Abstract
Induced systemic resistance (ISR) of plants against pathogens is a widespread phenomenon that has been intensively investigated with respect to the underlying signalling pathways as well as to its potential use in plant protection. Elicited by a local infection, plants respond with a salicylic‐dependent signalling cascade that leads to the systemic expression of a broad spectrum and long‐lasting disease resistance that is efficient against fungi, bacteria and viruses. Changes in cell wall composition, de novo production of pathogenesis‐related‐proteins such as chitinases and glucanases, and synthesis of phytoalexins are associated with resistance, although further defensive compounds are likely to exist but remain to be identified. In this Botanical Briefing we focus on interactions between ISR and induced resistance against herbivores that is mediated by jasmonic acid as a central signalling molecule. While many studies report cross‐resistance, others have found trade‐offs, i.e. inhibition of one resistance pathway by the other. Here we propose a framework that explains many of the thus far contradictory results. We regard elicitation separately from signalling and from production, i.e. the synthesis of defensive compounds. Interactions on all three levels can act independently from each other.
Key words: Cross‐talk, induced defence, induced resistance, jasmonic acid, octadecanoid signalling, salicylic acid, systemic acquired resistance
INTRODUCTION
Many plants respond to local attack by herbivores or pathogens with a de novo production of compounds reducing or inhibiting further attack by, or performance of, their enemies. Responses occur both in the plant organ originally attacked (local response) and in distant, yet unaffected, parts (systemic response). One of these responses is induced systemic resistance (ISR; or systemic acquired resistance, SAR) of plants against pathogens. Many excellent reviews on ISR have been published (Hunt et al., 1996; Schneider et al., 1996; Sticher et al., 1997; Mauch‐Mani and Métraux, 1998; Hammerschmidt, 1999a), so only a short overview on the phenomenon is given here. This Botanical Briefing will focus on ISR within the broader context of induced plant responses against a variety of different enemies.
ISR: THE PHENOMENON
Interactions between plants and pathogens can lead either to a successful infection (compatible response) or resistance (incompatible response). In incompatible interactions, infection by viruses, bacteria or fungi will elicit a set of localized responses in and around the infected host cells. These responses include an oxidative burst (Lamb and Dixon, 1997), which can lead to cell death (Kombrink and Schmelzer, 2001). Thus, the pathogen may be ‘trapped’ in dead cells and appears to be prevented from spreading from the site of initial infection. Further local responses in the surrounding cells include changes in cell wall composition that can inhibit penetration by the pathogen, and de novo synthesis of antimicrobial compounds such as phytoalexins (Kuc, 1995; Hammerschmidt, 1999b) and pathogenesis‐related (PR) proteins (see below).
Caused by—or at least regularly following—these local responses, a signal spreads through the plant and induces subtle changes in gene expression in yet uninfected plant parts. The systemic response involves the de novo production, in some cases, of phytoalexins and of PR proteins (van Loon, 1997; Neuhaus, 1999; van Loon and van Strien, 1999). While phytoalexins are mainly characteristic of the local response, PR proteins occur both locally and systemically. The functional role of both groups of compounds in resistance is a matter of continuing discussion.
Originally, PR proteins were detected and defined as being absent in healthy plants but accumulating in large amounts after infection (van Loon and van Kammen, 1970); they have now been found in more than 40 species belonging to at least 13 families (van Loon, 1999). Two groups of PR proteins can be distinguished. Acidic PR proteins are predominantly located in the intercellular spaces. Basic PR proteins are functionally similar but have different molecular weights and amino acid sequences and are mainly located intracellularly in the vacuole (Legrand et al., 1987; Niki et al., 1998; van Loon, 1999).
Some PR proteins have chitinase (Legrand et al., 1987) or β‐1,3‐glucanase activity. Chitinases are a functionally and structurally diverse group of enzymes that can hydrolyse chitin, and several are believed to contribute to the defence of plants against certain fungal pathogens (Sahai and Manocha, 1993; Jackson and Taylor, 1996). Chitinases exhibit pronounced antifungal activity (Schlumbaum et al., 1986), and plants over‐expressing chitinase show decreased susceptibility to infection by fungi with chitin‐containing cell walls (Broglie et al., 1991; Datta and Datta, 1999). In contrast, the function of other PR proteins is still unknown (van Loon and van Strien, 1999) and many of them may be functionally active only when combined. Some PR proteins, most prominently the basic ones, are also expressed constitutively in a tissue‐specific and developmentally controlled manner (e.g. during leaf senescence; van Loon, 1999; and pers. comm. from L. C. van Loon) and thus the functional significance of PR proteins with respect to plant defence is unresolved. Recent studies have demonstrated that the expression of typical ‘defence‐related’ genes such as PR‐1 and β‐glucanase 2 (which are often used as ISR markers) can be uncoupled from phenotypic pathogen resistance (Greenberg et al., 2000), indicating that these compounds are not absolutely necessary for an effective resistance phenotype.
PR proteins are generally used as ISR markers, but no antiviral or antibacterial activity has yet been reported for any PR protein. A similar situation exists for phytoalexins, for which, in general, only in vitro antibacterial or antifungal effects have been established: assumptions concerning their role in phenotypic plant resistance are mainly based on correlative evidence. Thomma et al. (1999) reported a phytoalexin (camalexin) deficient arabidopsis mutant to be more susceptible to infection by a necrotrophic fungus, but the same mutant showed no altered susceptibility to a bacterium and two biotrophic fungi.
Phenotypically, systemic resistance is manifested as a protection of the plant not only against the attacking pathogen, but also against other types of pathogens. Although some specificity has recently been described, the resistance seems to be rather non‐specific and long‐lasting. Most research has been conducted on a restricted number of model species (20; Schneider et al., 1996; Sticher et al., 1997), and differences in the biochemistry and efficacy exist among various resistance forms and remain to be investigated in detail. Yet, ISR is generally regarded as a widespread and conserved trait, since the phenomenon is known from species belonging to both Monocotyledonae and Dicotyledonae. The mechanisms underlying the resistance to viruses still remain to be determined, but ISR in general is regarded as being effective against pathogens from all three major groups (viruses, bacteria and fungi).
PRIMING
Some of the compounds normally associated with ISR (for example PR proteins) are expressed in uninfected tissue in response to a first infection. Other biochemical changes characteristic of ISR‐expressing plants become obvious only in response to a further infection and only in plant parts where an effective resistance is required. This phenomenon has been described as ‘priming’, ‘conditioning’ or ‘sensitization’ (Sticher et al., 1997; Conrath et al., 2001). Priming effects can be elicited by chemical ISR inducers, such as β‐aminobutyric acid (Jakab et al., 2001). Responses such as phytoalexin synthesis or cell wall lignification then occur more rapidly and more strongly than during the primary infection, thus enabling a more effective response to the new infection. The molecular mechanisms underlying priming and its importance in the overall plant resistance still remain to be investigated.
LOCAL AND SYSTEMIC SIGNALLING
Salicylic acid (SA) (Raskin, 1992) has an important role in the signalling pathway leading to ISR (Mauch‐Mani and Métraux, 1998; Cameron, 2000; Métraux, 2001). After infection, endogenous levels of SA increase locally and systemically, and SA levels increase in the phloem before ISR occurs (Malamy et al., 1990; Métraux et al., 1990; Rasmussen et al., 1991). SA is synthesized in response to infection both locally and systemically; de novo production of SA in non‐infected plant parts might therefore contribute to systemic expression of ISR (Meuwly et al., 1995). The level of resistance of plants exhibiting constitutive expression of SA is positively correlated with SA levels. This is true for natural cultivars of rice (Silverman et al., 1995), for within‐plant differences in SA levels in potato (Coquoz et al., 1995) and for arabidopsis plants expressing a novel hybrid enzyme with salicylate synthase (SAS) activity and thus having elevated SA levels (Mauch et al., 2001). Key experiments establishing a role for SA in certain forms of ISR have utilized transgenic plants expressing the bacterial nahG gene encoding for naphthalene hydroxylase G. Such plants cannot accumulate SA and are blocked in their ISR response (Delaney et al., 1994; Gaffney et al., 1994).
Experiments using reciprocal combinations of nahG and wild‐type shoots grafted onto nahG and wild‐type plants showed that ISR was elicited in the wild‐type tissue even when the nahG‐transformed part of the plant received the inducing infection, suggesting that the signal emanating from the inducing tissue is not SA (Vernooij et al., 1994). nahG plants might suffer from further, as yet unknown, defects (Cameron, 2000). Rasmussen et al. (1991) reported that time courses in induction and appearance of SA in the phloem combined with leaf‐removal experiments were not consistent with SA being the primary systemic signal in the investigated system (cucumber). These and other experiments suggest that both SA and other systemic signals are involved in ISR signalling (Sticher et al., 1997).
ALLOCATION COSTS
Compared with constitutive resistance, ISR has the disadvantage of leaving plants unprotected until resistance is expressed. Its selective advantage therefore demands an explanation. One possible explanation is fitness costs. If resistant plants reproduce less effectively than sensitive plants when compared under conditions where there is no benefit from resistance, then the disadvantages of any temporal delay in acquiring resistance may be outweighed by the benefit of not incurring these costs when resistance is unnecessary (Heil, 2001; Heil and Baldwin, 2002).
Fitness costs can result from the allocation of limited resources to resistance; resources that then cannot be used for growth or reproduction—allocation costs (Herms and Mattson, 1992). Initial experiments with wheat grown under nitrogen‐poor conditions and treated with BION®, a synthetic mimic of SA‐action, are consistent with the view that ISR expressed under pathogen‐free conditions can have negative effects on plant fitness when plants suffer from a shortage of nutrients (Heil et al., 2000). Amino acids released by the proteolytic degradation of photosynthetic proteins, which happens during induction of resistance, might be re‐utilized for the synthesis of defensive compounds (Weidhase et al., 1987; Reinbothe et al., 1994). Similarly, Somssich and Hahlbrock (1998) hypothesized that ‘the metabolic significance of gene repression concomitant with gene activation during pathogen defence is probably associated with the downregulation of all disposable cellular activities’. In tobacco, single PR proteins may constitute approx. 1 % of the soluble protein of an infected leaf (Antoniw and Pierpoint, 1978), and total PR proteins may constitute up to 10 % (van Loon, pers. comm.), a proportion that is likely to represent a relevant allocation cost under natural growing conditions which are often N‐limited. The observation that many resistance‐overexpressing arabidopsis plants show ‘stunted’ or ‘dwarfed’ and less fertile phenotypes is in line with the assumption that constitutive expression of inducible resistance incurs relevant costs (Heil and Baldwin, 2002).
INTERACTIONS WITH INDUCED HERBIVORE RESISTANCE
Plants growing under natural conditions encounter simultaneous challenges from different external stresses so that different signalling pathways enabling specific responses have evolved (Walling, 2000). Signalling pathways can interact either synergistically or antagonistically (Fidantsef et al., 1999; Pieterse and Van Loon, 1999; Stout and Bostock, 1999; Stout et al., 1999; Walling, 2000; Bostock et al., 2001). The evidence for both ‘cross‐resistance’ and ‘trade‐offs’ between induced resistance against herbivores and induced resistance to pathogens is mixed. Specificity of the induced responses should be distinguished from specificity of the effect of the responses against various attackers. These are not necessarily the same, since several induced compounds can have effects against very different plant enemies, while different compounds can exhibit similar antibiotic effects. Here, we focus mainly on the effects of induced responses. This perspective does not discriminate between different signalling pathways that exhibit similar resistance phenotypes. Thus, some causal relationships, established by genetic and biochemical methods, might be obscured. However, ecological interactions take place at the level of the phenotype and it is at this level upon which selective forces will act.
Induced resistance against herbivores (IRH)
Insect feeding has been reported to elicit local, as well as systemic, responses in more than 100 plant species (Karban and Baldwin, 1997). These responses might function either as direct resistance (physical or chemical traits that act directly against further attack or reduce herbivore performance) or as indirect resistance. The latter is based on the attraction of ‘enemies of the plant’s enemies’ (Price et al., 1980).
A central signalling molecule in induced responses against herbivores is jasmonic acid (JA) (Creelman and Mullet, 1997; Wasternack and Parthier, 1997). In response to wounding and/or insect feeding, linolenic acid is released from membrane lipids and then converted enzymatically into JA. JA, in turn, causes the transcriptional activation of genes encoding proteinase inhibitors (PIs) and of enzymes involved in the production of volatile compounds, or of secondary compounds such as nicotine and numerous phenolics, and other defence‐related compounds (Creelman and Mullet, 1997; Karban and Baldwin, 1997; Wasternack and Parthier, 1997; Boland et al., 1999).
Oligosaccharides (Bishop et al., 1981) and oligogalacturonides (Doares et al., 1995b; Norman et al., 1999) released from damaged cell walls might play a role in the elicitation of the general wound response, but specific elicitors such as systemin have also been reported (Pearce et al., 1991). Systemin is an 18‐amino acid polypeptide that is released upon wounding from a 200‐amino acid precursor (‘prosystemin’) and that leads to the release of linolenic acid. This activates the octadecanoid signalling cascade (Ryan, 2000). Both JA (Zhang and Baldwin, 1997) and systemin (Ryan, 2000) can be transported in the phloem and thus might act as systemic signals. To date, systemin has been described for tomato only, and not even for other solanaceous plants such as tobacco (Ryan, 2000; León et al., 2001). The importance of cell wall fragments in elicitation was supported by the finding that cellulysin, a mixture of several cell wall‐degrading enzymes from the plant parasitic fungus Trichoderma viride, can induce several JA‐responsive volatiles in lima bean (Phaseolus lunatus) (Piel et al., 1997). The action of cellulysin is followed by a rapid increase in endogenous JA (Koch et al., 1999).
Cross‐talk
Many studies have assumed the existence of at least two main signalling pathways: SA‐dependent ISR involved in resistance caused by and effective against pathogens, and JA‐dependent IRH effective against herbivores [but see Pieterse et al. (2001) for a JA‐dependent pathogen resistance elicited by rhizobacteria, and Walling (2000) for an overview on further signalling pathways]. We therefore regard resistance elicited by one group of enemies and active (also) against another as cross‐resistance (e.g. resistance against pathogens induced by herbivores and vice versa).
Cross‐resistance has been found in different systems. Feeding by thrips and aphids reduced infection of watermelon by the fungus Colletotrichum orbiculare (Russo et al., 1997). Padgett et al. (1994) reported that defoliation of soybean by Pseudoplusia includens (soybean looper) reduces the severity of two different fungal infections. Beetle grazing can induce resistance against fungal infections in Rumex obtusifolius (Hatcher and Paul, 2000). Helicoverpa zea (corn earworm) feeding can increase resistance of tomato plants to an aphid species (Macrosiphum euphorbiae), a mite species (Tetranychus urticae), another noctuid species (corn earworm, Spodoptera exigua) and to a bacterial phytopathogen, Pseudomonas syringae pv. tomato (Stout et al., 1998a; Bostock et al., 2001). P. syringae pv. tomato can induce PIs in tomato leaves, which are characteristic of a wound‐response and induced herbivore resistance rather than of ISR. Noctuid larvae feeding on leaves of Pseudomonas‐induced plants performed significantly less well than on control leaves (Bostock et al., 2001).
Trade‐offs
Other studies have reported ‘trade‐offs’, i.e. compromised resistance against one group of enemies when the plant is in the induced stage against the other group (Felton et al., 1999; Bostock, 1999). In this context, many studies have been conducted on tomato, Lycopersicon esculentum (for reviews, see Thaler, 1999; Bostock et al., 2001). These studies have demonstrated that chemical induction of ISR decreases the plants’ ability to express wound‐inducible PIs (Doares et al., 1995a; Fidantsef et al., 1999). Similarly, treating leaves with acibenzolar (synthetic benzothiadiazole, the active component of BION®) increased their suitability for herbivorous caterpillars (Thaler et al., 1997; Stout et al., 1999). SA‐treatment inhibits wound‐ and JA‐induced responses in the same plant (Stout et al., 1998b), and application of JA partially reduced the efficacy of chemical ISR elicitors (Thaler et al., 1997).
Acetylsalicylic acid applied to tomato plants inhibits the synthesis of PIs in response to wounding (Doherty et al., 1988). In both tobacco and tomato, SA inhibits synthesis of JA and thereby the expression of JA‐regulated genes in response to wounding, but not the induction of the same genes in response to exogenously applied JA (Pena‐Cortes et al., 1993; Baldwin et al., 1996; Baldwin et al., 1997). The inhibition of the octadecanoid pathway by acetylsalicylic acid appears to occur, at least in part, at the conversion of 13S‐hydroperoxylinolenic acid to oxophytodienoic acid (Pena‐Cortes et al., 1993). Doares et al. (1995a) reported that acetylsalicylic acid strongly reduced PI accumulation in tomato in response to wounding or to the action of systemin. Similarly, the synthesis of PIs induced by exposure to methyljasmonate (MeJA) vapours was inhibited by acetylsalicylic acid, and several other proteins that specifically responded to JA did not accumulate in response to JA in the presence of SA. These results point to an SA‐mediated inhibition of octadecanoid signalling that is localized downstream of JA. In lima bean, SA blocks the octadecanoid pathway downstream of OPDA, but before JA (Engelberth et al., 2000). Taken together, these results demonstrate that SA can inhibit the octadecanoid signalling cascade at different steps that are located both upstream and downstream of JA (Fig. 1).
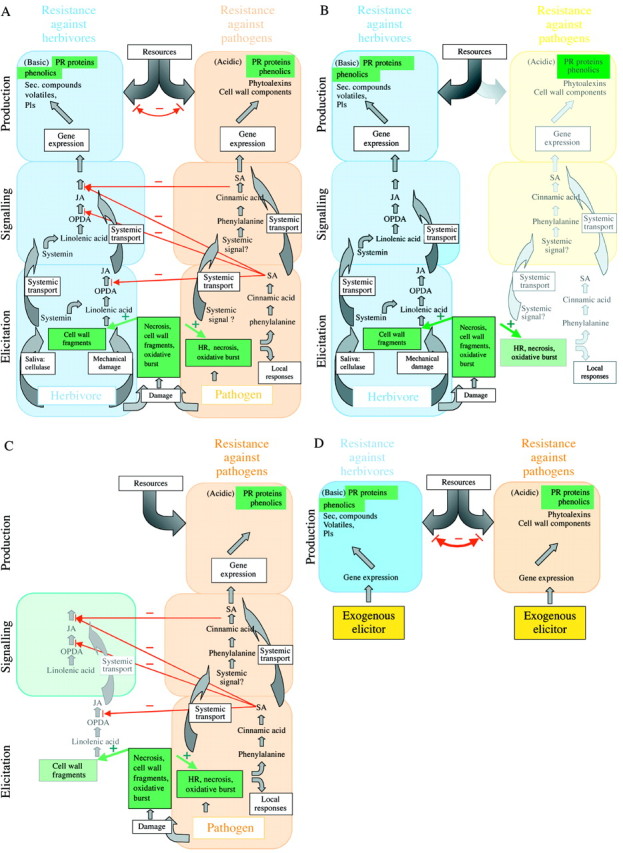
Fig. 1. Variable outcomes of cross‐talk between ISR (induced systemic resistance) and IRH (induced resistance against herbivores) signalling. A, Overview of interactions. On the level of elicitation and production several ‘common factors’ (in green boxes) appear in both signalling pathways (necrosis, cell wall fragments and oxidative burst during elicitation; phenolics and PR proteins on the production level) and might represent factors leading to cross‐resistance phenomena. B, Elicitation by a herbivore. While inducing mainly the octadecanoid pathway, the ‘common’ elicitors might lead to partial induction of ISR signalling. Resources are mainly allocated to herbivore resistance, but some resistance against pathogens is expressed, too. C, Elicitation by a pathogen. The partial induction of the octadecanoid pathway by the ‘common’ elicitors might lead to the occurrence of some early metabolites such as OPDA, but later, the pathway is blocked by the inhibitory effects of SA. On the phenotypic level, only resistance against pathogens is expressed. D, Exogenous elicitation bypasses regulatory mechanisms on the elicitation and the signalling level. The competition between both pathways for limiting resources therefore dominates the outcome and leads to phenotypically visible trade‐offs when both pathways are induced at the same time. See text for further details.
Evidence for inhibition of SA‐signalling by the action of JA is less common. Doares et al. (1995a) found no effect of MeJA or JA on the expression of two SA‐responsive PR proteins. In contrast, Niki et al. (1998) studied the expression of acidic and basic PR‐1, PR‐2 and PR‐3 genes in tobacco and reported that all acidic PR genes tested were induced by SA, while their induction in response to SA was inhibited by MeJA. On the other hand, basic PR genes were induced in response to wounding and MeJA and were inhibited by SA. Induction and inhibition in response to both elicitors occurred in a dose‐responsive manner (Niki et al., 1998).
General patterns
The data published to date reveal mixed evidence, and a general pattern is difficult to discern. Several studies have tried to find integrating concepts (Sticher et al., 1997; Bostock, 1999; Pieterse and Van Loon, 1999; Walling, 2000; Bostock et al., 2001; León et al., 2001), but a generally accepted model for the interplay between ISR and IRH has not yet been presented. Published results might be strongly biased since experiments demonstrating cross‐resistance are much more likely to appear in print than experiments failing to reveal a significant effect. Finally, results from one plant–pathogen or plant–herbivore system are not necessarily representative of other species (León et al., 2001). In order to provide a starting point, we have tried to find general patterns in the published data. First, studies eliciting or detecting resistance using biological methods (living organisms as inducers and testing the resistance by determining the effects on living organisms) must be distinguished from those eliciting or detecting the resistance using chemical or biochemical approaches. Secondly, herbivores of different feeding guilds should be considered separately. By doing this, several general trends become apparent.
‘Biol–biol’ (biological induction and biological detection).
Many of the studies that use induction by herbivores and then challenge with pathogens have reported cross‐resistance (i.e. herbivores induced resistance to herbivores and to pathogens). This is true for herbivores belonging to the group of leaf chewers, since beetles or caterpillars can induce resistance against fungal (Padgett et al., 1994; Hatcher et al., 1995; Hatcher and Paul, 2000) or bacterial pathogens (Stout et al., 1998a), and for thrips and aphids that induce resistance against a fungal pathogen (Russo et al., 1997). Sucking insects in particular cause only very local damage and seem to be recognized by the plants as ‘pathogens’ rather than as ‘classical herbivores’, thus eliciting ISR (Walling, 2000). Cases in which pathogens have been reported to induce resistance against herbivore feeding are much less common (but see Bostock et al., 2001).
‘Biol–chem’.
Several studies have been carried out with biological induction and biochemical markers for resistance (e.g. by detecting PR proteins as typical ‘ISR‐markers’) and in most cases cross‐resistance was reported, e.g. induction of PR proteins by the action of caterpillars (Padgett et al., 1994), whiteflies (Inbar et al., 1999), aphids (Fidantsef et al., 1999; Stout et al., 1999) and leaf miners (Inbar et al., 1999). Many defensive compounds (or groups of compounds) have effects against both pathogens and herbivores. Prominent examples are quinolizidine alkaloids (Petterson et al., 1991), gossypol, glucosinolates, nonproteinogenic amino acids (Bennett and Wallsgrove, 1994) and furanocoumarins (Berenbaum and Zangerl, 1999). When only defensive chemicals are quantified, induction of all of these compounds could be interpreted as ‘cross‐resistance’.
‘Chem–biol’.
Some studies have used chemical elicitation and reported cross‐resistance. For example, treatment with acibenzolar, but not SA itself, reduced the density of leaf miner larvae on tomato (Inbar et al., 1998), and JA can induce resistance against Phytophthora infestans in potato and tomato (Cohen et al., 1993). However, most studies using chemical induction have revealed trade‐offs rather than cross‐resistance (see work on tomato discussed above). No effect of SA treatment on future herbivore feeding could be detected for Helicoverpa zea feeding on cotton, although feeding by this herbivore does lead to higher SA levels in the same plant (Bi et al., 1997). Studies based on exogenous application of elicitors often suffer from physiologically unrealistic within‐plant concentrations and distributions of these elicitors and thus should be interpreted with caution (see ‘Outlook’ and Heil and Baldwin, 2002).
Unifying model for the cross‐talk between ISR and IRH
Three levels in the induction pathway should be distinguished: elicitation, signalling and ‘production’, i.e. gene expression and synthesis of enzymes and other proteins involved in the establishment of the resistance phenotype. Assuming that interactions can occur independently on all three levels, most of the so far contradictory results can be interpreted within the same framework (Fig. 1).
(1) Elicitation.
SA is synthesized in response to mechanical damage, necrosis and oxidative stress. Compounds resulting from the degradation of cells or cell walls might be involved in eliciting the systemic signal and ISR can thereby be induced by different types of enemies. Correspondingly, JA can be induced in response to cell wall degradation (see above). Further elicitors reported in the context of both wound‐response and ISR include the development of reactive oxygen species [‘oxidative burst’; see Lamb and Dixon (1997) for its involvement in ISR, and Orozco‐Cardenas and Ryan (1999) for its association with IRH], chitosan (ISR; Benhamou, 1996; IRH; Doares et al., 1995b), membrane depolarization (Engelberth et al., 2000) and Ca2+ fluxes (León et al., 2001). Thus, any factor leading to necrosis or otherwise activating some of these factors might elicit, at least partly, both the IRH‐ and the ISR‐pathway (Fig. 1A and B). Therefore, events at the elicitation level will mainly lead to the expression of a rather non‐specific cross‐resistance.
(2) Signalling.
Further interactions can occur at the signalling level. Different activities of the various intermediates have been reported for the octadecanoid cascade, thus leading to a large diversity of potential outcomes (Koch et al., 1999). Similar regulatory properties might characterize the SA‐dependent signalling. An inhibition of the JA‐pathway by SA has been described in different plant species. While herbivores can induce both ISR and IRH (Fig. 1B), an induction by pathogens (although probably eliciting early steps of the octadenanoid pathway) leads to synthesis of high concentrations of SA and thus blocks later steps in octadecanoid signalling. Phenotypically, pathogen attack thus induces mainly (or only) ISR compounds (Fig. 1C). At the signalling level, an elicitation of ‘ISR‐typical’ compounds and pathogen resistance by IRH‐ as well as by ISR‐elicitors, but an inhibition of the JA‐pathway by compounds of the ISR pathway, appear to have evolved.
(3) Production.
The trade‐offs found in other studies (Fidantsef et al., 1999; Thaler, 1999; Bostock et al., 2001) might, in contrast, occur mainly at the production level (i.e. signal–response coupling; Fig. 1D). Production of defensive compounds can be limited by the supply of available precursors such as amino acids, ATP and other biosynthetic cofactors, and so does not depend only on the outcome of events at the signalling level (see ‘Allocation costs’). Niki et al. (1998) reported that the accumulated mRNA‐levels for SA‐responsive acidic types and for JA‐responsive basic types of PR‐1 genes in the presence of various JA‐ and SA‐concentrations were mirror images: conditions that induce basic PR gene transcripts reduce the expression of acidic PR transcripts and vice versa. Induction of SA‐responsive and of JA‐responsive genes appeared to occur each at the cost of the other group, since ‘plants might simply be compromised in the total amounts of defensive compounds which can be produced during a limited time span’ (Heil, 2001). Yet the study by Niki et al. (1998) was based on the detection of mRNAs. These are only a rough predictor of amounts of synthesized PR proteins, which are likely to represent the most important cost factor. Legrand et al. (1987) reported acidic chitinases to occur at higher quantities (approx. 4–6 µg g–1 f. wt) than basic chitinases (1–3 µg–1 f. wt), results that are somewhat contradictory to the idea that total amounts of both types of PR proteins might be compromised. However, PR protein amounts depend strongly on the degree of infection (van Loon et al., 1987). Further studies carefully quantifying total amounts of PR proteins are required to verify the assumption that this is one example in which resource limitation might be the reason for inhibitory effects of chemical ISR or IRH elicitation on the respective other pathway.
For several of the ‘broad‐spectrum’ defensive compounds that have been reported to lead to resistance against both pathogens and herbivores, studies have looked at whole groups of compounds rather than single substances (but see references for gossypol). Furanocoumarins exhibit effects against bacteria, fungi, viruses, insects, molluscs and vertebrates (Berenbaum and Zangerl, 1999), yet this does not necessarily mean that any single furanocoumarin causes resistance against all these groups. Metabolic competition is likely to occur among compounds having most precursors in common. While single ‘broad‐spectrum’ defensive compounds such as gossypol clearly lead to cross‐resistance, these groups of compounds, also appearing as ‘cross‐resistance’ when quantified as a whole chemical group, might in fact be the source of ‘trade‐offs’ in biologically defined resistance.
Evolutionary background for the cross‐talk between ISR and IRH
Any form of induced resistance can be of selective advantage only if the eliciting attack has a predictive value and thus can be used as a cue to indicate future attack by a given enemy (Karban et al., 1999 and references therein). This is true for insect herbivores since they are unlikely to move away from a plant as long as they are not forced to do so. The same remains true for pathogens, many of which can move systemically within a plant or infect new plant parts from the site of initial infection, and thus predict, to some degree, their own occurrence in the near future. However, herbivore feeding can facilitate pathogen infection, and herbivores act as vectors for pathogens (Harris and Maramorosh, 1980). This is especially so for phloem‐feeding whiteflies and aphids, which establish long‐lasting and intimate associations with their host; these two groups of herbivores are generally reported to elicit ISR (Walling, 2000). Herbivore feeding can be predictive of disease in such cases, and ISR induced by herbivore feeding might provide plants with strong selective advantages. Pathogen resistance elicited in response to herbivory has been found in many studies (see above). On the other hand, the octadecanoid signalling can be induced, at least partly, by different forms of damage, including mechanical wounding, chewing or sucking herbivores, and herbivore‐ or pathogen‐caused cell death. However, pathogen attack has only a low (if any) predictive value for future herbivore attack. Since ‘superfluous’ induction of resistance might use up limited resources (‘allocation costs’), a down regulation or inhibition of the JA‐dependent pathway in case of damage caused non‐specifically by pathogens would provide strong selective advantages and is evident in the form of the SA‐caused inhibition of octadecanoid signalling.
OUTLOOK
More than 3000 articles on induced pathogen resistance have been published since 1995. However, many questions are still unanswered and require further investigation. Strong efforts are required to identify the compounds causing resistance, and future studies should quantify these compounds in combination with the biologically detectable resistance to characterize the induced stage. A variety of resistance compounds should be quantified instead of focusing on a few selected markers, and resistance against biologically relevant organisms (i.e. those pests that challenge plants under natural conditions) should be conducted instead of using target organisms selected mainly due to their ease of use under laboratory conditions.
Most studies revealing contradictory results with respect to positive or negative interactions between ISR and IRH have focused on different levels of the pathways and/or have used different methods, with ‘biological’ induction obviously leading to cross‐resistance, while chemical elicitation mainly results in trade‐offs. Several differences have already been reported between chemically and biologically induced resistance (Schweizer et al., 1997; Molina et al., 1999). Studies using external application of elicitors might suffer strongly from physiologically unrealistic concentrations and spatial distributions of the resistance elicitors. There are probably mechanisms regulating the interplay among resistance pathways in response to natural elicitation that are bypassed when resistance is elicited chemically. Studies integrating the molecular, physiological and ‘biological’ aspect of resistance that take into account different forms of resistance at the same time are required to rule out the dependencies between molecular and physiological events and the phenotypically occurring resistance, and to find general patterns in the interactions among resistance pathways. Experiments thoroughly exploring signalling conflicts and synergies in plant–herbivore and plant–pathogen interactions will be essential to realise fully the potential of inducible resistance strategies in agricultural pest management.
ACKNOWLEDGEMENTS
We thank Wilhelm Boland and L. C. van Loon for valuable comments. Financial support to M.H. by the DFG (research grant He3169/1–1) and to R.M.B. (USDA National Research Initiative Competitive Grants Program and by CEPRAP, the NSF Center for Engineering Plants for Resistance Against Pathogens) is gratefully acknowledged.
Supplementary Material
Received: 31 August 2001; Returned for revision: 9 November 2001; Accepted: 18 January 2002.
References
- AntoniwJF, Pierpoint WS.1978. The purification and properties of one of the ‘b’ proteins from virus‐infected tobacco plants. Journal of General Virology 39: 343–350. [Google Scholar]
- BaldwinIT, Schmelz EA, Zhang Z‐P.1996. Effects of octadecanoid metabolites and inhibitors on induced nicotine accumulation in Nicotiana sylvestris Journal of Chemical Ecology 22: 61–74. [DOI] [PubMed] [Google Scholar]
- BaldwinIT, Zhang Z‐P, Diab N, Ohnmeiss TE, McCloud ES, Lynds GY, Schmelz EA.1997. Quantification, correlations and manipulation of wound‐induced changes in jasmonic acid and nicotine in Nicotiana sylvestris Planta 201: 397–404. [Google Scholar]
- BenhamouN.1996. Elicitor‐induced plant defence pathways. Trends in Plant Science 1: 233–240. [Google Scholar]
- BennettRN, Wallsgrove RM.1994. Tansley Review No. 72: secondary metabolites in plant defence mechanisms. New Phytologist 127: 617–633. [DOI] [PubMed] [Google Scholar]
- BerenbaumMR, Zangerl AR.1999. Coping with life as a menu option: inducible defenses of the wild parsnip. In: Tollrian R, Harvell CD, eds. The ecology and evolution of inducible defenses Princeton: Princeton University Press, 10–32. [Google Scholar]
- BiJL, Murphy JB, Felton GW.1997. Does salicylic acid act as a signal in cotton for induced resistance to Helicoverpa zea? Journal of Che micalEcology 23: 1805–1818. [Google Scholar]
- BishopPD, Makus DJ, Pearce G, Ryan CA.1981. Proteinase inhibitor‐inducing factor activity in tomato leaves resides in oligosaccharides enzymatically released from cell walls. Proceedings of the National Academy of Sciences of the USA 78: 3536–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BolandW, Koch T, Krumm T, Piel J, Jux A.1999. Induced biosynthesis of insect semiochemicals in plants. In: Chadwick DJ, Goode JA, eds. Insect–plant interactions and induced plant defence Chichester, New York: Wiley, 110–131. [DOI] [PubMed] [Google Scholar]
- BostockRM.1999. Signal conflicts and synergies in induced resistance to multiple attackers. Physiological and Molecular Plant Pathology 55: 99–109. [Google Scholar]
- BostockRM, Karban R, Thaler JS, Weyman PD, Gilchrist D.2001. Signal interactions in induced resistance to pathogens and insect herbivores. European Journal of Plant Pathology 107: 103–111. [Google Scholar]
- BroglieK, Chet I, Holliday M, Cressman R, Biddle P, Kowlton S, Mauvais C, Broglie R.1991. Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani Science 254: 1194–1197. [DOI] [PubMed] [Google Scholar]
- CameronRK.2000. Salicylic acid and its role in plant defense responses: what do we really know? Physiological and Molecular Plant Pathology 56: 91–93. [Google Scholar]
- CohenY, Gisi U, Niderman T.1993. Local and systemic protection against Phytophtora infestans induced in potato and tomato plants by jasmonic acid and jasmonic methyl‐ester. Phytopathology 83: 1054–1062. [Google Scholar]
- ConrathU, Thulke O, Katz V, Schwindling S, Kohler A.2001. Priming as mechanism in induced systemic resistance of plants. European Journal of Plant Pathology 107: 113–119. [Google Scholar]
- CoquozJL, Buchala AJ, Meuwly P, Métraux JP.1995. Arachidonic acid treatment of potato plants induces local synthesis of salicylic acid and confers systemic resistance to Phytophthora infestans and Alternaria soltani Phythopathology 85: 1219–1224. [Google Scholar]
- CreelmanRA, Mullet JE.1997. Biosynthesis and action of jasmonates in plants. Annual Review of Plant Physiology and Plant Molecular Biology 48: 355–381. [DOI] [PubMed] [Google Scholar]
- DattaKN, Datta SK.1999. Expression and function of PR‐protein genes in transgenic plants. In: Datta S, Muthukrishnan S, eds. Pathogenesis‐related proteins in plants Boca Raton: CRC Press, 261–277. [Google Scholar]
- DelaneyTP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut‐Rella M, Kessmann H, Ward E, Rylas J.1994. A central role of salicylic acid in plant disease resistance. Science 266: 1247–1249. [DOI] [PubMed] [Google Scholar]
- DoaresSH, Narváez‐Vásquez J, Conconi A, Ryan CA.1995a Salicylic acid inhibits synthesis of proteinase‐inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiology 108: 1741–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DoaresSH, Syrovets T, Weiler EW, Ryan CA.1995b Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proceedings of the National Academy of Sciences of the USA 92: 4095–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DohertyHM, Selvendran RR, Bowles DJ.1988. The wound response of tomato plants can be inhibited by aspirin and related hydroxy‐benzoic acids. Physiological and Molecular Plant Pathology 33: 377–384. [Google Scholar]
- EngelberthJ, Koch T, Kühnemann F, Boland W.2000. Channel‐forming peptaibols are a novel class of potent elicitors of plant secondary metabolism and tendril coiling. Angewandte Chemie 112: 1928–1930. [DOI] [PubMed] [Google Scholar]
- FeltonGW, Korth KL, Bi JL, Wesley SV, Huhman DV, Mathews MC, Murphy JB, Lamb C, Dixon RA.1999. Inverse relationship between systemic resistance of plants to microorganisms and to insect herbivory. Current Biology 9: 317–320. [DOI] [PubMed] [Google Scholar]
- FidantsefAL, Stout MJ, Thaler JS, Duffey SS, Bostock RM.1999. Signal interactions in pathogen and insect attack: expression of lipoxygenase, proteinase inhibitor II, and pathogenesis‐related protein P4 in the tomato, Lycopersicon esculentum. Physiological and Molecular Plant Pathology 54: 97–114. [Google Scholar]
- GaffneyT, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J.1994. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261: 754–756. [DOI] [PubMed] [Google Scholar]
- GreenbergJT, Silverman FP, Liang H.2000. Uncoupling salicylic acid‐dependent cell death and defense‐related responses from disease resistance in the Arabidopsis mutant acd5 Genetics 156: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HammerschmidtR.1999a Induced disease resistance: how do induced plants stop pathogens? Physiological and Molecular Plant Pathology 55: 77–84. [Google Scholar]
- HammerschmidtR.1999b Phytoalexins: what have we learned after 60 years? Annual Review of Phytopathology 37: 285–306. [DOI] [PubMed] [Google Scholar]
- HarrisKF, Maramorosh K.1980. Vectors of plant pathogens. New York: Academic Press. [Google Scholar]
- HatcherPE, Paul ND.2000. Beetle grazing reduces natural infection of Rumex obtusifolius by fungal pathogens. New Phytologist 146: 325–333. [DOI] [PubMed] [Google Scholar]
- HatcherPE, Ayres PG, Paul ND.1995. The effect of natural and simulated insect herbivory, and leaf age, on the process of infection of Rumex crispus L. and R. obtusifolius L. by Uromyces rumicis (Schum.) Wint. New Phytologist 130: 239–249. [Google Scholar]
- HeilM.2001. The ecological concept of costs of induced systemic resistance (ISR). European Journal of Plant Pathology 107: 137–146. [Google Scholar]
- HeilM, Baldwin IT.2002. Fitness costs of induced resistance – the emerging experimental support for a slippery concept. Trends in Plant Science 7: 61–67. [DOI] [PubMed] [Google Scholar]
- HeilM, Hilpert A, Kaiser W, Linsenmair KE.2000. Reduced growth and seed set following chemical induction of pathogen defence: does systemic acquired resistance (SAR) incur allocation costs? Journal of Ecology 88: 645–654. [Google Scholar]
- HermsDA, Mattson WJ.1992. The dilemma of plants: to grow or to defend. The Quarterly Review of Biology 67: 283–335. [Google Scholar]
- HuntMD, Neuenschwander UH, Delaney TP, Weymann KB, Friedrich LB, Lawton KA, Steiner H‐Y, Ryals JA.1996. Recent advances in systemic acquired resistance research – a review. Gene 179: 89–95. [DOI] [PubMed] [Google Scholar]
- InbarM, Doostdar H, Leibee GL, Mayer RT.1999. The role of plant rapidly induced responses in asymmetric interspecific interactions among insect herbivores. Journal of Chemical Ecology 25: 1961–1979. [Google Scholar]
- InbarM, Doostdar H, Sonoda RM, Leibee GL, Mayer RT.1998. Elicitors of plant defensive systems reduce insect densities and disease incidence. Journal of Chemical Ecology 24: 135–149. [Google Scholar]
- JacksonAO, Taylor CB.1996. Plant–microbe interactions: life and death at the interface. The Plant Cell 8: 1651–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JakabG, Cottier V, Toquin V, Rigoli G, Zimmerli L, Métraux J‐P, Mauch‐Mani B.2001. β‐Aminobutyric acid‐induced resistance in plants. European Journal of Plant Pathology 107: 29–37. [Google Scholar]
- KarbanR, Baldwin IT.1997. Induced responses to herbivory. Chicago and London: University of Chicago Press. [Google Scholar]
- KarbanR, Agrawal AA, Thaler JS, Adler LS.1999. Induced plant responses and information content about risk of herbivory. Trends in Ecology and Evolution 14: 443–447. [DOI] [PubMed] [Google Scholar]
- KochT, Krumm T, Jung V, Engelberth J, Boland W.1999. Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoid‐signaling pathway. Plant Physiology 121: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KombrinkE, Schmelzer E.2001. The hypersensitive response and its role in local and systemic disease resistance. European Journal of Plant Pathology 107: 69–78. [Google Scholar]
- KucJ.1995. Phytoalexins, stress metabolism, and disease resistance in plants. Annual Review of Phytopathology 33: 275–297. [DOI] [PubMed] [Google Scholar]
- LambC, Dixon RA.1997. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology 48: 251–275. [DOI] [PubMed] [Google Scholar]
- LegrandM, Kauffmann S, Pierrette G, Fritig B.1987. Biological function of pathogenesis‐related proteins: Four tobacco pathogenesis‐related proteins are chitinases. Proceedings of the National Academy of Sciences of the USA 84: 6750–6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeónJ, Rojo E, Sánchez‐Serrano JJ.2001. Wound signalling in plants. Journal of Experimental Botany 52: 1–9. [DOI] [PubMed] [Google Scholar]
- MalamyJ, Carr JP, Klessig DF, Raskin I.1990. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250: 1002–1004. [DOI] [PubMed] [Google Scholar]
- MauchF, Mauch‐Mani B, Gaille C, Kull B, Haas D, Reimmann C.2001. Manipulation of salicylate content in Arabidopsis thaliana by the expression of an engineered bacterial salicylate synthase. The Plant Journal 25: 67–77. [DOI] [PubMed] [Google Scholar]
- Mauch‐ManiB, Métraux J‐P.1998. Salicylic acid and systemic acquired resistance to pathogen attack. Annals of Botany 82: 535–540. [Google Scholar]
- MétrauxJ‐P.2001. Systemic acquired resistance and salicylic acid: current state of knowledge. European Journal of Plant Pathology 107: 13–18. [Google Scholar]
- MétrauxJ‐P, Signer H, Ryals J, Ward E, Wyss‐Benz M, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverardi B.1990. Increase in salicylic acid at the onset of systemic acquired resistance. Science 250: 1004–1006. [DOI] [PubMed] [Google Scholar]
- MeuwlyP, Mölders W, Buchala A, Métraux JP.1995. Local and systemic biosynthesis of salicylic acid in infected cucumber plants. Plant Physiology 109: 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MolinaA, Görlach J, Volrath S, Ryals J.1999. Wheat genes encoding two types of PR‐1 proteins are pathogen inducible, but do not respond to activators of systemic acquired resistance. Molecular Plant‐Microbe Interactions 12: 53–58. [DOI] [PubMed] [Google Scholar]
- NeuhausJM.1999. Plant chitinases (PR‐3, PR‐4, PR‐8, PR‐11). In: Datta SK, Muthukrishnan S, eds. Pathogenesis‐related proteins in plants Boca Raton: CRC Press, 77–105. [Google Scholar]
- NikiT, Mitsuhara I, Seo S, Ohtsubo N, Ohashi Y.1998. Antagonistic effects of salicylic acid and jasmonic acid on the expression of pathogenesis‐related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiology 39: 500–507. [Google Scholar]
- NormanC, Vidal S, Palva ET.1999. Oligogalacturonide‐mediated induction of a gene involved in jasmonic acid synthesis in response to the cell‐wall‐degrading enzymes of the plant pathogen Erwinia carotovora Molecular Plant‐Microbe Interactions 12: 640–644. [DOI] [PubMed] [Google Scholar]
- Orozco‐CardenasM, Ryan CA.1999. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proceedings of the National Academy of Sciences of the USA 96: 6553–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PadgettGB, Russin JS, Snow JP, Boethel DJ, Berggren GT.1994. Interactions among the soybean looper (Lepidoptera: Noctuidae), threecorned alfalfa hopper (Homoptera: Membracidae), stem canker, and red crown rot in soybean. Journal of Entomological Science 29: 110–119. [Google Scholar]
- PearceG, Strydom D, Johnson S, Ryan CA.1991. A polypeptide from tomato leaves induces wound‐inducible proteinase inhibitor proteins. Science 253: 895–898. [DOI] [PubMed] [Google Scholar]
- Pena‐CortesH, Albrecht T, Prat S, Weiler EW, Willmitzer L.1993. Aspirin prevents wound‐induced gene‐expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta 191: 123–128. [Google Scholar]
- PettersonDS, Harris DJ, Allen DG.1991. Alkaloids. In: D’Mello JPF, Duffus CM, Duffus JH, eds. Toxic substances in crop plants Cambridge: The Royal Society of Chemistry, 148–179. [Google Scholar]
- PielJ, Atzorn R, Gäbler R, Kühnemann F, Boland W.1997. Cellulysin from the plant parastic fungus Trichoderma viride elicits volatile biosynthesis in higher plants via the octadecanoid signaling cascade. FEBS Letters 416: 143–148. [DOI] [PubMed] [Google Scholar]
- PieterseCMJ, van Loon LC.1999. Salicylic acid‐independent plant defence pathways. Trends in Plant Science 4: 52–58. [DOI] [PubMed] [Google Scholar]
- PieterseCMJ, van Pelt JA, van Wees SCM, Ton J, Léon‐Kloosterziel K, Keurentjes JJB, Verhagen BWM, Knoester M, van der Sluis I, Bakker PAHM, van Loon LC.2001. Rhizobacteria‐mediated induced systemic resistance: triggering, signalling and expression. European Journal of Plant Pathology 107: 51–61. [Google Scholar]
- PricePW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE.1980. Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annual Review of Ecology and Systematics 11: 41–65. [Google Scholar]
- RaskinI.1992. Role of salicylic acid in plants. Annual Review of Plant Physiology and Plant Molecular Biology 43: 439–463. [Google Scholar]
- RasmussenJB, Hammerschmidt R, Zook MN.1991. Systemic induction of salicylic acid accumulation in cucumber after inoculation with Pseudomonas syringae pv syringae Plant Physiology 97: 1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ReinbotheS, Mollenhauer B, Reinbothe C.1994. JIPs and RIPs: the regulation of plant gene expression by jasmonates in response to environmental cues and pathogens. The Plant Cell 6: 1197–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RussoVM, Russo BM, Peters M, Perkins‐Veazie P, Cartwright B.1997. Interaction of Colletotrichum orbiculare with thrips and aphid feeding on watermelon seedlings. Crop Protection 16: 581–584. [Google Scholar]
- RyanCA.2000. The systemin signaling pathway: differential activation of plant defensive genes. Biochimica et Biophysica Acta 1477: 112–121. [DOI] [PubMed] [Google Scholar]
- SahaiAS, Manocha MS.1993. Chitinases of fungi and plants: their involvement in morphogenesis and host–parasite interaction. FEMS Microbiology Reviews 11: 317–338. [Google Scholar]
- SchlumbaumA, Mauch F, Vögeli U, Boller T.1986. Plant chitinases are potent inhibitors of fungal growth. Nature 324: 365–367. [Google Scholar]
- SchneiderM, Schweizer P, Meuwly P, Métraux JP.1996. Systemic acquired resistance in plants. In: Jeon KW, ed. International review of cytology, vol. 168 San Diego: Academic Press, 303–340. [Google Scholar]
- SchweizerP, Buchala A, Silverman P, Seskar M, Raskin I, Métraux J‐P.1997. Jasmonate‐inducible genes are activated in rice by pathogen attack without a concomitant increase in endogenous jasmonic acid levels. Plant Physiology 114: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SilvermanP, Seskar M, Kanter D, Schweizer P, Métraux J.1995. Salicylic acid in rice. Plant Physiology 108: 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SomssichIE, Hahlbrock K.1998. Pathogen defence in plants – a paradigm of biological complexity. Trends in Plant Science 3: 86–90. [Google Scholar]
- SticherL, Mauch‐Mani B, Métraux J‐P.1997. Systemic acquired resistance. Annual Review of Phytopathology 35: 235–270. [DOI] [PubMed] [Google Scholar]
- StoutMJ, Bostock RM.1999. Specificity of induced responses to arthropods and pathogens. In: Agrawal AA, Tuzun S, Bent E, eds. Induced plant defenses against pathogens and herbivores St Paul, Minnesota: The American Phytopathological Society Press, 183–209. [Google Scholar]
- StoutMJ, Fidantsef AL, Duffey SS, Bostock RM.1999. Signal interactions in pathogen and insect attack: Systemic plant‐mediated interactions between pathogens and herbivores of the tomato, Lycopersicon esculentum Physiological and Molecular Plant Pathology 54: 115–130. [Google Scholar]
- StoutMJ, Workman KV, Bostock RM, Duffey SS.1998a Specificity of induced resistance in the tomato, Lycopersicon esculentum Oecologia 113: 74–81. [DOI] [PubMed] [Google Scholar]
- StoutMJ, Workman KV, Bostock RM, Duffey SS.1998b Stimulation and attenuation of induced resistance by elicitors and inhibitors of chemical induction in tomato (Lycopersicon esculentum) foliage. Entomologia Experimentalis et Applicata 86: 267–279. [Google Scholar]
- ThalerJS.1999. Jasmonic acid mediated interactions between plants, herbivores, parasitoids, and pathogens: a review of field experiments in tomato. In: Agrawal AA, Tuzun S, Bent E, eds. Induced plant defenses against pathogens and herbivores: Biochemistry, ecology, and agriculture St Paul, Minnesota: The American Phytopathological Society Press, 319–334. [Google Scholar]
- ThalerJS, Fidantsef AL, Duffey SS, Bostock RM.1999. Trade‐offs in plant defense against pathogens and herbivores: a field demonstration of chemical elicitors of induced resistance. Journal of Chemical Ecology 25: 1597–1609. [Google Scholar]
- ThommaBPHJ, Nelissen I, Eggermont K, Broekaert WF.1999. Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola The Plant Journal 19: 163–171. [DOI] [PubMed] [Google Scholar]
- vanLoonLC.1997. Induced resistance in plants and the role of pathogenesis‐related proteins. European Journal of Plant Pathology 103: 753–765. [Google Scholar]
- vanLoonLC.1999. Occurrence and properties of plant pathogenesis‐related proteins. In: Datta SK, Muthukrishnan S, eds. Pathogenesis‐related proteins in plants Boca Raton: CRC Press, 1–19. [Google Scholar]
- vanLoonLC, van Kammen A.1970. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. ‘Samsun’ and ‘Samsun NN’ II. Changes in protein constitution after infection with tobacco mosaic virus. Virology 40: 199–211. [DOI] [PubMed] [Google Scholar]
- vanLoonLC, van Strien EA.1999. The families of pathogenesis‐related proteins, their activities, and comparative analysis of PR‐1 type proteins. Physiological and Molecular Plant Pathology 55: 85–97. [Google Scholar]
- vanLoonLC, Gerritsen YAM, Ritter CE.1987. Identification, purification and characterization of pathogenesis‐related proteins from virus‐infected Samsun NN tobacco leaves. Plant Molecular Biology 9: 593–609. [DOI] [PubMed] [Google Scholar]
- VernooijB, Friedrich L, Morse A, Reist R, Kolditz‐Jawhar R, Ward E, Uknes S, Kessmann H, Ryals J.1994. Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. Plant Cell 6: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WallingLL.2000. The myriad plant responses to herbivores. Journal of Plant Growth Regulation 19: 195–216. [DOI] [PubMed] [Google Scholar]
- WasternackC, Parthier B.1997. Jasmonate‐signalled plant gene expression. Trends in Plant Science 2: 302–307. [Google Scholar]
- WeidhaseRA, Kramell HM, Lehmann J, Liebisch HW, Lerbs W, Parthier B.1987. Methyl jasmonate‐induced changes in the polypeptide pattern of senescing barley leaf segments. Plant Science 51: 177–186. [Google Scholar]
- ZhangZ‐P, Baldwin IT.1997. Transport of (2‐14C) jasmonic acid from leaves to roots mimics wound‐induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris Planta 203: 436–441. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


