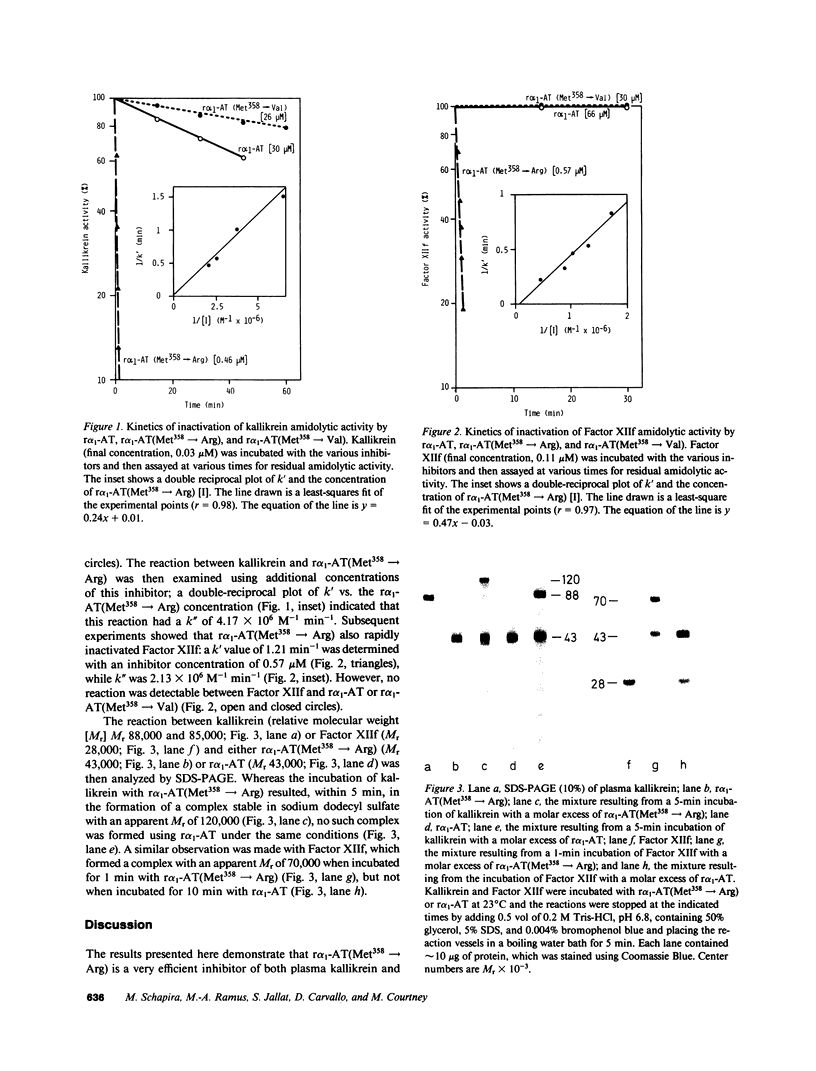
Abstract
In normal plasma, the serine protease inhibitor alpha 1-antitrypsin (alpha 1-AT) plays little or no role in the control of plasma kallikrein or activated Factor XII fragment (Factor XIIf), this function being performed by Cl-inhibitor. Recently, an alpha 1-AT variant was described with a Met----Arg mutation at the reactive center P1 residue (position 358) which altered the specificity of inhibition from the Met- or Val-specific protease neutrophil elastase to thrombin, an Arg-specific protease. We have now examined the inhibition of plasma kallikrein and Factor XIIf, both Arg-specific enzymes, with recombinant alpha 1-AT(Met358----Arg) produced by an Escherichia coli strain carrying a mutated human alpha 1-AT gene. The engineered protein was a very efficient inhibitor of both enzymes. It was more effective than Cl-inhibitor by a factor of 4.1 for kallikrein and 11.5 for Factor XIIf. These results suggest that recombinant alpha 1-AT(Met358----Arg) has therapeutic potential for disease states where activation of the plasma kinin-forming system is observed, for example in hereditary angioedema or septic shock.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Courtney M., Buchwalder A., Tessier L. H., Jaye M., Benavente A., Balland A., Kohli V., Lathe R., Tolstoshev P., Lecocq J. P. High-level production of biologically active human alpha 1-antitrypsin in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(3):669–673. doi: 10.1073/pnas.81.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M., Jallat S., Tessier L. H., Benavente A., Crystal R. G., Lecocq J. P. Synthesis in E. coli of alpha 1-antitrypsin variants of therapeutic potential for emphysema and thrombosis. Nature. 1985 Jan 10;313(5998):149–151. doi: 10.1038/313149a0. [DOI] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. H., Iammarino R. M., Spero J. A., Hasiba U. Antithrombin Pittsburgh: an alpha1-antitrypsin variant causing hemorrhagic disease. Blood. 1978 Jan;51(1):129–137. [PubMed] [Google Scholar]
- Mason J. W., Kleeberg U., Dolan P., Colman R. W. Plasma kallikrein and Hageman factor in Gram-negative bacteremia. Ann Intern Med. 1970 Oct;73(4):545–551. doi: 10.7326/0003-4819-73-4-545. [DOI] [PubMed] [Google Scholar]
- Nagase H., Barrett A. J. Human plasma kallikrein. A rapid purification method with high yield. Biochem J. 1981 Jan 1;193(1):187–192. doi: 10.1042/bj1930187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. C., Brennan S. O., Lewis J. H., Carrell R. W. Mutation of antitrypsin to antithrombin. alpha 1-antitrypsin Pittsburgh (358 Met leads to Arg), a fatal bleeding disorder. N Engl J Med. 1983 Sep 22;309(12):694–698. doi: 10.1056/NEJM198309223091203. [DOI] [PubMed] [Google Scholar]
- Purdon A. D., Schapira M., De Agostini A., Colman R. W. Plasma kallikrein and prorenin in patients with hereditary angioedema. J Lab Clin Med. 1985 Jun;105(6):694–699. [PubMed] [Google Scholar]
- Schapira M., Gardaz J. P., Py P., Lew P. D., Perrin L. H., Suter P. M. Prekallikrein activation in the adult respiratory distress syndrome. Bull Eur Physiopathol Respir. 1985 May-Jun;21(3):237–241. [PubMed] [Google Scholar]
- Schapira M., Scott C. F., Colman R. W. Contribution of plasma protease inhibitors to the inactivation of kallikrein in plasma. J Clin Invest. 1982 Feb;69(2):462–468. doi: 10.1172/JCI110470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M., Scott C. F., Colman R. W. Protection of human plasma kallikrein from inactivation by C1 inhibitor and other protease inhibitors. The role of high molecular weight kininogen. Biochemistry. 1981 May 12;20(10):2738–2743. doi: 10.1021/bi00513a006. [DOI] [PubMed] [Google Scholar]
- Schapira M., Scott C. F., James A., Silver L. D., Kueppers F., James H. L., Colman R. W. High molecular weight kininogen or its light chain protects human plasma kallikrein from inactivation by plasma protease inhibitors. Biochemistry. 1982 Feb 2;21(3):567–572. doi: 10.1021/bi00532a024. [DOI] [PubMed] [Google Scholar]
- Schapira M., Silver L. D., Scott C. F., Colman R. W. New and rapid functional assay for C1 inhibitor in human plasma. Blood. 1982 Apr;59(4):719–724. [PubMed] [Google Scholar]
- Schapira M., Silver L. D., Scott C. F., Schmaier A. H., Prograis L. J., Jr, Curd J. G., Colman R. W. Prekallikrein activation and high-molecular-weight kininogen consumption in hereditary angioedema. N Engl J Med. 1983 May 5;308(18):1050–1053. doi: 10.1056/NEJM198305053081802. [DOI] [PubMed] [Google Scholar]
- de Agostini A., Lijnen H. R., Pixley R. A., Colman R. W., Schapira M. Inactivation of factor XII active fragment in normal plasma. Predominant role of C-1-inhibitor. J Clin Invest. 1984 Jun;73(6):1542–1549. doi: 10.1172/JCI111360. [DOI] [PMC free article] [PubMed] [Google Scholar]