their first year’s growth in a simulated polar light regime (equivalent to latitude = 69°) are reported. Utilizing this facility, we tested the hypotheses that elevated CO2 (1) stimulates photosynthetic carbon gain and carbohydrate storage; (2) lowers the potential for photoinhibition during polar summers; and (3) suppresses rates of plant respiration. The experimental results have been incorporated into a process‐based model of conifer forest growth (Osborne and Beerling, 2000) to provide a first assessment of the possible consequences of the interactions between CO2 and light for the productivity of mid‐Cretaceous north Alaskan coniferous forests.
Table 1.
Woody plant species grown under polar light conditions in a simulated Cretaceous environment with and without CO2 enrichment
| Species | Family | Life form | Native habitat |
| Ginkgo biloba (maidenhair tree) | Ginkgoaceae | Deciduous tree | Uncertain, widely planted in urban environments, but probably sub‐tropical forests of southern China* |
| Metasequoia glyptostroboides (dawn redwood) | Taxodiaceae | Deciduous conifer | Mesothermal forested regions of S.E. China† |
| Sequoia sempervirens (coastal redwood) | Taxodiaceae | Evergreen conifer | Low hills, near coastal regions of California‡ |
| Taxodium distichum (swamp cypress) | Taxodiaceae | Deciduous conifer | Tidal creeks, flood plains, southern USA |
| Araucaria araucana (monkey‐puzzle) | Araucariaceae | Evergreen conifer | Forests in the southern Andes, Chile§ |
| Nothofagus cunninghamii (southern beech) | Nothofagaceae | Evergreen tree | Cool/temperate forests, Australia/Tasmania¶ |
* Tredici et al. (1992).
† Chaney (1948); Chu and Cooper (1950).
‡ Waring and Franklin (1979).
§ De Laubenfels (1988).
¶ Read (1999).
MATERIALS AND METHODS
Experimental design and performance
The controlled environment facility (Tapton Experimental Gardens, University of Sheffield, UK) consists of four glasshouses, each divided into two isolated sections. This provides eight independent replicated environments: four maintained at ambient CO2 (400 µmol mol–1) and four at the target CO2 concentration (800 µmol mol–1). Control of temperature, humidity and CO2 in each section was achieved using a programmable datalogger (CR10 Measurement and Control System; Campbell Scientific, Inc., Logan, Utah, USA). CO2 was measured with an infrared gas analyser (IRGA) mounted in each glasshouse (CO2 Gas Monitor, ADC 2000 Series; The Analytical Development Company Limited, Hoddesdon, Herts., UK). The calibration of each IRGA was checked monthly using a volumetrically mixed reference gas (Certified Standard ± 5 %; BOC Gases, Guildford, Surrey, UK). The CO2 concentration was increased by frequent injection of pure CO2 gas (BOC Gases, UK) into the circulating air of each section. During the first year of operation (treatments began in April 2000), daily mean CO2 concentrations were maintained to within ± 5 % of the target values (Fig. 2A).
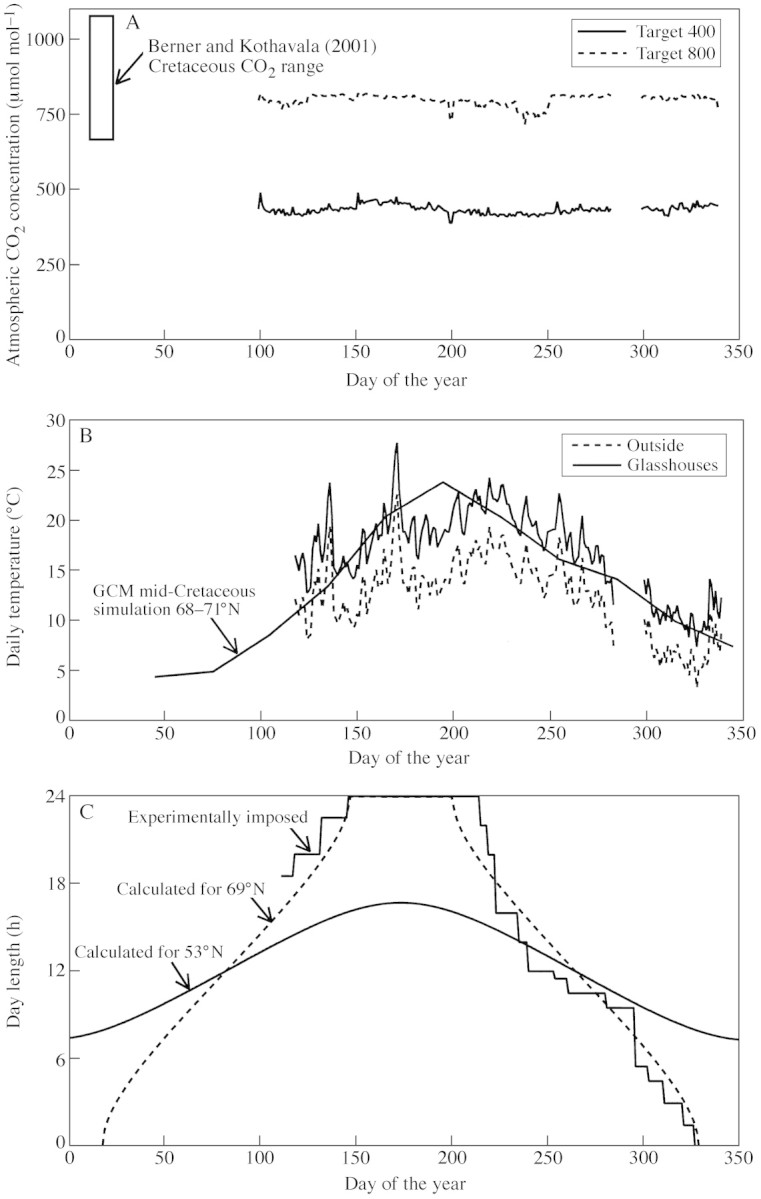
Fig. 2. A, Measurements of atmospheric CO2 concentrations in the ambient and elevated CO2 glasshouses during the first year (2000) of treatment. Values are means of four replicates at each concentration. The vertical box denotes the range of uncertainty in CO2 predictions of a geochemical model for the mid‐Cretaceous. B, Seasonal temperature (solid line) within both the ambient and elevated CO2 glasshouses during 2000. For comparison, the outside air temperature in Sheffield (UK) is shown (broken line). Temperatures at 69°N from a climate modelling study for the mid‐Cretaceous (Valdes et al., 1996) are shown for comparison. C, Calculated and experimentally imposed changes in day length at 69°N. Also shown, for comparison, is the seasonal change in daylength experienced at Sheffield (53°N).
Air temperature was measured in the immediate vicinity of plants using a shaded, ventilated copper–constantan thermocouple, and controlled in each section by air‐conditioner units (Bradley Refrigeration, Sheffield, UK), which also served to mix and circulate air. Growth temperature within all sections was modified to track outside ambient values, but with a +5 °C warming to mimic the seasonal cycle simulated in high latitudes by general circulation modelling (GCM) studies for the mid‐Cretaceous (Valdes et al., 1996; Price et al., 1997; Beerling, 2000). A minimum winter temperature of 5 °C prevents plants being damaged by frosts. The system provides an effective means of simulating an annual climate representative of the Cretaceous (Fig. 2B).
Atmospheric humidity near the plants was measured using a ventilated wet–dry bulb psychrometer with copper–constantan thermocouples, and the relative humidity within each glasshouse section was maintained to a minimum of 75 % using a horticultural misting system (LBS Horticulture, Colne, Lancs., UK).
Seasonal changes in daylength at 69°N were achieved by complete replacement of glass in the glasshouses with opaque, insulated panels and the installation of an automated lighting system to regulate the intensity and duration of lighting throughout the year. Two water‐cooled light units (Sunbeam Hydrostar; Avon Gro‐Lite Systems, Bristol, UK) were deployed in each of the eight sections, each unit providing 300–400 µmol m–2 s–1 of photosynthetically active radiation (PAR) over approx. 2 m2 ground area. Thus lighting was supplied and controlled artificially all year round. Daylength was adjusted weekly to simulate the seasonal changes calculated from standard equations (Monteith and Unsworth, 1990) for 69°N (Fig. 2C).
Plant materials
The tree species (Table 1) were grown from seed at Llangwm Arboretum (Usk, UK), and 1‐year‐old saplings were acclimated to the Sheffield climate in a polythene tunnel for 2 months before transfer to the glasshouses in April 2000. Each tree species was represented by two individuals per glasshouse, a total of eight per CO2 treatment. Plants were grown in 2 l pots in a medium designed to have good water holding and pH‐buffering capacity, and a high mineral content, and consisting of lime‐free silica sand (Pioneer Supamix Ltd, Nuneaton, UK), fine vermiculite (LBS Horticulture) and peat (Midland Irish Peat Moss Ltd, Rathlowen, Co. Westmeath, Ireland) in the ratio 13 : 5 : 2. As gymnosperms are naturally associated with vesicular–arbuscular (VA) mycorrhizas (Khan and Valder, 1972), even in Antarctic Triassic fossils (Stubblefield et al., 1987); and contemporary Nothofagus is commonly ectomycorrhizal (Warcup, 1980), mycorrhizal symbioses were established in the experiment by inoculating roots with spores of generalist fungal species during potting (MycorTree Root Dip; Plant Health Care, Berkhamsted, Herts., UK).
Plants were drip irrigated regularly using an automated system (Nutriculture Ltd, Mawdsley, Lancs., UK), and supplied weekly during the growing season with a nutrient solution appropriate for VA mycorrhizal systems (10 % Rorison’s Nutrient Solution).
Leaf gas exchange and carbohydrate status
Leaf photosynthetic responses and carbohydrate contents of five tree species grown with either 400 or 800 µmol mol–1 CO2 were determined during their first year under a polar light regime. All measurements on leaves were made using a portable open gas exchange system (CIRAS‐1; PP‐Systems, Hitchen, Herts., UK) on foliage that developed under the experimental conditions. Gas exchange was measured at a PAR flux of 600 µmol m–2 s–1, previously shown to saturate photosynthesis (data not shown), a leaf temperature of 25 °C and a leaf‐air vapour pressure deficit of 1.0 kPa. Measurements were made during the summer (plants under continuous light) and the autumn (plants under a daylength of 10 h), at CO2 concentrations of 400 and 800 µmol mol–1, i.e. at the growth CO2 concentrations.
Following the method of Scholes et al. (1994), leaf carbohydrate content was determined three times during the year: in summer and autumn, in parallel with leaf photosynthesis measurements and in midwinter, during continuous darkness. For photosynthesis and carbohydrate measurements, the mean of two leaves, i.e. one leaf sampled from each of two plants, was used as the replicate, giving n = 4 measurements at each CO2 concentration for each species.
Photoinhibition
The maximum quantum efficiency of photosystem II photochemistry (Genty et al., 1989) was assessed in situ using periodic measurements of the dark‐adapted variable to maximum fluorescence ratio (Fv/Fm) using a portable modulated system (FMS‐2 Field Fluorescence Monitoring System; Hansatech Instruments Ltd, King’s Lynn, UK), with replication as for photosynthesis. To determine the effects of growth CO2 concentration on Fv/Fm, plants were exposed for 2 h to warm temperatures (32 °C) and a PAR of 1000 µmol m–2 s–1, under the CO2 concentration in which they grew (i.e. either 400 or 800 µmol mol–1). A baseline set of measurements was established before and after this treatment from plants placed in cool (22 °C), low light (PAR = 150 µmol m–2 s–1) conditions under growth CO2 concentrations.
Plant respiration
Measurements were made on Sequoia sempervirens and Nothofagus cunninghamii, as evergreen taxa representing elements of the northern and southern hemisphere polar forests, respectively, using a portable open gas exchange system (CIRAS‐1; PP‐Systems, Hitchin, Herts., UK). A mean value for each leaf was obtained from 30 measurements made at 1‐min intervals under the mean ambient temperature for January (7 °C), with replication as for photosynthesis. Direct or indirect effects of CO2 were measured by reciprocal transfer of plants grown in ambient CO2 to elevated CO2, and of plants grown in elevated CO2 to ambient CO2. The CO2 efflux from leaves was measured as before, immediately following the transfers.
Rates of root respiration were measured for Araucaria araucana and S. sempervirens during early and late autumn, when daylengths were 12 and 3 h, respectively. These two species were selected because of their differential accumulation of carbohydrates in leaves, which could influence root carbohydrate content (Ekblad and Högberg, 2001). Respiration rates of excised roots were measured as the quantity of O2 consumed using a liquid phase oxygen electrode (Model LD 2/2 oxygen electrode; Hansatech Instruments, King’s Lynn, UK) maintained at a constant temperature of 20 °C. Roots were then dried at 40 °C for 48 h to obtain dry mass, and respiration rates were expressed as µmol O2 consumed g–1 dry matter h–1.
RESULTS
Leaf gas exchange and carbohydrate status
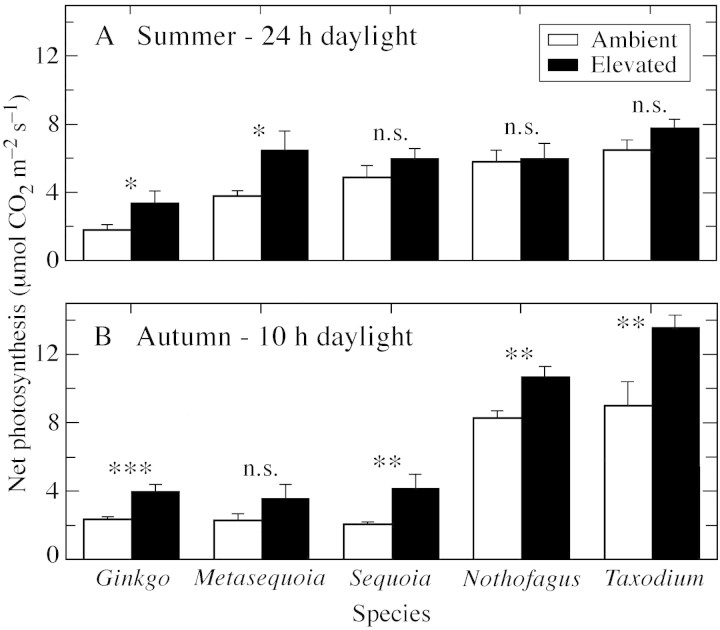
After 4 weeks of continuous light during the polar summer, CO2 enrichment stimulated photosynthesis by 47 % in Ginkgo biloba and 42 % in Metasequoia glyptostroboides (P = 0.05). However, this did not occur in S. sempervirens, N. cunninghamii and Taxodium distichum (Fig. 3A), indicating marked photosynthetic acclimation in these species. In contrast, photosynthetic rates in the autumn were significantly higher in elevated than in ambient CO2 for all species except M. glyptostroboides (which was showing visible signs of leaf senescence by this time) (Fig. 3B). The response to CO2 in species previously showing acclimation shows that acclimation is reversible, and suggests that the earlier acclimation was not caused by limited rooting volume. In addition, photosynthetic rates in N. cunninghamii and T. distichum were higher in autumn than in summer (Fig. 3A, B), suggesting that photosynthetic activity was limited during the summer in these species irrespective of CO2 treatment. Responses to CO2 were not related to leaf habit, either in the summer or autumn.
Fig. 3. Response of net photosynthesis to CO2 enrichment in five tree species during their first exposure to a continuous PAR flux of the polar summer light regime (A) and the short days of autumn (B).
The effect of CO2 on total non‐structural carbohydrate (TNC) content of leaves in the polar summer was an increase in G. biloba and M. glyptostroboides by 73 % and 59 %, respectively, compared with their counterparts in ambient CO2 (Fig. 4A), but there were no significant effects in the other species. This pattern mirrors the photosynthetic responses (Fig. 3A). By contrast, TNC content in autumn was similar in all species (Fig. 4B) with no effects of growth in elevated CO2, a pattern seemingly unrelated to the autumnal photosynthetic responses (Fig. 3B). In winter, the TNC contents of the evergreen species were depleted relative to the summer and autumn (Fig. 4C) due to respiration under continuous darkness without photosynthetic replenishment. Growth CO2 concentration had no effect on the TNC content of leaves of S. sempervirens and N. cunninghamii, but increased it in the leaves of A. araucana (Fig. 4C).
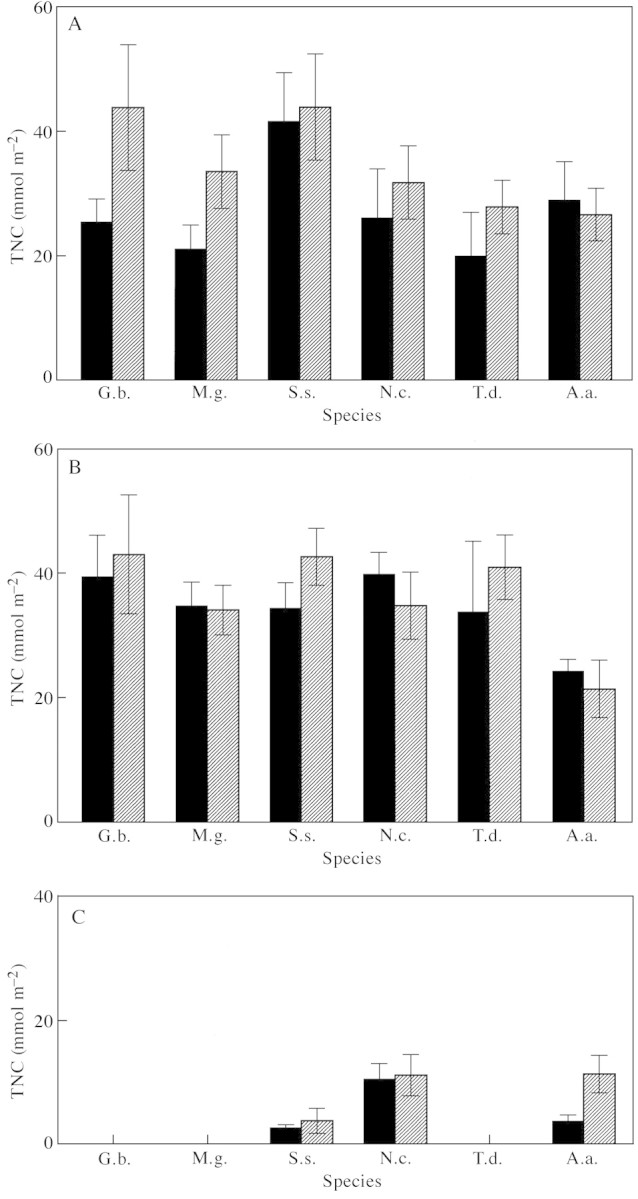
Fig. 4. Effects of CO2 enrichment on leaf total non‐structural carbohydrate (TNC) content. Results are shown for six tree species during their first year of exposure to the continuous days of a polar summer light regime (A), the shorter days during the autumn (B) and for evergreen taxa during the winter (C). A.a., Araucaria araucana; G.b., Ginkgo biloba; M.g., Metasequoia glyptostroboides; N.c., Nothofagus cunninghamii; S.s., Sequoia sempervirens; T.d., Taxodium distichum.
Effects of CO2 on photoinhibition
All species, from both ambient and elevated CO2, showed a progressive time‐dependent reduction in Fv/Fm on exposure to saturating irradiance, reflecting a decrease in the efficiency of photochemical energy dissipation. The decrease in Fv/Fm was largely reversed after a return to cool, low light conditions (Fig. 5). Four of the six tree species grown in elevated CO2 showed no significant (P < 0.05) differences in the response of Fv/Fm to high irradiance when compared with their ambient CO2‐grown equivalents (Fig. 5). The two species showing differences in Fv/Fm due to CO2 treatment responded in different ways: in S. sempervirens, Fv/Fm was greater in elevated CO2 conditions on exposure to high irradiance compared with ambient CO2 controls, whereas in T. distichum, Fv/Fm was lower in plants grown in elevated CO2 (Fig. 5).
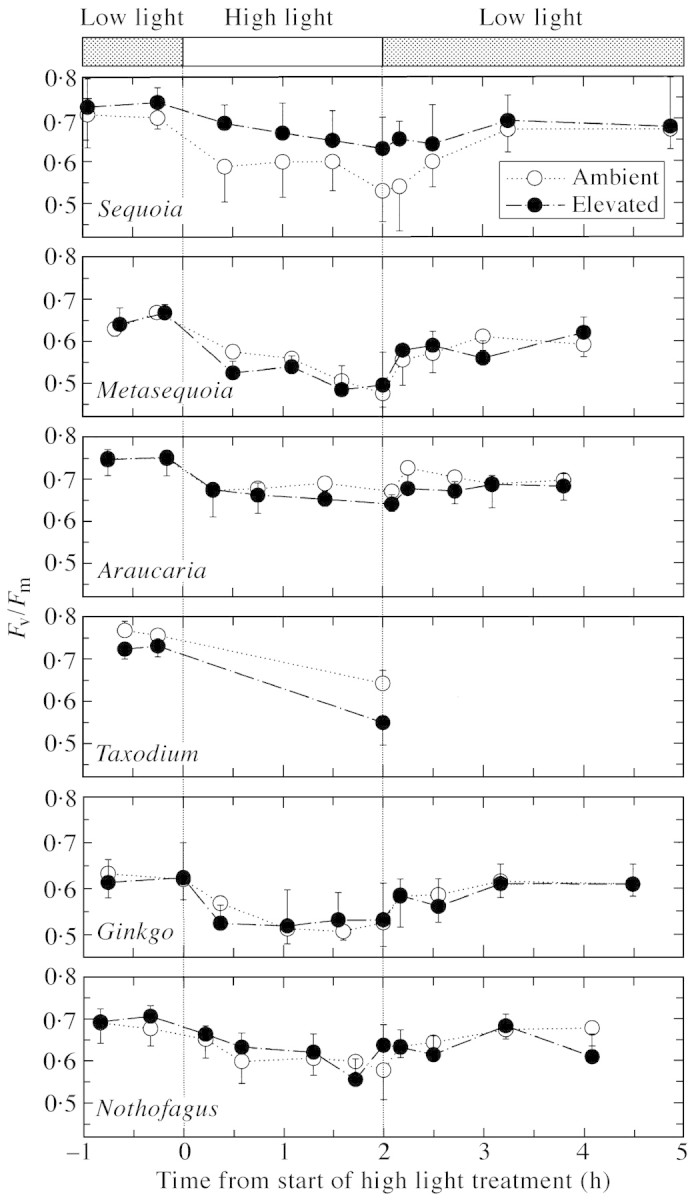
Fig. 5. Effects of CO2 enrichment on the variable to maximum fluorescence ratio (Fv/Fm) of six tree species exposed to high irradiance. CO2 effects were significant (P < 0.05) for Sequoia and Taxodium only.
Plant respiration
After 2 months of permanent darkness in elevated CO2, dark respiration rates of S. sempervirens leaves were reduced by 61 % (P < 0.05) compared with plants grown under ambient CO2 (Table 2). N. cunninghamii responded similarly, with respiration rates being reduced by 38 %, although this was not statistically significant because of large variability between individuals (Table 2). Transfer of plants grown in ambient CO2 to elevated CO2 markedly suppressed rates of leaf respiration in both S. sempervirens and N. cunninghamii, whilst the transfer from elevated‐ to ambient‐CO2 increased them (Table 2).
Table 2.
. Responses of leaf respiration (R, µmol m–2 s–1) of Sequoia sempervirens and Nothofagus cunninghamii to CO2 enrichment in a polar light regime during midwinter
| CO2 concentration during growth | ||||
| Ambient CO2 (400 µmol mol–1) | Elevated CO2 (800 µmol mol–1) | |||
| CO2 concentration during measurement | S. sempervirens | N. cunninghamii | S. sempervirens | N. cunninghamii |
| Ambient CO2 | 0.13 ± 0.02 | 0.32 ± 0.01 | 0.11 ± 0.03 | 0.26 ± 0.10 |
| Elevated CO2 | 0.05 ± 0.01 | 0.19 ± 0.06 | 0.05 ± 0.01 | 0.20 ± 0.06 |
Values are means ± s.e. of eight plants. Two‐way analysis of variance indicated a significant (P < 0.01) effect of CO2 concentration during measurement on leaf respiration of S. sempervirens, but no effect of the CO2 concentration during growth. CO2 concentrations during growth and measurement had no significant effects on the respiration rates of N. cunninghamii leaves. There were no significant interactions between measurement and growth CO2.
Respiration rates of roots of S. sempervirens and A. araucana grown in elevated CO2 were lower in both the early and late autumn than those of plants grown in ambient CO2 (Table 3). Overall, root respiration was suppressed by 10 and 40 % in early and late autumn, respectively, in both species, but as with leaf respiration, the large variation between individuals resulted in a significant effect of CO2 treatment on A. araucana during the autumn (Table 3).
Table 3.
Effect of CO2 enrichment on root respiration rates, measured as O2 consumption (µmol g–1 dry matter h–1) at 20 °C, for two evergreen conifer taxa during contrasting day lengths (12 and 3 h)
| Early autumn | Late autumn | |||
| Species | Ambient CO2(400 µmol mol–1) | Elevated CO2(800 µmol mol–1) | Ambient CO2(400 µmol mol–1) | Elevated CO2(800 µmol mol–1) |
| Sequoia sempervirens | 85.1 ± 7.2 | 73.5 ± 15.0 | 82.0 ± 19.7 | 52.8 ± 10.9 |
| Araucaria araucana | 59.6 ± 6.6 | 55.4 ± 4.4 | 40.9 ± 3.7 | 30.5 ± 0.9 |
Values are means of eight replicates ± 1 s.d. Analysis of variance indicated CO2 effects on respiration were significant (P < 0.05) for Araucaria araucana in the late autumn. All other CO2 effects were not significant at P = 0.05.
DISCUSSION
Understanding polar forest ecology of the Mesozoic requires that key aspects of tree physiology, and particularly the effects of light, atmospheric CO2 and their interactions with physiology be considered (Beerling, 1998, 2000). To do so requires information on plant taxa of this extinct biome. As this is impossible, we have used closely related extant species. Our experiment is designed to provide data allowing us to address this fundamental requirement by assessing three determinants of the plant carbon balance (photosynthesis, photoinhibition and respiration). Contrary to expectations, we found that stimulation of leaf photosynthesis by elevated CO2 was not generally evident during the polar summer, i.e. under conditions of continuous sunlight (24 h), but did occur under shorter days (10 h). Measured TNC contents of leaves were not directly correlated with absence of CO2 stimulation of photosynthesis (Figs 3 and ), in contrast with previous studies (Drake et al., 1997; Curtis and Wang, 1998). The reasons for the unresponsiveness of TNC to CO2 are uncertain, but possibly the storage capacity of leaves was similar in both ambient and elevated CO2 treatments, and saturated during the continuous light of the polar summer. If this is the case, the TNC content of trunk wood might have increased in elevated CO2, since the trunk is a major site for storage in trees (Larcher, 1995).
Growth in elevated CO2 can increase photochemical energy use when there is a large demand for assimilates but not when demand is low (Hymus et al., 1999). This indicates the potential for increased photoinhibition during periods of carbohydrate accumulation. Measured TNC contents of leaves were highest in the polar summer (Fig. 4) under conditions of continuous irradiance. Our measurements of Fv/Fm were therefore made a time when the risk of CO2‐induced photoinhibition was high. Despite this, there was scant evidence to suggest that growth in an elevated CO2 atmosphere, representative of the Mesozoic, affected photoinhibition during the polar summer in either evergreen or deciduous taxa (Fig. 5).
The observed reductions in rates of leaf (Table 2) and root (Table 3) respiration for plants grown with CO2 enrichment are consistent with results from numerous studies of plants exposed to elevated CO2 (Lambers et al., 1996; Norby et al., 1999). Reductions in the respiration rates of roots of plants grown in elevated CO2 are likely to be caused by acclimation of the respiratory system, by decreased supply of respiratory substrate or by decreased demand for the products of respiration (Ryan, 1991; Amthor, 2000). Interestingly, however, the effect of elevated CO2 on leaf respiration (50 % decrease) in our polar experiment was greater than that shown by a number of tree species (mean 19 %) in experiments with regular 12–16 h daylengths (Drake et al., 1999). This difference is consistent with the idea (Bunce, 2001) that the CO2‐sensitivity of mitochondrial respiration is greatest after a period of prolonged darkness, when respiration becomes substrate‐limited (Fig. 4). Data from the reciprocal transfer experiment (Table 2) indicate that dark respiration rates of leaves of S. sempervirens and N. cunninghamii were directly suppressed by CO2 inhibiting the biochemistry of respiratory pathways, possibly by reducing activity of cytochrome‐c‐oxidase and succinate dehydrogenase (Drake et al., 1999). Analyses of carbohydrates in leaves suggest that reserves accumulated during the polar summer were largely depleted during the winter in these two species (Fig. 4C). Thus, limited stores of TNC, and of respiratory substrate in leaves during winter, may explain why no indirect effects of CO2 on leaf respiration rates were observed then.
Suppression of respiration of above‐ and below‐ground plant organs by elevated CO2, if a consistent feature of the experimental treatment, implies that the high concentration of atmospheric CO2 in the Mesozoic played an important role in decreasing respiratory demand of an evergreen canopy during the mild winters. Furthermore, it is possible that plants acclimated with decreased respiration to a warm winter climate, as seen in the field (e.g. Mooney and Brayton, 1966) and in controlled environment studies (Tranquillini et al., 1986). Suppression of plant respiration by the joint action of a high CO2 atmosphere (Tables 2 and ), and physiological acclimation to warmer climates, suggests that the deciduous habit in northern hemisphere polar forests may not have been such a crucial adaptation for reducing canopy respiratory CO2 losses as previously suggested (Axelrod, 1984). Critical to this conclusion is further investigation of CO2‐related effects on leaf and root respiration, especially in the longer term (Bunce, 2001).
A more complete assessment of the effects of CO2 on whole plant carbon balance, particularly the distribution of dry matter between above‐ and below‐ground parts, is clearly required. The complexity of whole tree responses to elevated CO2 is exemplified by controlled environment microcosm experiments with Pseudotsuga menziesii (Lin et al., 2001). They showed that increased root growth under elevated CO2 more than compensated for reduced respiration rates per unit weight of root, so that the total respiration of the rhizosphere increased under high CO2. In the context of polar forests, measurements throughout the year of carbon fluxes associated with shoots and roots of trees and with the soil are an important requirement of future studies, and will be made as the experiment progresses.
Simulation modelling
Collectively, the effects of CO2 on carbon gain by photosynthesis and carbon loss by respiration in extant species show the potential of CO2 to modify the primary production of ancient polar forests. We assessed this potential using a process‐based generic model of coniferous forest productivity (Osborne and Beerling, 2000), and a simulated Mid‐Cretaceous climate for northern Alaska (72°N), as in the study conditions (Beerling, 2000). Northern Alaska was selected because analyses of the fossil record indicate that Cretaceous forests in this region included a mixture of deciduous and evergreen taxa (Falcon‐Lang, 2000; Falcon‐Lang and Cantrill, 2000, 2001). The model of Osborne and Beerling mathematically describes forest carbon, nitrogen and water fluxes by scaling up widely applicable relationships between leaf lifespan and function (Reich et al., 1997). It is therefore uniquely sensitive to prescribed leaf lifespan, and includes representation of the environmental influences on photosynthesis, respiration and stomatal activity, and is sensitive to soil nutrient and water status. From climatic and soil information it predicts the structural (leaf area index) and functional [net primary productivity (NPP), canopy transpiration, etc.] characteristics of coniferous forests.
Five simulations were performed to account for the separate and combined effects of elevated CO2. In each case, leaf lifespans of either 8 (deciduous) or 60 months (evergreen) were used in the model.
Simulation 1.
Control, no changes to the conifer growth model, CO2 concentration of 800 µmol mol–1.
Simulation 2.
As for the control, but with a 50 % reduction in leaf respiration rates (Table 2).
Simulation 3.
As for the control, but with a 25 % reduction in root respiration rates (Table 3).
Simulation 4.
As for the control, but without photosynthetic CO2 stimulation during the polar summer, when daylength was greater than 20 h (Fig. 3).
Simulation 5.
As for the control, but with effects 2–4 applied together.
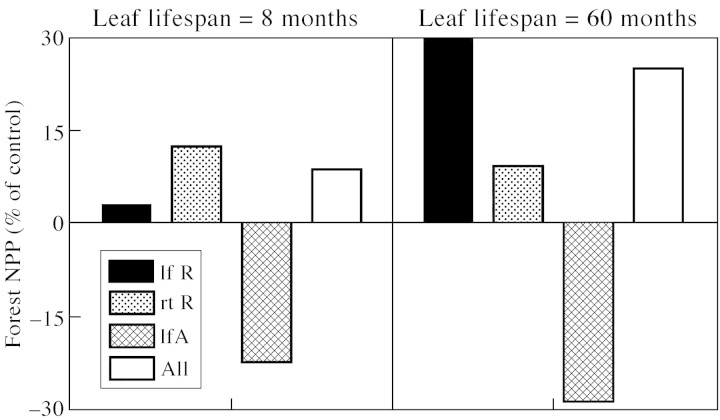
Each effect of elevated CO2 has a different impact on the simulated productivity of north Alaskan coniferous forests (Fig. 6). Reductions in leaf and root respiration by elevated CO2 increased forest NPP, whilst the absence of stimulation of photosynthesis by CO2 during the summer reduced NPP (Fig. 6). These effects of CO2 are largest for trees with long‐lived foliage (evergreen) (Fig. 6). Decreased leaf respiration increases forest NPP, relative to the control simulation, because it reduces the light compensation point of photosynthesis. This allows a positive net carbon gain for a longer period of each day (Long and Drake, 1991; Osborne et al., 1997) and in a greater fraction of the canopy (Osborne et al., 1998). Reduced root respiration rates decrease the demand for carbohydrates, allowing root growth to increase, with a resulting improvement in nutrient and water uptake efficiency. If photosynthesis is not increased by elevated CO2 during the warm polar summer, NPP is reduced (Fig. 6), because stimulation of photosynthesis by elevated CO2 is strongly dependent upon temperature (Long, 1991), and is therefore greatest in summer. When all three physiological effects of CO2—leaf and root respiration and photosynthesis—operate together, their non‐linear interactions result in a net increase in forest productivity with the decreased respiratory costs more than compensating for loss of high CO2–high temperature interactions during the polar summer (Fig. 6).
Fig. 6. Simulated changes in the net primary productivity (NPP) of evergreen and deciduous Cretaceous coniferous forests resulting from the inclusion of the observed effects of CO2 on respiration and photosynthesis in a simulation model. Changes are expressed relative to the control simulation. Codes for each simulation are: the effects of a 50 % decrease in leaf respiration (lf R); the effects of a 25 % reduction in root respiration (rt R); absence of CO2 stimulation of photosynthesis during the polar summer (lfA); and all three of the previous treatments combined (all).
We conclude that the effects of elevated CO2 observed in six species of deciduous and evergreen trees, representing some ancient taxa of polar forests, are likely to be important modifiers of whole tree carbon balance. According to the results of our simulations, trees with long‐lived foliage will show the greatest benefit from growth in a high CO2 environment (Fig. 6). This is because the growing season of above‐ and below‐ground plant organs is longer in evergreen than in deciduous trees. They are therefore better placed to exploit the interaction between high CO2 and warm spring and autumnal temperatures to increase photosynthetic carbon gain, whilst at the same time incurring reduced respiratory costs of fine root production. These considerations provide some physiological clues that might explain the geographical separation between the northern and southern hemispheres of polar forests with a predominately evergreen or deciduous leaf habit.
ACKNOWLEDGEMENTS
We thank Howard Falcon‐Lang for comments on the manuscript, Coralie Hopwood and Barry Lomax for performing leaf tissue carbohydrate analyses and Paul Quick for advice on, and assistance with, root respiration measurements. D.J.B. gratefully acknowledges funding through a Royal Society University Research Fellowship, NERC (GR3/11900), a Royal Society Equipment grant for the Hanstech chlorophyll fluorescence system, and the Leverhulme Trust.
Supplementary Material
Received: 2 August 2001; Returned for revision: 10 October 2001; Accepted: 30 November 2001.
References
- AmthorJS.2000. The McCree‐de Wit‐Penning de Vries‐Thornley respiration paradigms: 30 years later. Annals of Botany 86: 1–20. [Google Scholar]
- ArchangelskyS.1963. A new Mesozoic flora from Tico, Santa Cruz Province, Argentina. Bulletin of the British Museum (Natural History) Geology 8: 47–92. [Google Scholar]
- AxelrodDL.1984. An interpretation of Cretaceous and Tertiary biota in polar regions. Palaeogeography, Palaeoclimatology, Palaeoecology 45: 105–147. [Google Scholar]
- BeerlingDJ.1998. The future as the key to the past for palaeobotany? Trends in Ecology and Evolution 13: 311–316. [DOI] [PubMed] [Google Scholar]
- BeerlingDJ.2000. Global terrestrial productivity in the Mesozoic era. In: Hart MB, ed. Climates: past and present Geological Society of London Special Publication 181: 17–32. [Google Scholar]
- BernerRA, Kothavala Z.2001. Geocarb III: a revised model of atmospheric CO2 over Phanerozoic time. American Journal of Science 301: 182–204. [Google Scholar]
- BunceJA.2001. Effects of prolonged darkness on the sensitivity of leaf respiration to carbon dioxide concentration in C3 and C4 species. Annals of Botany 87: 463–468. [Google Scholar]
- ChaneyRW.1948. The bearing of living Metasequoia on problems of Tertiary paleobotany. Proceedings of the National Academy of Sciences of the USA 34: 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChuKL, Cooper WS.1950. An ecological reconnaissance of the native home of Metasequoia glyptostroboides Ecology 31: 260–278. [Google Scholar]
- CreberGT, Chaloner WG.1985. Tree growth in the Mesozoic and early Tertiary and the reconstruction of palaeoclimates. Palaeogeography, Palaeoclimatology, Palaeoecology 52: 35–60. [Google Scholar]
- CurtisPS, Wang X.1998. A meta‐analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113: 299–313. [DOI] [PubMed] [Google Scholar]
- De LaubenfelsDJ.1988. Araucariaceae. Flora Malesiana Series 10 3: 419–442. [Google Scholar]
- DeLuciaEH, Hamilton JG, Naidu SL, Thomas, RB, Andrews JA, Finzi A, Lavine M, Matamala R, Mohan JE, Hendrey GR, Schlesinger WH.1999. Net primary productivity of a forest ecosystem with experimental CO2 enrichment. Science 284: 1177–1179. [DOI] [PubMed] [Google Scholar]
- DouglasJG, Williams GE.1982. Southern polar forests: the early Cretaceous floras of Victoria and their palaeoclimatic significance. Palaeogeography, Palaeoclimatology, Palaeoecology 39: 171–185. [Google Scholar]
- DrakeBG, Gonzalez‐Meler M, Long SP.1997. More efficient plants: a consequence of rising atmospheric CO2? Annual Review of Plant Physiology and Plant Molecular Biology 49: 609–639. [DOI] [PubMed] [Google Scholar]
- DrakeBG, Azcon‐Bieto J, Berry J, Bunce J, Dijkstra P, Farrar J, Gifford RM, Gonzalez‐Meler MA, Koch G, Lambers H, Siedow J, Wullschleger S. 1999. Does elevated atmospheric CO2 concentration inhibit mitochondrial respiration in green plants? Plant, Cell and Environment 22: 649–657. [Google Scholar]
- EkbladA, Högberg P.2001. Natural abundance of 13C in CO2 respired from forest soils reveals speed of link between tree photosynthesis and root respiration. Oecologia 127: 305–308. [DOI] [PubMed] [Google Scholar]
- Falcon‐LangHJ.2000. The relationship between longevity and growth ring markedness in modern conifer woods and its implications for palaeoclimatic studies. Palaeogeography, Palaeoclimatology, Palaeoecology 160: 317–328. [Google Scholar]
- Falcon‐LangHJ, Cantrill DJ.2000. Cretaceous (Late Albian) coniferales of Alexander Island, Antarctica. 1 Wood taxonomy: a quantitative approach. Review of Palaeobotany and Palynology 111: 1–17. [DOI] [PubMed] [Google Scholar]
- Falcon‐LangHJ, Cantrill DJ.2001. Leaf phenology of some mid‐Cretaceous polar forests, Alexander Island, Antarctica. Geological Magazine 138: 39–52. [Google Scholar]
- Falcon‐LangHJ, Cantrill DJ, Nichols GJ.2001. Biodiversity and terrestrial ecology of a mid‐Cretaceous, high latitude floodplain, Alexander Island, Antarctica. Journal of the Geological Society 158: 709–724. [Google Scholar]
- FrakesLA, Francis JE, Syktus JI.1992. Climate modes of the Phanerozoic. Cambridge: Cambridge University Press. [Google Scholar]
- FrancisJE.1986. Growth rings in Cretaceous and Tertiary wood from Antarctica and their palaeoclimatic implications. Palaeogeography, Palaeoclimatology, Palaeoecology 29: 665–684. [Google Scholar]
- GentyB, Briantais JM, Baker NR.1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- HymusGJ, Ellsworth DS, Baker NR, Long SP.1999. Does free‐air carbon dioxide enrichment affect photochemical energy use by evergreen trees in different seasons? A chlorophyll fluorescence study of mature loblolly pine. Plant Physiology 120: 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JeffersonTH.1982. Fossil forests from the Lower Cretaceous of Alexander Island, Antarctica. Palaeontology 25: 681–708. [Google Scholar]
- KhanAG, Valder PG.1972. The occurrence of root nodules in the Ginkgoales, Taxales, and Coniferales. Proceedings of the Linnean Society of New South Wales 97: 35–41. [Google Scholar]
- LaDeauSL, Clark JS.2001. Rising CO2 levels and the fecundity of forest trees. Science 292: 95–98. [DOI] [PubMed] [Google Scholar]
- LambersH, Stulen I, Van der Werf A.1996. Carbon use in root respiration as affected by elevated CO2 Plant and Soil 187: 251–263. [Google Scholar]
- LarcherW.1995. Physiological plant ecology. Berlin: Springer‐Verlag. [Google Scholar]
- LinG, Rygiewicz PT, Ehleringer JR, Johnson MG, Tingey DT.2001. Time‐dependent responses of soil CO2 efflux components to elevated atmospheric [CO2] and temperature in experimental forest mesocosms. Plant and Soil 229: 259–270. [Google Scholar]
- LongSP.1991. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated? Plant, Cell and Environment 14: 729–740. [Google Scholar]
- LongSP, Drake BG.1991. Effect of long‐term elevation of CO2 concentration in the field on the quantum yield of the C3 sedge, Scirpus olneyi Plant Physiology 96: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LongSP, Humphries S, Falkowski PG.1994. Photoinhibition of photosynthesis in nature. Annual Review of Plant Physiology and Plant Molecular Biology 45: 633–662. [Google Scholar]
- MonteithJL, Unsworth M.1990. Principles of environmental physics. London: Arnold. [Google Scholar]
- MooneyHA, Brayton R.1966. Field measurements of the metabolic responses of bristlecone pine and big sagebrush in the White Mountains. Botanical Gazette 127: 105–113. [Google Scholar]
- NorbyRJ, Wullschleger SD, Gunderson CA, Johnson DW, Ceulemans R.1999. Tree responses to rising CO2 in field experiments: implications for the future forest. Plant, Cell and Environment 22: 683–714. [Google Scholar]
- OsborneCP, Beerling DJ.2000. Modelling the distribution and composition of Mesozoic polar forests. Eos Transactions 81: (48 suppl.), F266. American Geophysical Union, Washington, DC. [Google Scholar]
- OsborneCP, Drake BG, LaRoche J, Long SP.1997. Does long‐term elevation of CO2 concentration increase photosynthesis in forest floor vegetation? Plant Physiology 114: 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OsborneCP, LaRoche J, Garcia RL, Kimball BA, Wall GW, Pinter PJ, LaMorte RL, Hendrey GR, Long SP.1998. Does leaf position within a canopy affect acclimation of photosynthesis to elevated CO2? Analysis of a wheat crop under free‐air CO2 enrichment. Plant Physiology 117: 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning de VriesFWT.1975. The cost of maintenance processes in plant cells. Annals of Botany 39: 77–92. [Google Scholar]
- PoleM.1999. Structure of a near‐polar latitude forest from the New Zealand Jurassic. Palaeogeography, Palaeoclimatology, Palaeoecology 147: 121–139. [Google Scholar]
- PriceGD, Valdes PJ, Sellwood BW.1997. Quantitative palaeoclimate GCM validation: Late Jurassic and mid‐Cretaceous case studies. Journal of the Geological Society 154: 769–772. [Google Scholar]
- ReadJ.1999. Rainforest ecology. In: Reid JB, Hill RS, Brown MJ, Hovenden MJ, eds. Vegetation of Tasmania. Canberra: Australian Biological Resources Study, 160–197. [Google Scholar]
- ReadJ, Francis J.1992. Responses of some Southern hemisphere tree species to a prolonged dark period and their implications for high‐latitude Cretaceous and Tertiary floras. Palaeogeography, Palaeoclimatology, Palaeoecology 99: 271–290. [Google Scholar]
- ReichPB, Walters MB, Ellsworth DS.1997. From the tropics to the tundra: global convergence in plant functioning. Proceedings of the National Academy of Sciences of the USA 94: 13730–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RodenJS, Egerton JJG, Ball MC.1999. Effect of elevated [CO2] on photosynthesis and growth of snow gum (Eucalyptus pauciflora) seedlings during winter and spring. Australian Journal of Plant Physiology 26: 37–46. [Google Scholar]
- RoyerDL, Berner RA, Beerling DJ.2001. Phanerozoic atmospheric CO2 change: evaluating geochemical and paleobiological approaches. Earth‐Science Reviews 54: 349–392. [Google Scholar]
- RyanGR.1991. Effects of climate change on plant respiration. Ecological Applications 1: 157–167. [DOI] [PubMed] [Google Scholar]
- ScholesJD, Lee PJ, Horton P, Lewis DH.1994. Invertase: understanding changes in the photosynthetic and carbohydrate metabolism of barley leaves infected with powdery mildew. New Phytologist 126: 213–222. [Google Scholar]
- SheenJ.1994. Feedback control of gene‐expression. Photosynthesis Research 39: 427–438. [DOI] [PubMed] [Google Scholar]
- SpicerRA, Chapman JL.1990. Climate change and the evolution of high‐latitude terrestrial vegetation and floras. Trends in Ecology and Evolution 5: 279–284. [DOI] [PubMed] [Google Scholar]
- SpicerRA, Herman AB.2001. The Albanian–Cenomanian flora of the Kukpowruk River, western North Slope, Alaska: stratigraphy, palaeofloristics, and plant communities. Cretaceous Research 22: 1–40. [Google Scholar]
- StubblefieldSP, Taylor TN, Trappe JM.1987. Vesicular–arbuscular mycorrhizae from the Triassic of Antarctica. American Journal of Botany 74: 1904–1911. [Google Scholar]
- TerryAC, Quick WP, Beerling DJ.2000. Long‐term growth of Ginkgo with CO2 enrichment increases leaf ice nucleation temperatures and limits recovery of the photosynthetic system from freezing. Plant Physiology 124: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ThornV.2001. Vegetation communities of a high palaeolatitude Middle Jurassic forest in New Zealand. Palaeogeography, Palaeoclimatology, Palaeoecology 168: 273–289. [Google Scholar]
- TranquilliniW, Havranek WM, Ecker P.1986. Effects of humidity and acclimation temperature on the temperature response of photosynthesis in young Larix deciduas Mill. Tree Physiology 1: 37–45. [DOI] [PubMed] [Google Scholar]
- TrediciPD, Ling H, Yang G.1992. The Ginkgos of Tian Mu Shan. Conservation Biology 6: 202–209. [Google Scholar]
- ValdesPJ, Sellwood BW, Price GD.1996. Evaluating concepts of Cretaceous equability. Palaeoclimates: Data and Modelling 2: 139–158. [Google Scholar]
- WarcupJH.1980. Ectomycorrhizal associations of Australian indigenous plants. New Phytologist 85: 531–535. [DOI] [PubMed] [Google Scholar]
- WaringRH, Franklin JF.1979. Evergreen coniferous forests of the Pacific Northwest. Science 204: 1380–1386. [DOI] [PubMed] [Google Scholar]
- WullschlegerSD, Ziska LH, Bunce JA.1994. Respiratory responses of higher plants to atmospheric CO2 enrichment. Physiologia Plantarum 90: 221–229. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.