Abstract
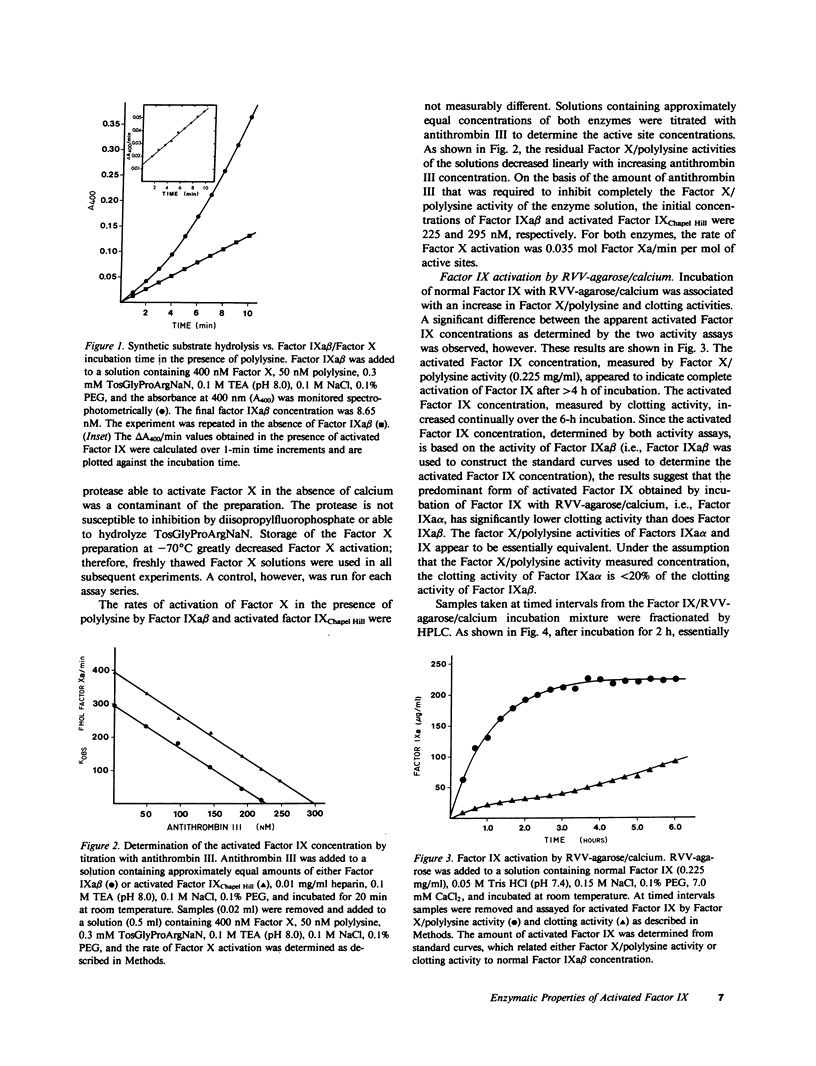
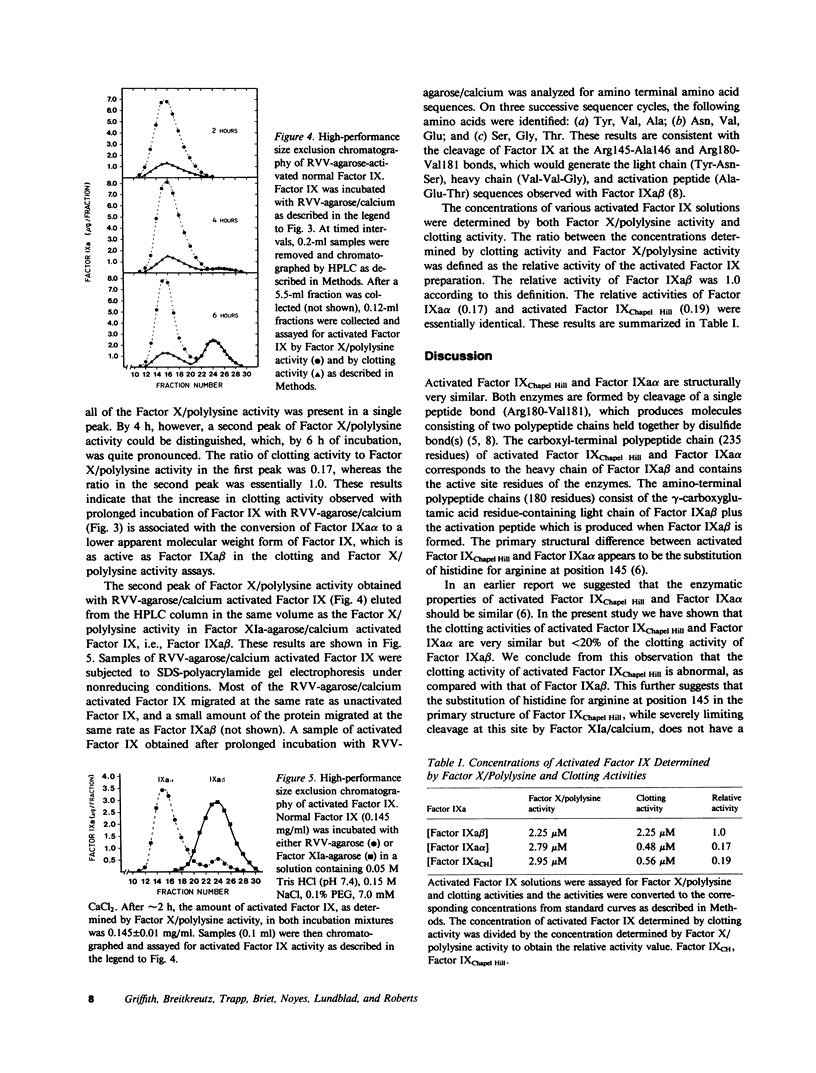
Two structurally different forms of activated human Factor IX (Factor IXa alpha and IXa beta) have been previously reported to have essentially identical clotting activity in vitro. Although it has been shown that activated Factor IX Chapel Hill, an abnormal Factor IX isolated from the plasma of a patient with mild hemophilia B, and normal Factor IXa alpha are structurally very similar, the clotting activity of activated Factor IX Chapel Hill is much lower (approximately fivefold) than that of normal Factor IXa beta. In the present study we have prepared activated Factor IX by incubating human Factor IX with calcium and Russell's viper venom covalently bound to agarose. Fractionation of the activated Factor IX by high-performance liquid chromatography demonstrated the presence of both Factors IXa alpha and IXa beta. On the basis of active site concentration, determined by titration with antithrombin III, the clotting activities of activated Factor IX Chapel Hill and IXa alpha were similar, but both activities were less than 20% of the clotting activity of Factor IXa beta. Activated Factor IX activity was also measured in the absence of calcium, phospholipid, and Factor VIII, by determination of the rate of Factor X activation in the presence of polylysine. In the presence of polylysine, the rates of Factor X activation by activated Factor IX Chapel Hill, Factor IXa alpha, and Factor IXa beta were essentially identical. We conclude that the clotting activity of activated Factor IX Chapel Hill is reduced when compared with that of Factor IXa beta but essentially normal when compared with that of Factor IXa alpha.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGGELER P. M., WHITE S. G., GLENDENING M. B., PAGE E. W., LEAKE T. B., BATES G. Plasma thromboplastin component (PTC) deficiency; a new disease resembling hemophilia. Proc Soc Exp Biol Med. 1952 Apr;79(4):692–694. doi: 10.3181/00379727-79-19488. [DOI] [PubMed] [Google Scholar]
- BIGGS R., DOUGLAS A. S., MACFARLANE R. G., DACIE J. V., PITNEY W. R., MERSKEY Christmas disease: a condition previously mistaken for haemophilia. Br Med J. 1952 Dec 27;2(4799):1378–1382. doi: 10.1136/bmj.2.4799.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein K. M., Noyes C. M., Griffith M. J., Lundblad R. L., Roberts H. R. Characterization of the defect in activation of factor IX Chapel Hill by human factor XIa. J Clin Invest. 1981 Dec;68(6):1420–1426. doi: 10.1172/JCI110393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K. S., Madar D. A., Goldsmith J. C., Kingdon H. S., Roberts H. R. Purification and characterization of an abnormal factor IX (Christmas factor) molecule. Factor IX Chapel Hill. J Clin Invest. 1978 Nov;62(5):1078–1085. doi: 10.1172/JCI109213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scipio R. G., Hermodson M. A., Yates S. G., Davie E. W. A comparison of human prothrombin, factor IX (Christmas factor), factor X (Stuart factor), and protein S. Biochemistry. 1977 Feb 22;16(4):698–706. doi: 10.1021/bi00623a022. [DOI] [PubMed] [Google Scholar]
- Di Scipio R. G., Kurachi K., Davie E. W. Activation of human factor IX (Christmas factor). J Clin Invest. 1978 Jun;61(6):1528–1538. doi: 10.1172/JCI109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M. J., Reisner H. M., Lundblad R. L., Roberts H. R. Measurement of human factor IXa activity in an isolated factor X activation system. Thromb Res. 1982 Aug 1;27(3):289–301. doi: 10.1016/0049-3848(82)90076-7. [DOI] [PubMed] [Google Scholar]
- Katayama K., Ericsson L. H., Enfield D. L., Walsh K. A., Neurath H., Davie E. W., Titani K. Comparison of amino acid sequence of bovine coagulation Factor IX (Christmas Factor) with that of other vitamin K-dependent plasma proteins. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4990–4994. doi: 10.1073/pnas.76.10.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist P. A., Fujikawa K., Davie E. W. Activation of bovine factor IX (Christmas factor) by factor XIa (activated plasma thromboplastin antecedent) and a protease from Russell's viper venom. J Biol Chem. 1978 Mar 25;253(6):1902–1909. [PubMed] [Google Scholar]
- Link R. P., Castellino F. J. Kinetic comparison of bovine blood coagulation factors IXa alpha and IXa beta toward bovine factor X. Biochemistry. 1983 Aug 16;22(17):4033–4041. doi: 10.1021/bi00286a007. [DOI] [PubMed] [Google Scholar]
- Lundblad R. L., Roberts H. R. The acceleration by polylysine of the activation of factor X by factor IXa. Thromb Res. 1982 Feb 15;25(4):319–329. doi: 10.1016/0049-3848(82)90232-8. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Noyes C. M., Griffith M. J., Roberts H. R., Lundblad R. L. Identification of the molecular defect in factor IX Chapel Hill: substitution of histidine for arginine at position 145. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4200–4202. doi: 10.1073/pnas.80.14.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Activation of 125I-factor IX and 125I-factor X: effect of tissue factor and factor VII, factor Xa and thrombin. Scand J Haematol. 1980 Mar;24(3):213–226. doi: 10.1111/j.1600-0609.1980.tb01329.x. [DOI] [PubMed] [Google Scholar]
- Pepper D. S., Prowse C. Chromatography of human prothrombin complex or dextran sulphate agarose. Thromb Res. 1977 Nov;11(5):687–692. doi: 10.1016/0049-3848(77)90026-3. [DOI] [PubMed] [Google Scholar]
- Roberts H. R., Grizzle J. E., McLester W. D., Penick G. D. Genetic variants of hemophilia B: detection by means of a specific PTC inhibitor. J Clin Invest. 1968 Feb;47(2):360–365. doi: 10.1172/JCI105732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


