Abstract
Objective
To determine risk of Down syndrome (DS) in multiple relative to singleton pregnancies, and compare prenatal diagnosis rates and pregnancy outcome.
Design
Population-based prevalence study based on EUROCAT congenital anomaly registries.
Setting
Eight European countries.
Population
14.8 million births 1990–2009; 2.89% multiple births.
Methods
DS cases included livebirths, fetal deaths from 20 weeks, and terminations of pregnancy for fetal anomaly (TOPFA). Zygosity is inferred from like/unlike sex for birth denominators, and from concordance for DS cases.
Main outcome measures
Relative risk (RR) of DS per fetus/baby from multiple versus singleton pregnancies and per pregnancy in monozygotic/dizygotic versus singleton pregnancies. Proportion of prenatally diagnosed and pregnancy outcome.
Statistical analysis
Poisson and logistic regression stratified for maternal age, country and time.
Results
Overall, the adjusted (adj) RR of DS for fetus/babies from multiple versus singleton pregnancies was 0.58 (95% CI 0.53–0.62), similar for all maternal ages except for mothers over 44, for whom it was considerably lower. In 8.7% of twin pairs affected by DS, both co-twins were diagnosed with the condition. The adjRR of DS for monozygotic versus singleton pregnancies was 0.34 (95% CI 0.25–0.44) and for dizygotic versus singleton pregnancies 1.34 (95% CI 1.23–1.46). DS fetuses from multiple births were less likely to be prenatally diagnosed than singletons (adjOR 0.62 [95% CI 0.50–0.78]) and following diagnosis less likely to be TOPFA (adjOR 0.40 [95% CI 0.27–0.59]).
Conclusions
The risk of DS per fetus/baby is lower in multiple than singleton pregnancies. These estimates can be used for genetic counselling and prenatal screening.
Keywords: Concordance, Down syndrome, monozygotic and dizygotic pregnancies, multiple births, pregnancy outcomes, twins
Introduction
Prenatal screening for Down syndrome (DS) uses maternal age-specific risk estimates combined with biochemical and/or ultrasound measurements to inform the decision whether to proceed to more invasive diagnostic tests. Lack of precise and maternal age-specific information regarding the risk of DS in multiple pregnancies1–3 potentially leads to misleading risk estimates. Amniocentesis is technically more difficult to carry out in multiple than in singleton pregnancies, with a potential risk of sampling the wrong fetus in discordant dizygotic pregnancies and incurring greater fetal loss.2 The excess risk of fetal loss following amniocentesis in multiple pregnancies is known to be more than double that in singleton pregnancies,1,4 but it is not known how much the risk increases for monozygotic and dizygotic pregnancies separately.5 Weighing the benefits and risks of such a procedure should depend on a solid evidence base.
A number of studies have reported a lower risk of DS in multiple births relative to singletons6–9 but this finding has not been reported universally, with some studies finding no statistical difference in risk.10–12 Nevertheless, the risk per fetus suggested for genetic counselling is taken by some investigators as similar in multiple and singleton pregnancies, with the caveat that in monozygotic multiple pregnancies both fetuses would be affected.2,13,14 Others propose that the risk per pregnancy is more appropriate in genetic counselling and, in recognition of the evidence of a lower than expected risk per fetus in multiple pregnancies, suggest that the risk for a twin pregnancy should be given as similar to the risk in singleton pregnancies.1,15 Recent NICE guidelines recommended counselling that the risk of DS per pregnancy should be considered higher in multiple pregnancies, without specifying how much higher.16 French guidelines recommend using singleton risk estimate tables without clearly addressing the pregnancy fetus issue.17
The prevalence of DS has increased in association with an increase in average maternal age over the past two decades.18,19 The prevalence of multiple births, particularly dizygotic pregnancies, has also increased in association both with increasing maternal age and with the use of assisted reproductive technologies (ART).20–22 The risk of Down syndrome specifically in monozygotic and in dizygotic pregnancies, as opposed to multiple pregnancies in general, has not been reported.
Using data from EUROCAT, a network of European population-based registries of congenital anomaly, we previously demonstrated a lower than expected risk of chromosomal anomalies in babies from multiple births compared with singletons in 1990–2009, with the difference increasing over time.20 The purpose of this paper is to determine the maternal age-specific prevalence of DS in monozygotic and in dizygotic pregnancies, and to explore the risk for each relative to singleton pregnancies. The paper will also compare prenatal diagnosis and outcome of pregnancy (livebirths, stillbirths or termination of pregnancy) for DS fetuses in multiple and singleton pregnancies.
Methods
European Surveillance of Congenital Anomalies (EUROCAT) is a network of population-based registries of congenital anomaly in 21 countries of Europe. EUROCAT collects standardised data that can be used to assess changes in the epidemiology of congenital anomalies and associated risk factors.23,24 Only DS cases were used in this analysis. The methods of registry case ascertainment are fully described elsewhere.19,25 The database includes live born congenital anomaly cases (LB), stillborn cases and fetal deaths after 20 weeks’ gestation (FD), and prenatally diagnosed cases resulting in termination of pregnancy for fetal anomaly (TOPFA).
Affiliated to EUROCAT is The National Down Syndrome Cytogenetic Register (NDSCR), a registry which does not routinely contribute case data to the EUROCAT central database but supplied such data specifically for this study. The NDSCR ascertains all cases of DS from cytogenetic reports sent directly from all laboratories in England and Wales.18 For this study we used all reports in this database of LB and FD from 20 weeks’ gestational age and TOPFA. The NDSCR data replaced the data of five British regional EUROCAT registries.
Criteria for inclusion of other EUROCAT registries in the study were that they should ascertain ≥80% of DS cases according to the EUROCAT DS data quality indicator (DQI) 2005–2009,26 they should have complete data on multiple birth status for DS cases, and >80% completeness for maternal age for DS cases, and they should be able to provide data on population births by multiple birth status and maternal age. Ten registries (including the NDSCR, which represented 87% of the study population) in eight countries participated in this study (Table1). The total study population was 14 827 105 births between 1990 and 2009, of which 2.89% were from multiple births. Individual fetuses/babies affected with DS from multiple and singleton pregnancies are referred to as cases. Twin pairs where both co-twins have DS are considered ‘concordant’ pairs, and the pregnancy is assumed to be monozygotic for same sex co-twins and dizygotic for different sex co-twins. We found three concordant pairs of unlike sex. Two same-sex concordant pairs where one co-twin was dead at amniocentesis and the other was a TOPFA, were included as monozygotic pregnancies but only the co-twin who was a TOPFA was included as a case. We did not have information on the sex of unaffected co-twins in non-concordant pairs.
Table 1.
Number of births by participating country, with proportion (%) from multiple births, and number of Down syndrome (DS) cases from singleton and multiple births, 1990–99 and 2000–2009
| Country | Years | 1990–99 | 2000–2009 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Births n | Multiple births % | DS cases from singleton births n | DS cases from multiple births n | Births n | Multiple births % | DS cases from singleton births n | DS cases from multiple births n | ||
| Poland (Wielkopolska) | 2000–2007 | – | – | – | – | 274 658 | 2.27 | 404 | 10 |
| Malta | 1999–2007 | 4376 | 3.15 | 11 | 0 | 30 896 | 2.78 | 59 | 3 |
| Austria (Styria)* | 1990–2007 | 125 787 | 2.30 | 179 | 3 | 80 706 | 3.00 | 152 | 5 |
| England and Wales (NDSCR) | 1990–2009 | 6 666 234 | 2.66 | 12 102 | 245 | 6 315 113 | 3.02 | 15 037 | 318 |
| Ireland*,† | 1990–2007 | 240 092 | 2.51 | 514 | 13 | 269 918 | 3.03 | 621 | 16 |
| Switzerland (Vaud) | 1990–2007 | 76 975 | 2.60 | 179 | 5 | 55 848 | 3.15 | 189 | 3 |
| France (Paris)** | 1990–2007 | 371 437 | 3.63 | 1 125 | 40 | 216 558 | 3.62 | 865 | 14 |
| Denmark (Odense)* | 1990–2007 | 57 949 | 3.52 | 91 | 4 | 40 558 | 4.68 | 92 | 3 |
| Total | 1990–2009 | 7 542 850 | 2.71 | 14 201 | 310 | 7 284 255 | 3.02 | 17 419 | 372 |
Denominators available by 5-year data on maternal age but NOT by like and unlike sex so monozygotic and dizygotic denominators were extrapolated from proportions in other EUROCAT Registries.
The Parisian registry was able to provide denominator data by 5 years of maternal age for all years but by like and unlike sex for 1993, 1997, 2001 and 2005 only, the proportions were extrapolated to other years within 4-year bands when estimating monozygotic and dizygotic denominators.
There are three Irish registries: Dublin (1990–2007) Cork and Kerry (1996–2004) and South east Ireland (1997–2007) All other countries have one participating EUROCAT registry.
Denominator data on all births by both maternal age (<20, by 5-year age groups to >44) and multiple birth status were available from all 10 registries (see Table1).The Office of National Statistics (ONS) for England and Wales (for NDSCR) and four registries outside the UK were able to provide denominator data by maternal age and like and unlike sex (see Table1), covering 94.4% of our data. In total, 66.4% of twin pairs were like sex in the UK, and 66.0% outside the UK. Zygosity proportions were calculated using the Weinberg rule: DZ pairs = 2 (unlike sex pairs) and MZ pairs = all pairs – (DZ pairs).27,28 This meant that denominator data could be estimated for singleton, dizygotic and monozygotic twin deliveries. This was done for data from England and Wales, and for the combined data of the four non-UK countries for the years available, extrapolated to all seven non-UK countries for each 10-year period. When these denominators were used to estimate risk from monozygotic and dizygotic pregnancies relative to singletons, only DS cases from singleton and twin pregnancies were included, excluding cases from higher order multiple births (one case from each of 12 triplet pregnancies, all from England and Wales). These cases who were triplets were also excluded when concordance was estimated.
The ONS also supplied denominators for England and Wales by single year of maternal age for singleton and multiple births which were used for an additional analysis of the NDSCR data. All data were used for all the analyses unless otherwise stated.
Outcome of pregnancy was available for all EUROCAT cases. For NDSCR, 3.36% of cases for which outcomes were missing were excluded from the outcomes analysis only. Analysis of outcome of pregnancy was confined to 2000–2009, as this represents the most recent data. Data on early neonatal survival were not available for all NDSCR cases, so perinatal death rates could not be calculated.
Multiple birth is defined for cases in EUROCAT guidelines according to the ‘number of babies/fetuses delivered’24 and this definition was also used for ‘multiple pregnancy’ in this study. In the case of DS TOPFA where there was a selective feticide rather than a full termination of pregnancy, it is possible that the civil registration of the unaffected twin was as a singleton despite their classification as multiple in this study.
Statistical analysis
Statistical analyses were performed using STATA version 9.0 (Statacorp LP, College Station, TX, USA).
Weights representing the probability of fetal survival to 20 weeks’ GA19 were applied to each TOPFA/selective feticide case. They were based on gestational age at TOPFA/selective feticide in order to standardise the number of cases and prevalence to 20 weeks’ gestational age, and thus correct total prevalence rates for any artefact caused by screening-related differences.
‘Total prevalence of DS cases from multiple pregnancies per 10 000 births’ was calculated as:
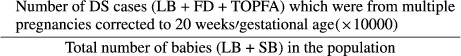 |
Total prevalence of DS cases per 10 000 multiple births was calculated as:
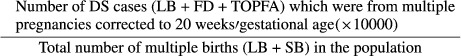 |
Similar definitions apply to singleton prevalence of DS. For monozygotic and dizygotic pregnancy-based analyses, cases and births are replaced with pregnancies in both numerator and denominator. A pregnancy is considered to be ‘affected’ when at least one co-twin is a DS case.
Relative risks (RR) with 95% confidence intervals (95% CI) were estimated using Poisson regression to represent the ratio of the prevalence of cases (or affected pregnancies) with DS among multiple births relative to the prevalence among singleton births. Poisson regression was used due to the rarity of the events studied and possibility of no events happening within a given time period.29 All RRs were adjusted for country and time period (1990–99 versus 2000–2009). Where stated, RRs were additionally adjusted for maternal age (grouped by 5-year intervals as described above for denominators). Logistic regression was used for analysis of prenatal diagnosis and pregnancy outcomes (2000–2009) based on cases only, and these analyses were adjusted for country and, where stated, for maternal age.
The Concordance proportion, calculated for twins only, was calculated as:
Results
The proportion of older mothers (35 years and over) giving birth to singletons and to multiples increased in the 2000s from the preceding decade in nearly all countries (Figure1). From 1990 to 1999, the total corrected prevalence of DS cases from multiple pregnancies per 10 000 births was 0.40 (95% CI 0.36–0.45), rising to 0.47 (95% CI 0.42–0.53) in 2000–2009 (P > 0.05). Overall (1990–2009) the prevalence of DS cases per 10 000 multiple births was 15.1 (95% CI 14.6–15.9) and prevalence of DS cases per 10 000 singleton births was 20.1 (95% CI 19.9–20.3). The prevalence of DS cases per 10 000 multiple births rose with maternal age up to 44 years but was always lower than the equivalent maternal age-specific DS prevalence among singletons (Table2). This finding was consistent across the two datasets used (Figure2). The relative risk of DS for babies from multiple births relative to those from singleton births, adjusted for maternal age, was 0.58 (95% CI 0.53–0.62). Within the 40–44-year maternal age group, DS prevalence per 10 000 multiple births was significantly lower in the 2000s than in the 1990s (P < 0.01, Table2).
Figure 1.
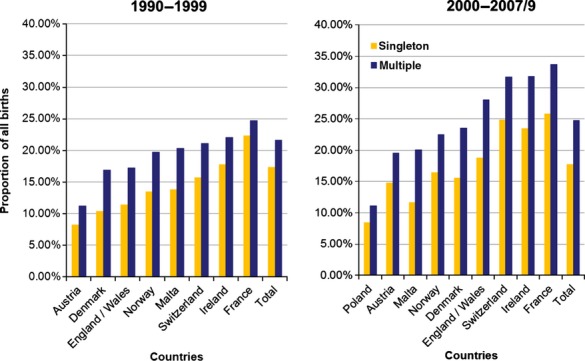
Proportion (%) of mothers aged >35 by multiple/singleton birth status according to time period (1990–99, 2000–2007/9) and country.
Table 2.
Prevalence of DS cases, adjusted to 20 weeks’ gestation, from multiple and singleton births per 10 000 births and relative risk of DS cases from multiple births relative to singleton births for two time periods, 10 EUROCAT registries
| Time | Maternal age group | DS cases from multiple births n | Total prevalence of DS per 10 000 multiple births (95% CI) | DS cases from singleton births n | Total prevalence of DS cases per 10 000 singleton births (95% CI) | RR multiple versus singleton (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|
| 1990–99 | <20 | 0 | 0 | 341 | 6.86 | (6.17–7.62) | 0 | 0 | |
| 20–24 | 10 | 3.45 | (1.85–6.43) | 1119 | 7.35 | (6.93–7.80) | 0.46 | (0.25–0.87) | |
| 24–29 | 35 | 5.39 | (3.95–7.67) | 2231 | 9.08 | (8.71–9.47) | 0.60 | (0.43–0.84) | |
| 30–34 | 82 | 11.9 | (9.55–14.7) | 3189 | 16.0 | (15.5–16.6) | 0.74 | (0.59–0.92) | |
| 35–39 | 116 | 36.9 | (30.8–44.2) | 4061 | 53.7 | (52.0–55.3) | 0.69 | (0.57–0.82) | |
| 40–44 | 59 | 133 | (103–172) | 2517 | 189 | (182–196) | 0.70 | (0.54–0.91) | |
| >44 | 0 | 0 | 229 | 357 | (313–406) | 0 | 0 | ||
| Total | 302 | 14.8 | (13.2–16.6) | 13 686 | 18.5 | (18.2–18.8) | 0.66* | (0.59–0.74) | |
| 2000–2009 | <20 | 3 | 4.61 | (1.49–14.3) | 297 | 6.19 | (5.52–6.93) | 0.74 | (0.24–2.31) |
| 20–24 | 11 | 4.16 | (2.30–7.52) | 905 | 6.60 | (6.19–7.05) | 0.63 | (0.35–1.14) | |
| 24–29 | 33 | 6.20 | (4.40–8.73) | 1600 | 8.14 | (7.75–8.55) | 0.76 | (0.54–1.07) | |
| 30–34 | 68 | 8.68 | (6.84–11.0) | 3398 | 16.2 | (15.6–16.7) | 0.54 | (0.42–0.68) | |
| 35–39 | 173 | 33.6 | (28.9–39.0) | 5655 | 50.1 | (48.8–51.4) | 0.67 | (0.56–0.78) | |
| 40–44 | 59 | 59.1 | (45.7–76.3) | 3768 | 166 | (161–172) | 0.35 | (0.27–0.46) | |
| >44 | 1 | 5.95 | (0.82–43.1) | 333 | 310 | (278–345) | 0.02 | (0.00–0.14 | |
| Total | 347 | 15.3 | (13.8–17.0) | 15 956 | 21.8 | (21.4–22.1) | 0.52* | 0.47–0.58 | |
| Combined 1990–2009 | <20 | 3 | 2.37 | (0.76–7.35) | 638 | 6.52 | (6.04–7.05) | 0.36** | (0.12–1.13) |
| 20–24 | 21 | 3.75 | (2.47–5.82) | 2024 | 7.00 | (6.70–7.31) | 0.54** | (0.35–0.83) | |
| 24–29 | 68 | 5.82 | (4.58–7.39) | 3831 | 8.66 | (8.39–8.94) | 0.67** | (0.52–0.85) | |
| 30–34 | 150 | 10.2 | (8.67–11.9) | 6587 | 16.1 | (15.7–16.5) | 0.63** | (0.54–0.74) | |
| 35–39 | 289 | 34.8 | (31.1–39.1) | 9716 | 51.5 | (50.5–52.6) | 0.68** | (0.60–0.76) | |
| 40–44 | 117*** | 81.9 | (68.4–98.2) | 6284*** | 175 | (170–179) | 0.47** | (0.39–0.56) | |
| >44 | 1 | 4.48 | (0.67–35.1) | 562 | 327 | (301–356) | 0.015** | (0.002–0.107) | |
| Total | 649 | 15.1 | (14.6–15.9) | 29 643*** | 20.1 | (19.9–20.3) | 0.58*** | (0.53–0.62) | |
Adjusted for maternal age.
Adjusted for time.
As all numbers of cases are adjusted to the number expected at 20 weeks’ gestation and have been rounded up to whole numbers, the combined 1990–2009 number is not exactly equal to the addition of 1990–199 and 2000–2009.
Figure 2.
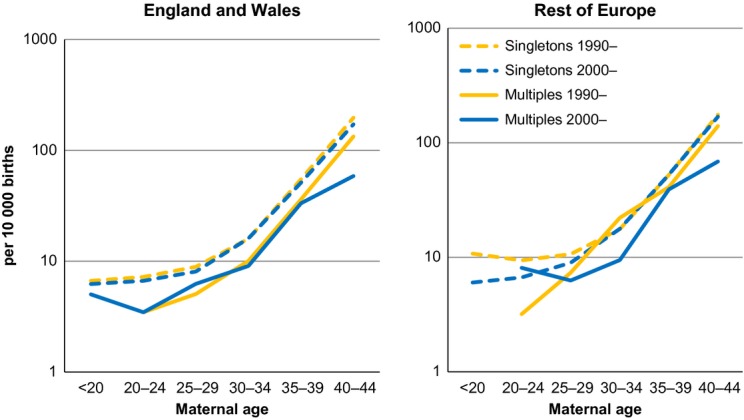
Prevalence of DS per 10 000 singleton births and per 10 000 multiple births, 1990–99 and 2000–2009, by 5 years of maternal age for England and Wales and for the rest of Europe (nine registries) separately.
Of the 19 397 babies born to mothers over 44 years of age, 2043 (10.5%) were from multiple births. In this maternal age group, only one fetus from a multiple pregnancy was a DS case, a prevalence of 4.48 (95% CI 0.67–35.1) per 10 000 multiple births, compared with 562 singleton DS cases: a prevalence of 327 (95% CI 301–356) per 10 000 singleton births (Table2 – RR 0.015 [95% CI 0.002–0.107]). Based on the number of multiple births in the >44 age group and the singleton DS prevalence in this age group, the expected number of DS cases from multiple births would have been 55.
When the NDSCR data were analysed using single year of maternal age for mothers 35 years and over, the reduced risk in DS cases from multiple births was more apparent and further reduced in the 2000s compared with the 1990s (Figure3; also see Table S1). There was also a slight decrease in DS prevalence among singleton births over the two time periods.
Figure 3.
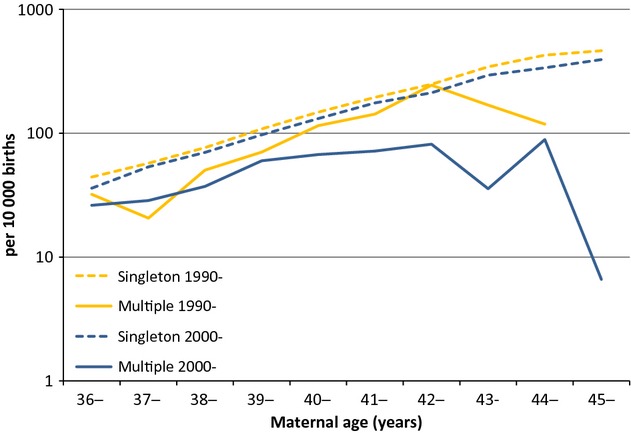
Prevalence of DS per per 10 000 singleton births and per 10 000 multiple births, 1990–99 and 2000–2009, by single year of maternal age, England and Wales.
In 8.7% (n = 54) of affected pairs the co-twins were concordant for DS, 51 same sex twin pairs (assumed to be monozygotic) and three unlike sex twin pairs. When analyses were carried out for monozygotic and dizygotic pregnancies separately, the maternal age-adjusted relative risk of a monozygotic pregnancy being affected was 0.34 (95% CI 0.25–0.44) compared with singleton pregnancies (Table3). There were no affected monozygotic twin pregnancies in the >44-year maternal age group and the relative risk did not vary significantly by maternal age for mothers under 45 (P > 0.05; Table3). For dizygotic pregnancies, the maternal age adjusted relative risk of at least one co-twin being affected was 1.34 (95% CI 1.23–1.46) compared with singleton pregnancies. The relative risk dropped after maternal age 44 to RR 0.04 (95% CI 0.01–0.27). There was no statistically significant variation in relative risk between maternal ages 20 and 44 (Table3; P > 0.05). The adjusted relative risk excluding the over 44-year maternal age group is unchanged compared with the all ages estimate.
Table 3.
Number and prevalence of pairs affected by DS for monozygotic and dizygotic pregnancies, corrected to 20 weeks’ gestation, and risk relative to singleton pregnancies, by maternal age, 10 EUROCAT registries
| Maternal age groups | Twin pregnancies with two same sex DS cases: assumed to be monozygotic n | Affected (MZ) pregnancies per 1000 monozygotic pregnancies (95% CI) | RR affected MZ relative to affected singleton pregnancies (95% CI) | Twin Pregnancies with at least one DS case assumed to be dizygotic n | Affected DZ pregnancies per 1000 dizygotic pregnancies (95% CI) | RR affected DZ relative to affected singleton pregnancies (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| <20 | 1 | 2.9 | (0.41–20.6) | 0.44 | (0.06–3.16) | 1 | 3.52 | (0.50–25.0) | 0.54 | (0.08–8.35) |
| 20–24 | 4 | 3.24 | (1.21–8.63) | 0.46 | (0.17–1.24) | 12 | 7.94 | (4.51–14.0) | 1.14 | (0.65–2.02) |
| 24–29 | 6 | 2.74 | (1.23–6.12) | 0.32 | (0.14–0.71) | 56 | 15.4 | (11.9–20.1) | 1.81 | (1.39–2.36) |
| 30–34 | 8 | 3.71 | (1.81–7.58) | 0.23 | (0.11–0.48) | 127 | 24.4 | (20.5–28.9) | 1.53 | (1.28–1.82) |
| 35–39 | 23* | 24.5 | (16.3–36.8) | 0.47 | (0.31–0.71) | 238 | 76.6 | (67.4–86.9) | 1.49 | (1.31–1.69) |
| 40–44 | 9* | 42.5 | (21.9–82.1) | 0.24 | (0.13–0.47) | 97 | 199 | (163–243) | 1.15 | (0.94–1.40) |
| >44 | 0 | 0 | (0.41–20.6) | 1 | 12.0 | (1.69–85.0) | 0.04 | (0.01–0.27) | ||
| Total | 51 | 7.34 | (5.47–9.65) | 0.34** | (0.25–0.44) | 532 | 37.2 | (34.1–40.4) | 1.34** | (1.23–1.46) |
Three concordant pregnancies where both co-twins were DS cases, but of different sexes, were found, one in the 35–39-year and two in the 40–44-year maternal age group. These pregnancies were assumed to be dizygotic.
Adjusted for maternal age.
The proportion of DS cases which were prenatally diagnosed rose with maternal age for cases from both singleton and multiple pregnancies (Table4).The proportion of DS cases which were prenatally diagnosed was lower for multiple than for singleton pregnancies at all maternal ages (Table4), giving an overall maternal age adjusted OR of 0.62 (95% CI 0.50–0.78; Table4).
Table 4.
Number and proportion of DS cases by pregnancy outcome, maternal age group, and multiple birth staus, 2000–2009; number and proportion of prenatally diagnosed cases and number and proportion of TOPFA, by maternal age group and multiple birth status: 10 EUROCAT registries, 2000–2009
| <20 | 20–24 | 25–29 | 30–34 | 35–39 | >39 | Total | |||
|---|---|---|---|---|---|---|---|---|---|
| Livebirths | Multiple | n (%) [% excluding TOPFA] | 3 (100) [100] | 8 (80.0) [100] | 26 (68.4) [96.3] | 38 (54.3) [86.4] | 110 (58.8) [96.5] | 33 (54.1) [91.7] | 218 (59.1) [94.0] |
| Singleton | n (%) [% excluding TOPFA] | 230 (74.9) [93.9] | 660 (68.6) [95.1] | 1064 (60.2) [95.3] | 1838 (5.1) [94.9] | 2208 (37.8) [93.6] | 1387 (32.9) [92.7] | 7387 (63.3) [94.1] | |
| Stillbirths/FD 20 weeks+ | Multiple | n (%) [% excluding TOPFA] | 0 | 0 | 1 (2.70) [3.70] | 6 (8.57) [13.6] | 4 (2.14) [3.50] | 3 (4.91) [8.30] | 14 (3.79) [6.00] |
| Singleton | n (%) [% excluding TOPFA] | 15 (4.89) [6.10] | 34 (3.53) [4.91] | 52 (2.96) [4.82] | 99 (2.75) [5.13] | 150 (2.57) [6.41] | 109 (2.59) [7.28] | 459 (3.93) [5.09] | |
| Odds (95% CI) of stillbirths/FD in cases from multiple relative singleton births (excluding TOPFA) | 0.86 (0.11–6.51) | 2.59 (1.06–6.31) | 0.58 (0.20–1.55) | 1.08 (0.32–3.60) | 1.03* (0.59–1.78) | ||||
| TOPFA | Multiple | n (%) | 0 | 2 (20.0) | 11 (28.9) | 26 (37.1) | 73 (44.4) | 25 (41.0) | 137 (37.1) |
| Singleton | n (%) | 62 (20.2) | 268 (27.9) | 642 (36.5) | 1657 (46.1) | 3488 (59.7) | 2713 (64.5) | 8830 (52.9) | |
| Odds (95% CI) of TOPFA in cases from multiple relative to singleton pregnancies | 0.86 (0.17–4.48) | 0.74 (0.35–1.54) | 0.59 (0.36–0.97) | 0.42 (0.30–0.57) | 0.41 (0.24–0.70) | 0.52* (0.41–0.65) | |||
| Prenatally diagnosed* | Multiple | n (%) | 0 | 2 (20.0) | 13 (34.2) | 32 (45.7) | 89 (47.6) | 30 (49.2) | 166 (45.0) |
| Singleton | n (%) | 88 (28.7) | 317 (33.0) | 688 (39.1) | 1763 (49.1) | 3612 (61.8) | 2813 (67.3) | 9281 (55.6) | |
| Odds (95% CI) of prenatal diagnosis in cases from multiple relative to singleton pregnancies | 0.63 (0.12–3.22) | 0.85 (0.40–1.78) | 0.71 (0.44–1.17) | 0.56 (0.32– 0.98 | 0.62* (0.50–0.78) | ||||
| TOPFA following prenatal diagnosis | Multiple | n (%) | 0 | 2 (100) | 11 (84.6) | 25 (78.1) | 72 (80.9) | 25 (83.4) | 135 (81.3) |
| Singleton | n (%) | 60 (68.2) | 256 (80.8) | 607 (88.2) | 1606 (91.1) | 3377 (93.5) | 2413 (92.8) | 8510 (91.7) | |
| Odds (95% CI) of TOPFA following prenatal diagnosis in cases from multiple relative to singleton pregnancies | 0.73 (0.16–3.37) | 0.35 (0.15–0.82) | 0.30 (0.17–0.51) | 0.47 (0.16 –1.37) | 0.40* (0.27–0.59) |
Adjusted for maternal age.
A prenatally diagnosed DS case from a multiple pregnancy was less likely to result in TOPFA following prenatal diagnosis than was a prenatally diagnosed DS case from a singleton pregnancy: maternal age adjusted OR 0.40 (95% CI 0.26–0.60; Table4). The overall proportion of cases from multiple pregnancies which were TOPFAs was lower than singletons at every maternal age, giving an overall maternal age adjusted OR of 0.52 (95% CI 0.41–0.65; Table4).
Overall, DS cases from multiple births were not more likely to be stillbirths/FD than from singleton births (after excluding TOPFAs): maternal age adjusted OR 1.03 (95% CI 0.59–1.78; Table4). In the NDSCR data there were 204 fetuses from singleton and eight from multiple pregnancies who were prenatally diagnosed but miscarried naturally before 20 weeks’ gestation and so were not included as cases in the main analyses. Of those eight from multiple pregnancies, two had an affected co-twin who became TOPFA and six had an unaffected co-twin. Overall, 3.8% of prenatally diagnosed fetuses who miscarried before 20 weeks were from multiple pregnancies.
Discussion
Main findings
Our results showing that fetus/babies from multiple pregnancies had 58% of the risk of DS than those from singleton pregnancies, after accounting for maternal age, strongly support the reports of a lower prevalence of DS in multiple relative to singleton births in several early studies.6–8 Several more recent studies10,11 found a similar risk in multiple births and singletons, but this may because their data only included livebirths, and a higher proportion of their singleton cases than their cases from multiple pregnancies may have proceeded to TOPFA and so been excluded from their studies, thus reducing the difference in risk. The other study finding a similar risk was very small.12 Fetus/baby-specific and pregnancy-specific risk estimates should be distinguished. In a dizygotic pregnancy, each co-twin has an individual risk of being a DS case and so the pregnancy-specific risk is almost twice the fetus/baby-specific risk. We estimated that dizygotic pregnancies are one-third more likely to have at least one DS case than their singleton counterparts after accounting for maternal age, much lower than the expected doubling of singleton risk. For a monozygotic pregnancy, the pregnancy-specific and fetus-specific risks are the same, but the former involves two affected fetuses rather than one. We found the pregnancy-specific risk of DS for monozygotic pregnancies to be one-third of the singleton risk.
With our large study population, we were also able to establish that the lower than expected number of DS cases is observed at all maternal ages up to 44. Over the age of 44 it becomes very rare for a mother of a multiple birth to have a baby diagnosed with Down syndrome.
We found that the lower than expected prevalence of DS related particularly to monozygotic twins, and this is supported by the low proportion of concordant pairs: 8.7% rather than the approximately 27% which would be expected if the ratio of monozygotic to dizygotic pairs was the same for DS cases from multiple pregnancies as the ratio in the birth population.
Prenatal diagnosis and TOPFA/selective feticides were less common for DS in multiple than singleton pregnancies.
Interpretation
A partial explanation for the very low risk of DS to mothers over 44 may be that these mothers are the most likely to have become pregnant through ART using either their own frozen or donor eggs, created at an earlier age than their age at the index pregnancy, giving them a risk which is lower than their age would suggest.30,31 Our risk estimates for women over 44 may therefore be underestimates for those naturally conceiving. It is likely that this also explains the increasing difference over time in DS risk between singletons and multiples for mothers in the 40–44-year age group. An obvious implication is that prenatal screening should make reference to the age of the mother/donor at the time the egg was harvested, rather than the mother’s age at the time of the current pregnancy. Use of a previously frozen egg/embryo would be more frequent with single embryo transfer policies, making this issue also pertinent to singleton pregnancies. Currently, preimplantation diagnosis and selection is rare, but in future this may also lower the prevalence of DS in multiple births from ART. As embryos with chromosomal anomalies are believed to be more fragile than their counterparts without chromosomal anomalies,32,33 one can also speculate that embryos without chromosomal anomalies tend to be chosen for implantation, even when no formal preimplantation diagnosis is performed, as they appear more robust during the ART process. ART (e.g. via ovarian stimulation) has been suggested as a possible risk factor for DS, but a recent study has not supported this34 and although we could not directly test this without data on the use of ART, there is nothing in our results to support this.
However, ART is not an explanation for the lower DS risk in multiples across all maternal ages, and the lower DS risk in monozygotic than dizygotic pregnancies. The most likely explanation is early fetal loss of DS in multiple pregnancies, particularly in pregnancies concordant for DS. Multiple pregnancy could be seen as co-morbidity, which makes it less likely for the DS-affected fetuses to survive to diagnosis. We postulate that at all maternal ages, but especially for those aged over 44 years, either the affected embryos/fetuses are too fragile to survive or one co-twin has been lost in very early pregnancy so that the remaining affected fetus is perceived to be a singleton. The literature speculates on a tendency for the unaffected twin of a dizygotic pair to be able to improve the survival chances of the affected twin35 This possibility is not borne out by our data, as the prevalence of DS cases from dizygotic multiple pregnancies is lower than that observed in singletons. The fragility of DS embryos/fetuses and vulnerability to stressors is supported by literature implicating low folate levels36 coffee and alcohol consumption and perhaps smoking with very early loss of the DS embryo. In the context of multiple pregnancy as a stressor,37,38 the higher survival of DS in dizygotic than monozygotic pairs may reflect the increased stress involved in carrying two DS cases in one pregnancy, combined with the increased delicacy of monochorionic relative to dichorionic pairs39 When one of a monozygotic concordant pair dies, the other is less likely to survive, as a result of their shared placenta.
The lower rates of prenatal diagnosis and TOPFA/selective feticides for DS in multiple than singleton pregnancies reflect in part the increased difficulty of the screening and diagnostic procedures. In addition to the technical difficulties involved in the procedures, prenatal diagnosis can present professionals and families with very difficult decisions, particularly in discordant pairs where there is an unaffected co-twin whose welfare must also be factored into decisions regarding selective feticide40 Although it is reported that women who have achieved pregnancy through ART are less likely to consent to invasive procedures,13 there is also anecdotal evidence that the decision to have a selective feticide is easier rather than harder for those women who have accessed ART procedures and have already had to make potentially difficult decisions about their fertility41 Where selective feticide is chosen, survival of the unaffected twin increases when the procedure is carried out in the first trimester of pregnancy,2 suggesting that reliable screening should be carried out as early as possible.
Strengths and limitations
The strengths of this study are the very large population size using standard definitions, with the ability thereby to stratify by maternal age and replicate results across two different types of registry. The weaknesses are that zygosity/chorionicity is not known and can only be inferred, and that ART is not known for the majority of cases and therefore could not be analysed. Therefore the zygosity-specific results must be treated as estimates. We considered same sex concordant pairs as monozygotic, but the three observed dizygotic different sex concordant DS pairs suggest that approximately three of the same sex concordant pairs might also be dizygotic. If we had reallocated these pairs, the adjusted risk of at least one co-twin in a dizygotic pair being affected relative to a singleton would have been essentially unchanged but the adjusted risk of both co-twins in a monozygotic pair relative to a singleton being affected would have been slightly smaller.
Risk estimates have been corrected to 20 weeks’ gestational age, which would be lower than the prevalence/risks in the first trimester of pregnancy, when DS is often diagnosed and decisions regarding TOPFA/selective feticide are made.
Conclusions
Individual fetuses from multiple pregnancies, whether monozygotic or dizygotic, are at less risk of DS than those from singleton pregnancies. The overall risk of a dizygotic pregnancy being affected by DS (one or both fetuses) is about a third higher than a singleton pregnancy at all maternal ages up to 44. The risk of DS in monozygotic multiple pregnancies is approximately one-third of the risk in singleton pregnancies for a mother of similar age although both fetuses will be affected. Twin pregnancies in mothers over 44 have a very low risk of DS.
Our estimates of the lower maternal age-specific DS risk in multiple pregnancies, when combined with the clinician’s knowledge of zygosity/chorionicity and maternal age at ovulation in the case of ART for individual women, should allow more accurate risk estimates to be used in genetic counselling and prenatal screening.
Disclosure of interest
The authors declare they have no conflict of interests.
Contribution to authorship
BB,JKM,HD defined the research question, designed the study and interpreted the analysis. BB and HD co-wrote the paper. BB also prepared and analysed the data. RMcC, EG and ML advised on the conduct of the study and interpretation of the results. EG, MCA, MG, MH, ALB, NL RMcD, CM and MO’M provided the data. All authors commented on drafts.
Details of ethics approval
EUROCAT has approval from the University of Ulster Research Ethics Committee. This study is part of a PhD project additionally approved by The University of Ulster School of Nursing Research Ethics Filter Committee on 31 October 2011. In addition, all registries have ethical approval appropriate to their national and local ethics guidelines.
Funding
BB was funded by a Northern Ireland Research and Development studentship from the Northern Ireland Public Health Agency. EUROCAT is co-funded by the EC, under the framework of the EU Health Programme 2008–2013, Grant Agreement 2010 22 04 (Executive Agency for Health & Consumers).
EUROCAT registries are funded as fully described in Paper 6 of EUROCAT Report 9 – ‘EUROCAT Member Registries: Organization and Activities’. The responsibility for the interpretation of data and/or information supplied is the authors’ alone.
Acknowledgments
We thank the many people throughout Europe involved in providing and processing information, including affected families, clinicians, health professionals, medical record clerks, and registry staff. We also thank Dr Inez Cooke, Queen’s University Belfast, for advice on assisted reproductive technologies.
Mini commentary on ‘Prevalence and risk of Down Syndrome in monozygotic and dizygotic multiple pregnancies in Europe: implications for prenatal screening’
Twin gestations now make up approximately 3% of births in the USA, and a comparable number in other developed countries. Given that the highest rate of increase has been seen in older mothers, accurate data regarding the risk of aneuploidy, the effectiveness of prenatal aneuploidy screening, and the risks of invasive diagnostic procedures are increasingly important.
Decisions regarding prenatal genetic testing in twins are complex for several reasons. The risk of aneuploidy is increased, based on the greater average maternal age, and is assumed to be nearly double for the pregnancy due to the risk for two fetuses. The background risk of pregnancy loss is higher in multiple gestations. Serum aneuploidy screening is less accurate in twins (Garchet-Beaudron A et al. Prenat Diagn 2008;28:1105–9), data on use of noninvasive prenatal testing remains limited (Canick JA et al. Prenat Diagn 2012;32:730–4), and the risk of procedure-related miscarriage following invasive diagnostic testing is not well documented (Agarwal et al. Ultrasound Obstet Gynecol 2012;40:128–34).
For these reasons, the data that we use to counsel patients regarding prenatal aneuploidy testing in singletons do not apply to twins. Key to prenatal genetic counseling is accurate knowledge of a priori risk of an affected fetus. This is important for women deciding about testing and also a component of multiple-marker screening risk algorithms. Although there is substantial data supporting the maternal age-based risks for chromosome abnormalities in singletons, there are limited data for twins. Many centres quote a ‘per fetus’ risk, assuming each twin has a risk comparable to a singleton at the same maternal age. Some centres use a formula that accounts for a percentage of monozygotic twins, but most quote risks based on such modeling rather than accumulated data.
Interestingly, some small studies have suggested that the risk of aneuploidy in twins is significantly lower than in singletons (Jamar M et al. Genet Couns 2003;14:395–400). The report in this edition of BJOG confirms these findings in a large population-based study. They found that the risk of Down syndrome in monozygotic twins was about one third of that in singletons, whereas the risk in dizygotic twins was about one third higher than in singletons – increased, but substantially less than the doubling of risk that is often used in counseling of pregnant patients (Boyle et al. BJOG).
Although not intuitive, these findings are certainly biologically plausible. The authors note that fetal loss can occur due to multiple ‘hits’, and that monochorionic placentation, older maternal age, and twinning all increase the risk of pregnancy loss, as does aneuploidy. In pregnancies in which multiple risk factors are present, the chance of a Down syndrome pregnancy surviving is decreased. This paper provides not just important data on aneuploidy risks in twin gestations but is also humbling as regards assumptions that we make that seem entirely reasonable but that are just that – opinions that may or may not be ultimately supported by data. Here, at last, are data useful in counseling our patients carrying twin gestations.
Disclosure of interests
I have no disclosures.
ME NortonDivision of Maternal-Fetal Medicine, Department of Obstetrics, Gynecology and Reproductive Sciences, University of California, San Francisco, San Francisco, CA, USA
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Prevalence of Down syndrome cases per 10 000 multiple and singleton births (95% confidence intervals) over time in England and Wales [Data displayed in Figure].
References
- 1.Cuckle H. Down’s syndrome screening in twins. J Med Screen. 1998;5:3–4. doi: 10.1136/jms.5.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Sebire NJ. Anomalous development in twins (including monozygotic duplication) In: Kilby MD, Baker P, Critchley H, Field D, editors. Multiple Pregnancy. 1st edn. London: RCOG; 2006. pp. 55–83. [Google Scholar]
- 3.Madsen HN, Ball S, Wright D, Torring N, Petersen OB, Nicolaides KH, et al. A reassessment of biochemical marker distributions in trisomy 21-affected and unaffected twin pregnancies in the first trimester. Ultrasound Obstet Gynecol. 2011;37:38–47. doi: 10.1002/uog.8845. [DOI] [PubMed] [Google Scholar]
- 4.Cahill AG, Macones GA, Stamilio DM, Dicke JM, Crane JP, Odibo AO. Pregnancy loss rate after mid-trimester amniocentesis in twin pregnancies. Am J Obstet Gynecol. 2009;200:257.e1–e6. doi: 10.1016/j.ajog.2008.09.872. [DOI] [PubMed] [Google Scholar]
- 5.Vink J, Fuchs K, D’Alton ME. Amniocentesis in twin pregnancies: a systematic review of the literature. Prenat Diagn. 2012;32:409–16. doi: 10.1002/pd.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay S, Wehrung DA. Congenital malformations in twins. Am J Hum Genet. 1970;22:662–78. [PMC free article] [PubMed] [Google Scholar]
- 7.Kallen B. Congenital malformations in twins: a population study. Acta Genet Med Gemellol (Roma) 1986;35:167–78. doi: 10.1017/s0001566000005687. [DOI] [PubMed] [Google Scholar]
- 8.Doyle PE, Beral V, Botting B, Wale CJ. Congenital malformations in twins in England and Wales. J Epidemiol Community Health. 1991;45:43–8. doi: 10.1136/jech.45.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamar M, Lemarchal C, Lemaire V, Koulischer L, Bours V. A low rate of trisomy 21 in twin-pregnancies: a cytogenetics retrospective study of 278 cases. Genet Couns. 2003;14:395–400. [PubMed] [Google Scholar]
- 10.Li SJ, Ford N, Meister K, Bodurtha J. Increased risk of birth defects among children from multiple births. Birth Defects Res A Clin Mol Teratol. 2003;67:879–85. doi: 10.1002/bdra.10093. [DOI] [PubMed] [Google Scholar]
- 11.Tang Y, Ma CX, Cui W, Chang V, Ariet M, Morse SB, et al. The risk of birth defects in multiple births: a population-based study. Matern Child Health J. 2006;10:75–81. doi: 10.1007/s10995-005-0031-5. [DOI] [PubMed] [Google Scholar]
- 12.Glinianaia SV, Rankin J, Wright C. Congenital anomalies in twins: a register-based study. Hum Reprod. 2008;23:1306–11. doi: 10.1093/humrep/den104. [DOI] [PubMed] [Google Scholar]
- 13.Gonce A, Borrell A, Fortuny A, Casals E, Martinez MA, Mercade I, et al. First-trimester screening for trisomy 21 in twin pregnancy: does the addition of biochemistry make an improvement? Prenat Diagn. 2005;25:1156–61. doi: 10.1002/pd.1304. [DOI] [PubMed] [Google Scholar]
- 14.Vandecruys H, Faiola S, Auer M, Sebire N, Nicolaides KH. Screening for trisomy 21 in monochorionic twins by measurement of fetal nuchal translucency thickness. Ultrasound Obstet Gynecol. 2005;25:551–3. doi: 10.1002/uog.1897. [DOI] [PubMed] [Google Scholar]
- 15.Wald NJ, Rish S. Prenatal screening for Down syndrome and neural tube defects in twin pregnancies. Prenat Diagn. 2005;25:740–5. doi: 10.1002/pd.1258. [DOI] [PubMed] [Google Scholar]
- 16.National Collaborating Centre for Women’s and Children’s health. 2011. pp. 81–8. Multiple pregnancy the management of twin and triplet pregnancies in the antinatal period. NICE (draft):
- 17.Vayssiere C, Benoist G, Blondel B, Deruelle P, Favre R, Gallot D, et al. Twin pregnancies: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF) Eur J Obstet Gynecol Reprod Biol. 2011;156:12–7. doi: 10.1016/j.ejogrb.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 18.Morris JK, Alberman E. Trends in Down’s syndrome live births and antenatal diagnoses in England and Wales from 1989 to 2008: analysis of data from the National Down syndrome Cytogenetic Register. BMJ. 2009;339:b3794. doi: 10.1136/bmj.b3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loane M, Morris J, Addor M, Arriola L, Budd J, Doray B, et al. Twenty-year trends in the prevalence of Down syndrome and other trisomies in Europe: impact of maternal age and prenatal screening. Eur J Hum Genet. 2013;21:27–33. doi: 10.1038/ejhg.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle B, McConkey R, Garne E, Loane M, Addor MC, Bakker MK, et al. Trends in the prevalence, risk and pregnancy outcome of multiple births with congenital anomaly: a registry-based study in 14 European countries 1984-2007. BJOG. 2013;120:707–16. doi: 10.1111/1471-0528.12146. [DOI] [PubMed] [Google Scholar]
- 21.Black M, Bhattacharya S. Epidemiology of multiple pregnancy and the effect of assisted conception. Semin Fetal Neonatal Med. 2010;15:306–12. doi: 10.1016/j.siny.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Kurinczuk J. Epidemiology of multiple pregnancy: changing effects of assisted conception. In: Kilby M, Baker P, Critchley H, Field D, editors. Multiple Pregnancy. 1st edn. London: RCOG; 2006. pp. 1–26. [Google Scholar]
- 23.Boyd PA, Haeusler M, Barisic I, Loane M, Garne E, Dolk H. Paper 1: The EUROCAT network–organization and processes. Birth Defects Res A Clin Mol Teratol. 2011;91(Suppl 1):S2–15. doi: 10.1002/bdra.20780. [DOI] [PubMed] [Google Scholar]
- 24.EUROCAT. 2005. EUROCAT Guide 1.3 and reference documents, instruction for the registration and surveillance of congenital. [anomalieshtt:// www.eurocatnetwork.eu/ABOUTUS/DataCollection/GuidelinesforRegistration/Guide1_3InstructionManualAvailable at: PDF. Accessed 11 July 2011.
- 25.Greenlees R, Neville A, Addor MC, Amar E, Arriola L, Bakker M, et al. Paper 6: EUROCAT member registries: organization and activities. Birth Defects Res A Clin Mol Teratol. 2011;91(Suppl 1):S51–100. doi: 10.1002/bdra.20775. [DOI] [PubMed] [Google Scholar]
- 26.Loane M, Dolk H, Garne E, Greenlees R EUROCAT Working Group. Paper 3: EUROCAT data quality indicators for population-based registries of congenital anomalies. Birth Defects Res A Clin Mol Teratol. 2011;91(Suppl 1):S23–30. doi: 10.1002/bdra.20779. [DOI] [PubMed] [Google Scholar]
- 27.Husby H, Holm NV, Gernow A, Thomsen SG, Kock K, Gurtler H. Zygosity, placental membranes and Weinberg’s rule in a Danish consecutive twin series. Acta Genet Med Gemellol (Roma) 1991;40:147–52. doi: 10.1017/s0001566000002579. [DOI] [PubMed] [Google Scholar]
- 28.Vlietinck R, Derom C, Derom R, Van den Berghe H, Thiery M. The validity of Weinberg’s rule in the East Flanders Prospective Twin Survey (EFPTS) Acta Genet Med Gemellol (Roma) 1988;37:137–41. doi: 10.1017/s0001566000004049. [DOI] [PubMed] [Google Scholar]
- 29.Kirkwood BA, Stern JAC. Essential Medical Statistics. 2nd edn. Oxford: Blackwell Science; 2003. [Google Scholar]
- 30.Forman EJ, Treff NR, Scott RT., Jr Fertility after age 45: from natural conception to Assisted Reproductive Technology and beyond. Maturitas. 2011;70:216–21. doi: 10.1016/j.maturitas.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Kurinczuk J, Hockley C. 2010. Fertility Treatment in 2006: a statistical analysis.
- 32.Stein Z, Susser M, Warburton D, Wittes J, Kline J. Spontaneous abortion as a screening device. The effect of fetal survival on the incidence of birth defects. Am J Epidemiol. 1975;102:275–90. doi: 10.1093/oxfordjournals.aje.a112163. [DOI] [PubMed] [Google Scholar]
- 33.Stein Z, Stein W, Susser M. Attrition of trisomies as a maternal screening device. An explanation of the association of trisomy 21 with maternal age. Lancet. 1986;1:944–7. doi: 10.1016/s0140-6736(86)91046-9. [DOI] [PubMed] [Google Scholar]
- 34.Massie JA, Shahine LK, Milki AA, Westphal LM, Lathi RB. Ovarian stimulation and the risk of aneuploid conceptions. Fertil Steril. 2011;95:970–2. doi: 10.1016/j.fertnstert.2010.07.1088. [DOI] [PubMed] [Google Scholar]
- 35.Hall JG. Twinning. Lancet. 2003;362:735–43. doi: 10.1016/S0140-6736(03)14237-7. [DOI] [PubMed] [Google Scholar]
- 36.James SJ, Pogribna M, Pogribny IP, Melnyk S, Hine RJ, Gibson JB, et al. Abnormal folate metabolism and mutation in the methylenetetrahydrofolate reductase gene may be maternal risk factors for Down syndrome. Am J Clin Nutr. 1999;70:495–501. doi: 10.1093/ajcn/70.4.495. [DOI] [PubMed] [Google Scholar]
- 37.Torfs CP, Christianson RE. Maternal risk factors and major associated defects in infants with Down syndrome. Epidemiology. 1999;10:264–70. [PubMed] [Google Scholar]
- 38.Torfs CP, Christianson RE. Effect of maternal smoking and coffee consumption on the risk of having a recognized Down syndrome pregnancy. Am J Epidemiol. 2000;152:1185–91. doi: 10.1093/aje/152.12.1185. [DOI] [PubMed] [Google Scholar]
- 39.Verp V. Pregnancy loss: Multiple pregnancy versus multiple birth. In: Blickstein I, Keith LG, editors. Multiple Pregnancy: Epidemiology, Gestation and Perinatal Outcome. Andover: Taylor and Francis; 2005. pp. 252–4. [Google Scholar]
- 40.Bryan E. Psychological aspects of prenatal diagnosis and its implications in multiple pregnancies. Prenat Diagn. 2005;25:827–34. doi: 10.1002/pd.1270. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert K. 2011. IVF Mom confesses: I would not have aborted my twin if I had conceived naturally [ http://www.lifesitenews.com/news/ivf-mom-confesses-aborting-twin-just-another-choice-in-consumerish-process/Accessed 22 June 2012.


