Abstract
Cardiovascular diseases are the leading cause of death among adults in developed countries. An increase in prevalent cardiovascular risk factors (e.g., obesity, hypertension and diabetes) has led to a concerted effort to raise awareness of the need to use evidence-based strategies to help patients at risk of developing cardiovascular disease and to reduce their likelihood of suffering a stroke. Sleep apnea has emerged as an important risk factor for the development of cardiovascular disease. Epidemiologic and clinical evidence has prompted the American Heart Association to issue a scientific statement describing the need to recognize sleep apnea as an important target for therapy in reducing cardiovascular disease risks. This article examines evidence supporting associations of sleep apnea with cardiovascular disease and considers evidence suggesting cardiovascular risk reductions through sleep apnea treatment. Perspectives on emerging therapeutic approaches and promising areas of clinical and experimental research are also discussed.
Keywords: cardiovascular disease, diabetes, dyslipidemia, hypertension, obesity, sleep apnea
Associations of obstructive sleep apnea with cardiovascular disease
The National Commission on Sleep Disorders Research estimated that obstructive sleep apnea (OSA) may account for 38,000 cardiovascular deaths yearly, with an associated expenditure of US $42 million dollars on related hospitalizations [1]. According to data from the Sleep Heart Health study, a National Heart, Lung and Blood Institute-sponsored project, the odds ratio (OR) of heart failure, stroke and coronary heart disease among patients with sleep apnea are 2.38 (95% CI: 1.22–4.62), 1.58 (95% CI: 1.02–2.46) and 1.27 (95% CI: 0.99–1.62), respectively [2]. Other reports based on data from the Sleep Heart Health cohort indicated that patients with sleep apnea had four times the ORs of atrial fibrillation (4.02; 95% CI: 1.03–15.74), three times the ORs of nonsustained ventricular tachycardia (3.40; 95% CI: 1.03–11.20) and nearly twice the ORs of complex ventricular ectopy (1.74; 95% CI: 1.11–2.74) [3]. Untreated sleep apnea is associated with an increased risk of fatal myocardial infarction and stroke (2.87; 95% CI: 1.17–7.51) and nonfatal cardiovascular events (3.17; 95% CI: 1.12–7.52) [4]. Evidence from a previous study conducted among male survivors of acute myocardial infarction suggested that the relative risk for myocardial infarction between the highest and lowest quartiles of apnea index was 23.3 (95% CI: 3.9–139.9) [5]. Individuals with sleep apnea initially referred for coronary angiography are nearly three times more likely (2.89; 95% CI: 1.37–6.09) to suffer a new stroke 10 years later [6].
In light of such findings, the American Heart Association/American College of Cardiology Foundation issued a scientific statement on sleep apnea and cardiovascular disease (CVD), highlighting the need for systematic research to explicate important associations between these two conditions with the desired goal to improve disease management, thereby reducing CVD morbidity and premature death [7]. Current evidence explains in part some of the mechanisms involved in the link between sleep apnea and CVD, but causal explanatory models are lacking owing to a dearth of published long-term control trials. New findings that have led to increased awareness that sleep apnea is a risk factor in the development and/or progression of CVD are discussed later.
Clinical & epidemiologic evidence
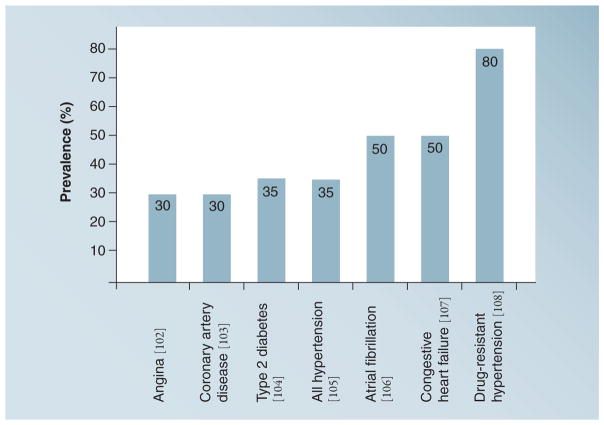
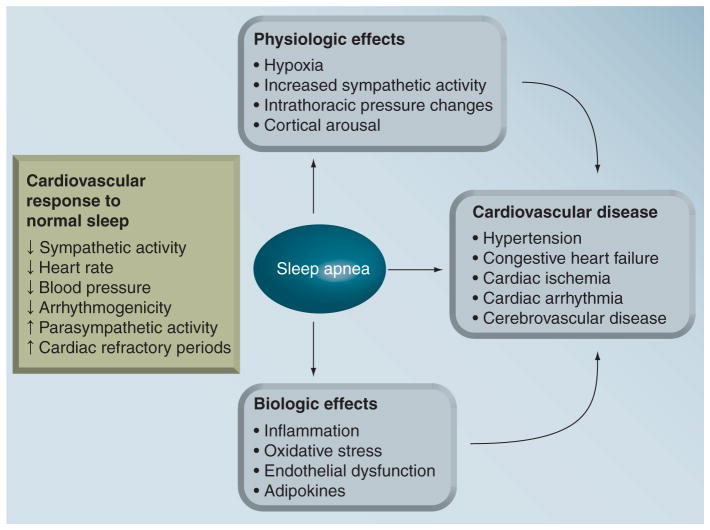
Epidemiologic and clinical studies have documented associations between OSA, the most prevalent sleep-disordered breathing, and CVD (Figure 1). OSA is a common medical condition characterized by repeated sleep-related breathing pauses owing primarily to complete or partial pharyngeal obstruction (Figure 2). It is recognized by the presence of several symptoms including loud snoring and loud gasps, as well as laboratory results including repetitive apneas and hypopneas, which accompany hypoxemia, sleep arousals and hemodynamic changes [8,9]. OSA causes significant sleep disturbances, leading to excessive daytime sleepiness and fatigue, often resulting in vehicular and industrial accidents [10], a gradual decline in cognitive ability and poor performance [11], as well as psychiatric comorbidity including depression, anxiety, post-traumatic stress disorder, psychosis and bipolar disorders [12]. OSA is also associated with all-cause mortality [13].
Figure 1.
Prevalence estimates of sleep apnea in several cardiovascular conditions based on published epidemiologic reports.
Figure 2.
The associations of obstructive sleep apnea with cardiovascular disease via increased physiologic and biologic dysfunctions resulting from repetitive apneic events.
Obstructive sleep apnea significantly increases the risk of stroke independently of other risk factors (i.e., age, sex, race, smoking, alcohol consumption, BMI, and the presence or absence of diabetes mellitus, dyslipidemia, atrial fibrillation and hypertension) [14]. Results of a multivariate analysis, which adjusted for known risk factors, showed that patients with an apnea/hypopnea index (AHI) of 20 or more had significantly greater ORs for stroke (OR: 4.33) relative to individuals without sleep apnea (AHI <5) [15]. Other evidence suggests that males with a respiratory disturbance index (RDI) over 30 had a significantly higher mortality hazard rate than males with a RDI of 1 or less [16]. AHI refers to the total number of apneas (complete cessation of breathing lasting ≥10 s) and hypopneas (≥30% reduction in airflow associated with either a cortical arousal or SaO2 desaturations) divided by the patient’s total sleep time. AHI provides a measure of the severity of sleep apnea. AHI less than 5 is considered normal; AHI values 5–15 are viewed as mild; AHI values 15–30 are moderate; and AHI values over 30 indicate severe sleep apnea. Hence, excess cardiovascular morbidity and mortality is more prevalent among individuals with greater sleep apnea indices.
Evidence from the Sleep Heart Health Study sampling 6424 individuals indicates an increased risk of coronary artery disease, congestive heart failure and stroke among patients with severe sleep apnea [2]. Analysis also showed that patients with OSA had four times the ORs for atrial fibrillation (adjusted OR: 4.02) [3]. This is in tandem with findings from a prospective study of consecutive patients undergoing electrocardioversion for atrial fibrillation, which showed that the adjusted OR for atrial fibrillation among patients with OSA was 2.19 [17]. Data from the Sleep Heart Health Study also revealed that ORs for coronary heart disease (OR: 4.02) and tachycardia (OR: 3.40) were also significantly greater among individuals with OSA [3]. It has been shown that the 10-year risk of coronary heart disease and stroke is approximately 30% among patients with sleep apnea [18].
Mechanisms underlying associations between OSA & CVD
Several intermediary mechanisms with the potential to explain associations between OSA and CVD have been advanced. These mechanisms include, but are not limited to, elevated sympathetic drive, negative intrathoracic pressure, oxidative stress and vascular inflammation [7,19]. Regarding the significance of elevated sympathetic drive, it is believed that repetitive apneic/hypopneic events along with ensuing arterial desaturation and hypercapnia activate chemoreflexes (through hypoxemia and CO2 retention) that activate the sympathetic nervous system (Figure 3). This then results in frequent increases in systemic and pulmonary pressure that might ultimately lead to hypertension. Among individuals with moderate-to-severe OSA (AHI >15), systolic and diastolic pressure can rise by up to 25% compared with baseline values [20]. This may also lead to vasoconstriction, myocardial ischemia, increased catecholamine levels, platelet activation and increased left ventricular afterload [21–25].
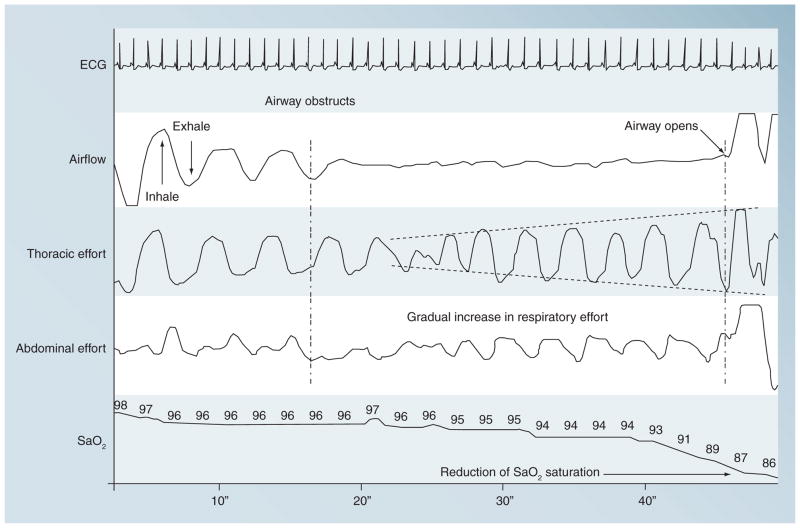
Figure 3. An obstructive sleep apnea event leading to oxyhemoglobin desaturation.
Adapted from the Sleep Academy Award Program sponsored by the National Sleep Foundation.
During the apnea recovery cycle, patients with OSA experience variations in intrathoracic pressure. This occurs as patients attempt to inspire while the pharynx is occluded (Müller maneuver), which could affect intrathoracic hemodynamics and left ventricular functions [26]. As a result of frequent hypoxia and reoxygenation, reactive oxygen species are formed, a process commonly referred to as oxidative stress [27,28]. With greater production of reactive oxygen species, an increase in circulating levels of adenosine and urinary uric acid is observed. Reactive oxygen species, however, regulate the activation of critical transcription factors that are redox sensitive, resulting in increased expression of genes that encode proteins that promote adaptation to hypoxia. It has been suggested that redox-sensitive transcription factors that elicit inflammatory pathways are also activated, thereby affecting inflammatory and immune responses by promoting the activation of endothelial cells, leukocytes and platelets [27]. Typically, patients with OSA have greater levels of endothelin and lower levels of nitric oxide (NO) than healthy sleepers [29]. Once activated, endothelial cells express adhesion molecules (ICAM-1) and proinflammatory cytokines (TNF-α and IL-6) that may lead to endothelial injury and dysfunction; this series of events inevitably leads to the development of cardiovascular morbidity [23,30,31].
Prevalence of OSA
Notwithstanding the risk for developing or exacerbating CVD among individuals with OSA, most individuals with OSA have not been evaluated. Estimates suggest that 82% of men and 93% of women with moderate-to-severe OSA are undiagnosed [32]. Lack of awareness of OSA risk factors (e.g., age, sex, ethnicity, body habitus, familial predisposition, alcohol consumption and chronic rhinitis) among patients and/or among health professionals could explain why the majority of individuals with sleep apnea have yet to receive a diagnosis [201]. Screening rates could improve through increased awareness of comorbid conditions (e.g., hypertension, diabetes and CVD).
Epidemiologic evidence shows that 2% of middle-aged women and 4% of middle-aged men have five or more apnea/hypopnea episodes per hour and meet criteria for OSA [32]. Using a different criterion (RDI ≥10), the Wisconsin Sleep Cohort Study, an epidemiological study surveying the US adult population, showed that sleep apnea affects as many as 15% of men and 5% of women between the ages of 30 and 60 years [33]. However, another study using laboratory polysomnography showed that 24% of men and 9% of women met criteria for sleep apnea [34]. Those estimates portend serious comorbid and psychosocial consequences for at-risk individuals, particularly those with an existing CVD diagnosis.
Pathophysiology of OSA
Obstructive sleep apnea is caused primarily by recurrent pharyngeal occlusion, causing partial or complete cessation of airflow. Clinical evidence points to upper airway obstruction among most patients with sleep apnea, occurring either in the nasopharynx or the oropharynx [35]. Both anatomical and physiological factors interplay in the development of pharyngeal collapse [36]. Most patients have a narrow airway owing to enlarged pharyngeal tissues caused by obesity, with increased fat deposits producing an enlarged soft palate and uvula. Other factors involved in airway obstruction include a large tongue, vascular congestion and pulse edema of the pharyngeal mucosa [35].
During periods of wakefulness, individuals counteract the effects of upper airway narrowing by a compensatory increase in the activity of upper airway dilator muscles. However, efforts to compensate for this narrowing among patients with OSA appear ineffective during sleep [35]. This then results in an upper airway closure, and the ensuing decrease in ventilation causes hypoxia and hypercapnia. This sets in motion a cascade of events beginning with increased ventilatory efforts leading to labored breathing. Subsequently, patients experience cortical arousals and/or abrupt awakenings as they endeavor to resume normal breathing. Upon awakening, the upper airway dilator muscles are reactivated, and airway patency and ventilation are restored. With adequate ventilation, patients can return to sleep (Figure 2).
Diagnosis of OSA
Standard of care for OSA screening typically entails a detailed sleep history, which is performed by a sleep clinician in a structured setting. Whenever possible, family members are queried regarding patients’ sleep patterns, since patients are often unaware that they are experiencing sleep-disordered breathing. Patients with positive screening results are routinely referred to a sleep clinic for a comprehensive assessment of sleep apnea using laboratory polysomnographic studies [37].
A nocturnal polysomnographic study incorporates assessment of sleep architecture, airflow and ventilatory effort, arterial blood saturation, ECG, body position and periodic limb movement [38]. Consistent with the need for an accurate diagnosis, a sleep specialist typically interprets the results of the study. Typically, a diagnosis of central sleep apnea is rendered when recordings show a lack of airflow in the absence of ventilatory effort [39]. The other type of sleep-disordered breathing, OSA, is by all accounts more prevalent and is characterized by closure of the upper airway, causing cessation of airflow despite persistent ventilatory effort.
Treatment of OSA
According to practice parameters established by the American Academy of Sleep Medicine, continuous positive airway pressure (CPAP) is the most effective noninvasive treatment for sleep apnea [202]. This therapy requires the patient to wear a sealed mask over the nose, or in certain cases both the nose and mouth, while sleeping. The patient receives forced room air via the mask, increasing the pressure in the oropharyngeal airway, which helps to maintain airway patency. As seen in Table 1, other therapeutic approaches may be used to alleviate OSA severity. Oral–dental devices may also be used in mild cases or for patients requiring treatment for primary snoring. These are intended to reposition the mandible, thereby maximizing the diameter of the oropharyngeal airway. Although patients often prefer the use of those devices, they are not very effective in reducing cardiovascular morbidity. CPAP is more effective than oral mandibular appliances in reducing respiratory disturbances [40]. However, in other cases surgical procedures (e.g., uvulopalatopharyngoplasty and laser-assisted uvuloplatoplasty) remain a viable option, albeit the least desired treatment owing to their invasiveness and complexity. Gastric bypass surgery is recommended in severe cases of sleep apnea, especially among patients with significant episodes of bradyarrhythmias or tachyarrhythmias [202].
Table 1.
Evidence-based treatment approaches to alleviate symptoms of obstructive sleep apnea.
| Treatment type | Device/procedure |
|---|---|
| Stenting the upper airway mechanically | Nasal CPAP, BiPAP, autoCPAP or dental devices |
| Altering the upper airway | Soft tissue or skeletal surgery |
| Bypassing the upper airway | Tracheostomy |
| Reducing weight | Diet and behavior modification or bariatric surgery |
| Positional therapy | Raise patient’s head off bed, alarm or tennis ball technique |
These therapeutic approaches have shown improvement in metabolic functioning including reduced blood pressure and weight, improved glucose homeostasis and lipid profile, as well as increased cardiac functions.
BiPAP: Bilevel positive airway pressure; CPAP: Continuous positive airway pressure.
The use of CPAP as a therapeutic modality for sleep apnea is often coupled with behavioral weight-reduction approaches [41]. Thus, the objectives of treatment are to eradicate not only physiologic abnormalities including sleep fragmentation, apneic episodes and oxygen desaturations, but also symptoms such as snoring and daytime sleepiness, and to reduce risks for comorbid conditions. Ultimately, weight loss interventions aiming to reduce total body weight by 7–10% seem to be the most effective lifestyle modification. Although it is often difficult for patients with OSA to initiate or maintain a weight management program, even modest weight loss may yield significant reduction in symptom severity [42].
Impact of CPAP studies on CVD & the metabolic syndrome
Intervention studies aiming to treat OSA with CPAP have further confirmed the link between OSA and CVD. Results of CPAP treatment studies among patients with CVD have been encouraging, with demonstrated positive effects not only on breathing-related disturbances during sleep, but also in diminishing several CVD risk markers. Available evidence points to both short- and long-term benefits of CPAP therapy [7,19].
Among patients with congestive heart failure, for instance, treatment of coexisting sleep apnea with CPAP improves left ventricular systolic function [43]. CPAP therapy eliminates apneic episodes and associated hemodynamic changes occurring during sleep [44]. A controlled study examining the effects of sleep apnea treatment on nonfatal (myocardial infarction, stroke and acute coronary syndrome requiring revascularization procedures) and fatal (death from myocardial infarction or stroke) cardiovascular events confirmed previous observations [45]. Investigators demonstrated that subsequent to adjustment for age, gender, cardiovascular risk factors and baseline comorbidities, sleep apnea treatment was associated with a cardiovascular risk reduction of 64%. This is consistent with data demonstrating that CPAP treatment reduces total cardiovascular events (i.e., mortality and new CVD combined) among patients receiving therapy (31 vs 18%) [46]. In addition, two independent studies ascertaining long-term (10-year) CVD outcomes among patients with OSA have found that CVD morbidity and mortality increase only among untreated patients [4,46]. Of note, although CVD outcomes are worse among untreated patients, the incidence of CVD may not necessarily be higher [46].
Furthermore, CPAP treatment has a positive effect on various CVD risk factors. Evidence shows that CPAP reverses endothelial dysfunction [47], an important marker in the pathogenesis of CVD among patients with OSA. CPAP studies also indicate that treatment might reduce elevated levels of C-reactive protein (CRP) [48], diminish levels of proinflammatory cytokines (TNF-α and IL-6) [49,50], and increase NO, a major vasodilator substance released by the endothelium and an important predictor of atherosclerosis [51].
CVD risk reduction targeting components of the metabolic syndrome
There is a growing interest in developing evidence-based therapeutic approaches aimed at reducing the risk of CVD for individuals with OSA presenting with the metabolic syndrome or its components [52]. Evidence suggests that associations between sleep apnea and CVD might be mediated by the metabolic syndrome [53,54]. The metabolic syndrome is characterized by central obesity, hypertension, diabetes and dyslipidemia; it represents a collection of interrelated risk factors of metabolic origin that increase the chances of developing heart disease, stroke and diabetes [55]. It increases the risk of coronary heart disease, and it is associated with prothrombotic and proinflammatory states, two common risk factors for coronary heart disease and diabetes mellitus [28]. The metabolic syndrome is an important contributor to the development of atherosclerosis and CVD. Individuals presenting with components of the metabolic syndrome are at increased risk of developing Type 2 diabetes [56], and those with diabetes are at increased risk of developing CVD [57]. OSA and the metabolic syndrome might have synergistic health risks, since both conditions are predictive of cardiovascular morbidity and mortality [56,57].
Cause and effect associations between OSA and the metabolic syndrome have not been demonstrated. However, preliminary data suggest an important role for the metabolic syndrome. Data from the Mayo Clinic (CA, USA) indicate higher prevalence of the metabolic syndrome among patients with sleep apnea (60%) than among individuals without sleep apnea (40%) [58]. The prevalence of the metabolic syndrome among clinic-attending males with OSA (mean age: 62 years) was 53%, with 98% of the patients characterized by increased waist circumference, 89% by high blood pressure, and 83% by decreased HDL-cholesterol [59]. Compared with patients presenting with the metabolic syndrome only, those with OSA and the metabolic syndrome have increased carotid intima media thickness (661 vs 767 mm), carotid–femoral pulse wave velocity (9.6 vs 10.6 ± m/s) and carotid diameter (6705 vs 7811 mm) [60]. It is not yet systematically ascertained whether cardiovascular events, morbidity and mortality differ significantly between patients with coexisting OSA and the metabolic syndrome, and those with OSA only [60].
Among patients with coexisting sleep apnea and the metabolic syndrome, a controlled trial showed that CPAP treatment improved insulin sensitivity, reduced systemic inflammation and oxidative stress, and reduced global CVD risk [50]. More specifically, patients using CPAP for at least 4 h a night showed a reduced 10-year risk of cardiovascular events using Framingham scores (from 18.8 to 13.9%). Other preliminary data showed that the prevalence of the metabolic syndrome decreased by 45% after 12 months of CPAP treatment, but mixed results were observed with regard to components of the syndrome [61]. Investigators in that study noted no significant difference for fasting blood glucose, triglyceride levels or systolic and diastolic blood pressure, but waist circumference, HDL-cholesterol and BMI improved after treatment.
OSA & obesity
It is well established that obesity, which affects 30% of Americans according to the National Institutes of Diabetes and Digestive and Kidney Diseases [203], plays an important role in the development of OSA. It is the most significant predictor of sleep apnea [62,63]. A study of obese men (BMI: 30 kg/m2) showing no major medical illnesses indicated that 60% met criteria for sleep-disordered breathing and 27% had OSA [64]. According to previous estimates, 60–90% of patients with sleep apnea are obese (obesity defined as a BMI >28 kg/m2); a BMI of 28 kg/m2 has a sensitivity of 93% and a specificity of 74% for sleep apnea [63]. The risk of having moderate-to-severe sleep apnea over a 4-year period increases sixfold among individuals gaining 10% excess weight [65].
There is considerable evidence that weight loss has positive effects on the severity of OSA. A study examining the effects of weight loss following laparoscopic-adjustable gastric banding found a significant reduction in AHI (baseline: 61.6; post-treatment: 13.4) [66]. The study also revealed improvement in sleep architecture and daytime sleepiness; fewer patients required CPAP after laparoscopic surgery. This is consistent with a more recent investigation suggesting that weight loss observed subsequent to laparoscopic surgery is associated with a significant reduction in AHI along with decreased CPAP needs [67]. Generally, weight-reduction procedures have shown significant resolution or improvement of Type 2 diabetes (60%), hypertension (43%), and dyslipidemia (70%) [68]. An important caveat in those clinical studies is that CPAP treatment alone does not necessarily lead to weight reduction [69]. Weight loss associated with improvement of OSA is perhaps best achieved when individuals participate in cognitive–behavioral weight-reduction programs [70].
OSA & hypertension
Understanding the pathophysiologic mechanisms underlying associations between OSA and CVD has been a challenge, owing in part to data suggesting that both conditions may be characterized by similar pathogenetic mechanisms [71]. Both conditions are linked to hypertension [33]. Results of several multivariate analytical models have indicated that sleep apnea represents an independent risk factor for hypertension [33,72,73], and hypertension constitutes a significant predictor of cardiopulmonary deaths among patients with OSA [72]. Data from the Wisconsin Sleep Cohort Study showed that for individuals with a BMI of 30 kg/m2, an AHI of 15 was associated with blood pressure increases of 3.6 mmHg for systolic and 1.8 mmHg for diastolic pressure [74]. Even with adequate adjustment for known confounders, analyses suggested the ORs for the presence of hypertension at follow-up were 1.42 with an AHI of 0.1–4.9 at baseline; 2.03 with an AHI of 5.0–14.9, and 2.89 with an AHI of 15.0 or more [74].
Clinical evidence suggests that treatment of sleep apnea results in a diminution of diurnal and nocturnal blood pressure [40,50,75,76], although it has not been conclusively demonstrated that CPAP treatment lowers blood pressure on a long-term basis. One study suggests that the average diurnal systolic and diastolic blood pressure may drop by as much as 6.7 and 4.9 mmHg, respectively, among patients receiving therapeutic CPAP treatment, compared with patients in a sham CPAP treatment condition [77]. According to published controlled studies and meta-analyses, CPAP therapy is associated with an average reduction of blood pressure of 2 mmHg, with greater reductions observed among patients with more severe OSA and/or among those exhibiting greater adherence to treatment [78,79].
OSA & diabetes
According to data from the Third National Health and Nutrition Examination Survey, 5.1% of US adults have a diagnosis of diabetes [80]. Analysis from the same research group indicated that 15.6% of American adults exhibited glucose intolerance (140 mg/dl) and 6.9% showed impaired fasting glucose levels (≥110 mg/dl) [80]. A growing body of evidence suggests that OSA may be involved in the pathogenesis of altered glucose metabolism, and OSA and insulin resistance might have a bidirectional relationship, as illustrated in a previous report [81]. Evidence shows that patients with OSA have increased glucose levels and insulin resistance [82], which might predispose afflicted individuals to developing Type 2 diabetes. Data suggest that OSA causes an increase in sympathetic activity [44], and increased sympathetic activity impairs glucose homeostasis by enhancing glycogen breakdown and gluconeogenesis [83]. Recurrent hypoxemia and abnormal sympathetic activity, commonly observed among patients with OSA, might mediate the relationships between insulin resistance and sleep apnea.
Data from the Sleep Heart Health Study indicate that the OR for having an abnormal glucose tolerance was 1.44 among patients with an AHI of 15 or more [84]. Results of that study also indicate that insulin resistance was also greatest among the latter group. These analyses provide evidence that apnea-induced hypoxemia is associated with glucose intolerance and insulin resistance [84], leading to the belief that CPAP therapy could improve these metabolic markers of diabetes.
To date, the preponderance of clinical evidence favors a positive effect of CPAP therapy on markers of diabetes. One study has shown that CPAP produced a significant improvement in insulin sensitivity and left ventricular function with a corresponding decrease in blood pressure [82]. Clinical evidence suggests that insulin sensitivity improved even among nondiabetic patients undergoing CPAP therapy [85]. Using a continuous glucose-monitoring system for concomitant measurement of interstitial glucose and polysomnographic sleep among patients with Type 2 diabetes and OSA, investigators found that the mean sleeping glucose decreased from baseline (122.0 mg/dl) to post-treatment (102.9 mg/dl) [86]. Another study found a significant reduction in hemoglobin A1c level (9.2% to 8.6%1.8%) subsequent to treatment [87]. CPAP therapy can also normalize leptin levels, thereby reducing central obesity, a common risk factor for diabetes [88].
OSA & dyslipidemia
Dyslipidemia is a known risk factor in the development of coronary artery disease [89]. Data from the Multi-Ethnic Study of Atherosclerosis indicated that 29.3% of adults met criteria for dyslipidemia [90]. Both factors involved in developing dyslipidemia (i.e., high triglyceride levels and LDL) are affected by obesity, a common predictor of OSA and cardiovascular morbidity. Patients with OSA exhibit greater HDL dysfunction and oxidized LDL levels compared with matched controls [91]. Of note, AHI explained 30% of the variance in HDL dysfunction in sleep apnea.
Clinical evidence indicates that patients with abnormal serum lipid/lipoprotein levels improved significantly with CPAP or bilevel positive airway pressure (BiPAP) therapy [92]. A follow-up (6-month) study of 127 patients showed that the mean HDL cholesterol serum level increased significantly by 5.8% [92]. This finding is in line with evidence that CPAP improved insulin secretion capacity and reduced leptin, total cholesterol and LDL levels among patients with moderate-to-severe OSA [50,88]. Such therapeutic interventions are consistent with the Third Report of the Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (ATP III), recommending that reduction of dyslipidemia is a potential secondary target of risk-reduction therapy [93]. Utilization of CPAP therapy has been shown to regress atherogenic plaque among patients with dyslipidemia [94].
Expert commentary
Epidemiologic and clinical evidence reviewed in this article constitutes a growing body of literature describing associations of sleep apnea with diverse systemic and metabolic abnormalities. Data explaining causal pathways among those metabolic conditions are lacking. Conceivably, associations between sleep apnea and other metabolic conditions point to a maladaptive autonomic response of chemoreceptors, reacting to hypoxia, hypercapnia and acidosis commonly found in sleep apnea [95]. Activation of the sympathetic nervous system through hypoxia and hypercapnia triggers an inflammatory response, resulting in several downstream consequences including hypertension, diabetes, and dyslipidemia [52,96], which are significant risk factors for cardiovascular morbidity or mortality.
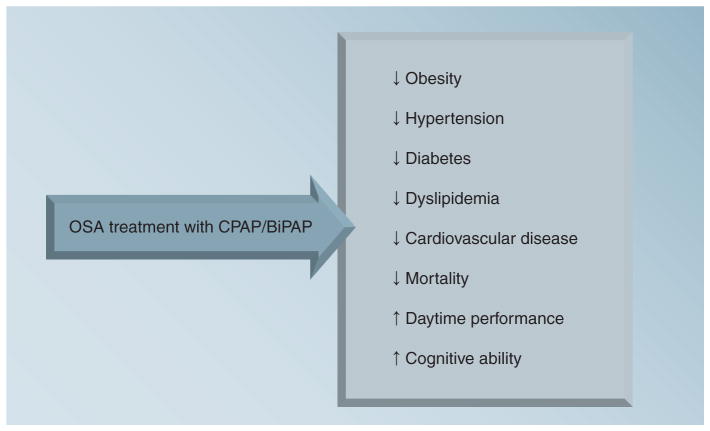
Clinical CPAP/BiPAP studies have demonstrated its benefits (Figure 4). This therapeutic modality is very effective in improving left ventricular ejection fraction and quality of life, lowers blood pressure and sympathetic activity, and reduces mortality among patients with congestive heart failure [75,97]. Among patients with coronary artery disease, it significantly reduces risks of cardiovascular death, acute coronary syndrome and hospitalization for heart failure [98]. Utilization of CPAP lowers blood pressure and improves mood among patients with a dual diagnosis of sleep apnea and stroke [99]. Moreover, CPAP therapy has significant positive effects on lipid levels [92], and it significantly improves insulin sensitivity and left ventricular function, with a corresponding decrease in blood pressure [100]. More conclusive interpretations of these clinical findings await results of long-term, randomized controlled trials.
Figure 4. Demonstrated benefits of treating obstructive sleep apnea with continuous positive airway pressure or bilevel positive airway pressure therapy.
BiPAP: Bilevel positive airway pressure; CPAP: Continuous positive airway pressure; OSA: Obstructive sleep apnea.
While evidence does not support large-scale referrals of individuals with CVD for sleep apnea assessment, it seems prudent that patients suspected of having sleep apnea and meeting criteria for the metabolic syndrome be properly screened. A detailed sleep evaluation is recommended for individuals with positive screening results. CPAP therapy should be considered in the management of individuals with sleep apnea to help mitigate negative CVD outcomes. Sleep apnea treatment is associated with a cardiovascular risk reduction of 64% [45].
The ATP III identifies the metabolic syndrome as a potential secondary target of risk-reduction therapy [93]. It is recommended that patients meeting criteria for the metabolic syndrome receive adequate, tailored treatment aimed at reducing obesity through lifestyle modifications including increased physical activity and improved dietary habits. Indeed, evidence from randomized controlled trials suggests that weight loss among overweight and obese individuals reduces blood pressure, improves lipid profiles and improves blood glucose levels. Weight loss diminishes the severity of sleep apnea, thereby improving overall health, daytime performance, cognitive ability and well-being.
Five-year view
Several systematic studies may be necessary to fully characterize the nature of the relationship between sleep apnea and CVD. Many observational findings have been reported, but there is a dearth of experimental data explaining causal pathways between OSA and CVD. There is also a concern regarding the design of randomized controlled trials, which might not incorporate methodologies to ensure that all participants receive the full benefits of available treatment regimens. These complexities are further compounded by findings suggesting that metabolic syndrome components might mediate the effects of OSA on CVD.
Available data have not yielded evidence to support which factor is the root cause of the co-occurrence of those inter-related metabolic conditions, but insulin resistance and obesity have been offered as potential candidates. Empirical studies testing causal models to explain links among sleep apnea, CVD and components of the metabolic syndrome are needed [19,101]. Future research is necessary to develop models ranking individuals with regards to their risk of sudden cardiac death and provide them with educational and medical intervention. Research is also needed to establish noninvasive markers with adequate sensitivity and specificity in predicting which patients are at greatest risk. It may be that patients with sleep apnea showing depressed left ventricular function are at greater risk for ventricular arrhythmias and other cardiovascular events. Identification and treatment of patients with overlapping syndromes (e.g., congestive heart failure and chronic obstructive pulmonary disease with sleep apnea) is crucial in the management of cardiovascular morbidity.
Key issues.
Sleep apnea is associated with components of the metabolic syndrome; that is, obesity, hypertension, diabetes and dyslipidemia.
Treatment of sleep apnea has beneficial effects on weight, blood pressure, insulin sensitivity and lipid profile.
Sleep apnea contributes to stroke, as over 80% of stroke victims reportedly have sleep apnea.
Sleep apnea is more prevalent among African–Americans; young African–Americans are at an increased risk.
Sleep apnea should be a key factor in the fight to reduce health disparities in cardiovascular disease, obesity, diabetes, hypertension and dyslipidemia.
Sleep apnea contributes to visceral obesity by increasing nocturnal cortisol and insulin, which promote visceral adiposity, metabolic abnormalities and cardiovascular dysfunction.
Individuals with sleep apnea develop leptin resistance, which in turn contributes to further weight gain.
Assessment of sleep apnea is recommended for patients with hypertension, diabetes, obesity and dyslipidemia, as well as those with congestive heart failure, acute coronary syndrome, cardiac arrhythmias and stroke.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This research was supported by funding from the NIH/NCMHD (grant no. R01MD004113 and P20MD005092). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.National Commission on Sleep Disorders Research. The National Commission on Sleep Disorders Research. Wake up America: a national sleep alert. US Government Printing Office; Washington DC, USA: 2008. [Google Scholar]
- 2.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 3.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 5.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336(8710):261–264. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 6.Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, Franklin KA. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation. 2008;118(9):955–960. doi: 10.1161/CIRCULATIONAHA.108.783290. [DOI] [PubMed] [Google Scholar]
- 7••.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52(8):686–717. doi: 10.1016/j.jacc.2008.05.002. Reported the concensus statement on the association between sleep apnea and cardiovascular disease, which is sanctioned by the American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee. [DOI] [PubMed] [Google Scholar]
- 8.Coccagna G, Mantovani M, Brignani F, Parchi C, Lugaresi E. Tracheostomy in hypersomnia with periodic breathing. Bull Physiopathol Respir (Nancy) 1972;8(5):1217–1227. [PubMed] [Google Scholar]
- 9.Coccagna G, Mantovani M, Brignani F, Parchi C, Lugaresi E. Continuous recording of the pulmonary and systemic arterial pressure during sleep in syndromes of hypersomnia with periodic breathing. Bull Physiopathol Respir (Nancy) 1972;8(5):1159–1172. [PubMed] [Google Scholar]
- 10.Stoohs RA, Guilleminault C, Itoi A, Dement WC. Traffic accidents in commercial long-haul truck drivers: the influence of sleep-disordered breathing and obesity. Sleep. 1994;17(7):619–623. [PubMed] [Google Scholar]
- 11.El Ad B, Lavie P. Effect of sleep apnea on cognition and mood. Int Rev Psychiatry. 2005;17(4):277–282. doi: 10.1080/09540260500104508. [DOI] [PubMed] [Google Scholar]
- 12.Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405–1411. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 13.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 15.Aartz M, Young T, Finn L, Skatrud JB, Bradley D. Association of sleep-disordered breathing and the occurence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J. 2005;25(3):514–520. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]
- 17.Gami AS, Pressman G, Caples SM. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;27(364):364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 18.Kiely JL, McNicholas WT, Zgierska A, et al. Cardiovascular risk factors in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2000;16(1):128–133. doi: 10.1034/j.1399-3003.2000.16a23.x. [DOI] [PubMed] [Google Scholar]
- 19.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 20.Shepard JW, Jr, Garrison MW, Grither DA, Evans R, Schweitzer PK. Relationship of ventricular ectopy to nocturnal oxygen desaturation in patients with chronic obstructive pulmonary disease. Am J Med. 1985;78(1):28–34. doi: 10.1016/0002-9343(85)90457-7. [DOI] [PubMed] [Google Scholar]
- 21.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290(14):1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 22.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyken ME, Somers VK, Yamada T, Ren ZY, Zimmerman MB. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27(3):401–407. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- 24.Sanner BM, Konermann M, Tepel M, Groetz J, Mummenhoff C, Zidek W. Platelet function in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2000;16(4):648–652. doi: 10.1034/j.1399-3003.2000.16d14.x. [DOI] [PubMed] [Google Scholar]
- 25.Franklin KA, Nilsson JB, Sahlin C, Naslund U. Sleep apnoea and nocturnal angina. Lancet. 1995;345(8957):1085–1087. doi: 10.1016/s0140-6736(95)90820-x. [DOI] [PubMed] [Google Scholar]
- 26.Bradley TD. Right and left ventricular functional impairment and sleep apnea. Clin Chest Med. 1992;13(3):459–479. [PubMed] [Google Scholar]
- 27.Lavie L. Obstructive sleep apnoea syndrome – an oxidative stress disorder. Sleep Med Rev. 2003;7(1):35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 28.Kasasbeh E, Chi DS, Krishnaswamy G. Inflammatory aspects of sleep apnea and their cardiovascular consequences. South Med J. 2006;99(1):58–67. doi: 10.1097/01.smj.0000197705.99639.50. [DOI] [PubMed] [Google Scholar]
- 29.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162(6):2166–2171. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 30.von Kanel R, Loredo JS, Powell FL, Adler KA, Dimsdale JE. Short-term isocapnic hypoxia and coagulation activation in patients with sleep apnea. Clin Hemorheol Microcirc. 2005;33(4):369–377. [PubMed] [Google Scholar]
- 31.Mills PJ, von Kanel R, Norman D, Natarajan L, Ziegler MG, Dimsdale JE. Inflammation and sleep in healthy individuals. Sleep. 2007;30(6):729–735. doi: 10.1093/sleep/30.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young T, Finn L. Epidemiological insights into the public health burden of sleep disordered breathing: sex differences in survival among sleep clinic patients. Thorax. 1998;53(Suppl 3):S16–S19. doi: 10.1136/thx.53.2008.s16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. Demonstrated the associations between hypertension and sleep apnea. [PubMed] [Google Scholar]
- 34.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 35.Remmers JE, Launois S, Feroah T, Whitelaw WA. Mechanics of the pharynx in patients with obstructive sleep apnea. Prog Clin Biol Res. 1990;345:261–268. [PubMed] [Google Scholar]
- 36.Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med. 1976;27(465):465–484. doi: 10.1146/annurev.me.27.020176.002341. [DOI] [PubMed] [Google Scholar]
- 37.Rechtshaffen A, Kales A. A manual of standardized terminology, techniques, and scoring systems of sleep stages of human subjects. UCLA Brain Information Service/ Brain Research Institute; Los Angeles, USA: 1968. [Google Scholar]
- 38.Chesson AL, Jr, Ferber RA, Fry JM, et al. The indications for polysomnography and related procedures. Sleep. 1997;20(6):423–487. doi: 10.1093/sleep/20.6.423. [DOI] [PubMed] [Google Scholar]
- 39.Kryger M, Roth T, Dement W. Principles and Practice of Sleep Medicine. WB Saunders Co; PA, USA: 2002. pp. 1–1336. [Google Scholar]
- 40.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PubMed] [Google Scholar]
- 41.Stepnowsky CJ, Palau JJ, Gifford AL, Ancoli-Israel S. A self-management approach to improving continuous positive airway pressure adherence and outcomes. Behav Sleep Med. 2007;5(2):131–146. doi: 10.1080/15402000701190622. [DOI] [PubMed] [Google Scholar]
- 42.Strobel RJ, Rosen RC. Obesity and weight loss in obstructive sleep apnea: a critical review. Sleep. 1996;19(2):104–115. doi: 10.1093/sleep/19.2.104. [DOI] [PubMed] [Google Scholar]
- 43.Malone S, Liu PP, Holloway R, Rutherford R, Xie A, Bradley TD. Obstructive sleep apnoea in patients with dilated cardiomyopathy: effects of continuous positive airway pressure. Lancet. 1991;338(8781):1480–1484. doi: 10.1016/0140-6736(91)92299-h. [DOI] [PubMed] [Google Scholar]
- 44.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176(12):1274–1280. doi: 10.1164/rccm.200611-1588OC. Provided convincing evidence that treatment of sleep apnea leads to cardiovascular risk reduction. [DOI] [PubMed] [Google Scholar]
- 46.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127(6):2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 47.Ohike Y, Kozaki K, Iijima K, et al. Amelioration of vascular endothelial dysfunction in obstructive sleep apnea syndrome by nasal continuous positive airway pressure – possible involvement of nitric oxide and asymmetric NG, NG-dimethylarginine. Circ J. 2005;69(2):221–226. doi: 10.1253/circj.69.221. [DOI] [PubMed] [Google Scholar]
- 48.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107(8):1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 49.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112(17):2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 50.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134(4):686–692. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 51.Schulz R, Schmidt D, Blum A, et al. Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: response to CPAP therapy. Thorax. 2000;55(12):1046–1051. doi: 10.1136/thorax.55.12.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5(2):207–217. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 53.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 54.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28(11):2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 55.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 56.Grundy SM. A constellation of complications: the metabolic syndrome. Clin Cornerstone. 2005;7(2–3):36–45. doi: 10.1016/s1098-3597(05)80066-3. [DOI] [PubMed] [Google Scholar]
- 57.Alexander CM. The coming of age of the metabolic syndrome. Diabetes Care. 2003;26(11):3180–3181. doi: 10.2337/diacare.26.11.3180. [DOI] [PubMed] [Google Scholar]
- 58.Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3(5):467–472. [PMC free article] [PubMed] [Google Scholar]
- 59.Ambrosetti M, Lucioni AM, Conti S, Pedretti RF, Neri M. Metabolic syndrome in obstructive sleep apnea and related cardiovascular risk. J Cardiovasc Med (Hagerstown) 2006;7(11):826–829. doi: 10.2459/01.JCM.0000250873.01649.41. [DOI] [PubMed] [Google Scholar]
- 60.Drager LF, Bortolotto LA, Maki-Nunes C, et al. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis. 2010;208(2):490–495. doi: 10.1016/j.atherosclerosis.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Oktay B, Akbal E, Firat H, Ardic S, Kizilgun M. CPAP treatment in the coexistence of obstructive sleep apnea syndrome and metabolic syndrome, results of one year follow up. Acta Clin Belg. 2009;64(4):329–334. doi: 10.1179/acb.2009.051. [DOI] [PubMed] [Google Scholar]
- 62.Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17(9):533–540. [PubMed] [Google Scholar]
- 63.Kushida CA, Efron B, Guilleminault C. A predictive morphometric model for the obstructive sleep apnea syndrome. Ann Intern Med. 1997;127(8 Pt 1):581–587. doi: 10.7326/0003-4819-127-8_part_1-199710150-00001. [DOI] [PubMed] [Google Scholar]
- 64.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165(5):677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 65•.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. Demonstrated the associations between hypertension and sleep apnea. [DOI] [PubMed] [Google Scholar]
- 66.Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond) 2005;29(9):1048–1054. doi: 10.1038/sj.ijo.0802960. [DOI] [PubMed] [Google Scholar]
- 67.Rao A, Tey BH, Ramalingam G, Poh AG. Obstructive sleep apnoea (OSA) patterns in bariatric surgical practice and response of OSA to weight loss after laparoscopic adjustable gastric banding (LAGB) Ann Acad Med Singapore. 2009;38(7):587–587. [PubMed] [Google Scholar]
- 68.Cunneen SA. Review of meta-analytic comparisons of bariatric surgery with a focus on laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2008;4(3 Suppl):S47–S55. doi: 10.1016/j.soard.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Redenius R, Murphy C, O’Neill E, Al Hamwi M, Zallek SN. Does CPAP lead to change in BMI? J Clin Sleep Med. 2008;4(3):205–209. [PMC free article] [PubMed] [Google Scholar]
- 70.Kajaste S, Brander PE, Telakivi T, Partinen M, Mustajoki P. A cognitive-behavioral weight reduction program in the treatment of obstructive sleep apnea syndrome with or without initial nasal CPAP: a randomized study. Sleep Med. 2004;5(2):125–131. doi: 10.1016/j.sleep.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 71.Marcus DM, Lynn J, Miller JJ, Chaudhary O, Thomas D, Chaudhary B. Sleep disorders: a risk factor for pseudotumor cerebri? J Neuroophthalmol. 2001;21(2):121–123. doi: 10.1097/00041327-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 72.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. Br Med J. 2000;320(7233):479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohayon MM, Guilleminault C, Priest RG, Zulley J, Smirne S. Is sleep-disordered breathing an independent risk factor for hypertension in the general population (13,057 subjects)? J Psychosom Res. 2000;48(6):593–601. doi: 10.1016/s0022-3999(00)00142-2. [DOI] [PubMed] [Google Scholar]
- 74•.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. Demonstrated the associations between hypertension and sleep apnea. [DOI] [PubMed] [Google Scholar]
- 75.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 76.Mayer J, Becker H, Brandenburg U, Penzel T, Peter JH, von Wichert P. Blood pressure and sleep apnea: results of long-term nasal continuous positive airway pressure therapy. Cardiology. 1991;79(2):84–92. doi: 10.1159/000174864. [DOI] [PubMed] [Google Scholar]
- 77.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29(4):720–727. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 78.Duran-Cantolla J, Aizpuru F, Martinez-Null C, Barbe-Illa F. Obstructive sleep apnea/hypopnea and systemic hypertension. Sleep Med Rev. 2009;13(5):323–331. doi: 10.1016/j.smrv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Alajmi M, Mulgrew AT, Fox J, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung. 2007;185(2):67–72. doi: 10.1007/s00408-006-0117-x. [DOI] [PubMed] [Google Scholar]
- 80.Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21(Suppl 3):C11–C14. doi: 10.2337/diacare.21.3.c11. [DOI] [PubMed] [Google Scholar]
- 81.Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med. 2003;254(1):32–44. doi: 10.1046/j.1365-2796.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- 82.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 2005;99(5):1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 83.Punjabi NM, Ahmed MM, Polotsky VY, Beamer BA, O’Donnell CP. Sleep-disordered breathing, glucose intolerance, and insulin resistance. Respir Physiol Neurobiol. 2003;136(2–3):167–178. doi: 10.1016/s1569-9048(03)00079-x. [DOI] [PubMed] [Google Scholar]
- 84.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 85.Lam JC, Lam B, Yao TJ, et al. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J. 2010;35(1):138–145. doi: 10.1183/09031936.00047709. [DOI] [PubMed] [Google Scholar]
- 86.Dawson A, Abel SL, Loving RT, et al. CPAP therapy of obstructive sleep apnea in Type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4(6):538–542. [PMC free article] [PubMed] [Google Scholar]
- 87.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165(4):447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 88.Cuhadaroglu C, Utkusavas A, Ozturk L, Salman S, Ece T. Effects of nasal CPAP treatment on insulin resistance, lipid profile, and plasma leptin in sleep apnea. Lung. 2009;187(2):75–81. doi: 10.1007/s00408-008-9131-5. [DOI] [PubMed] [Google Scholar]
- 89.Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT) JAMA. 1986;256(20):2823–2828. [PubMed] [Google Scholar]
- 90.Goff DC, Jr, D’Agostino RB, Jr, Haffner SM, Otvos JD. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. Results from the Insulin Resistance Atherosclerosis Study. Metabolism. 2005;54(2):264–270. doi: 10.1016/j.metabol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 91.Tan KC, Chow WS, Lam JC, et al. HDL dysfunction in obstructive sleep apnea. Atherosclerosis. 2006;184(2):377–382. doi: 10.1016/j.atherosclerosis.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 92.Borgel J, Sanner BM, Bittlinsky A, et al. Obstructive sleep apnoea and its therapy influence high-density lipoprotein cholesterol serum levels. Eur Respir J. 2006;27(1):121–127. doi: 10.1183/09031936.06.00131304. [DOI] [PubMed] [Google Scholar]
- 93.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 94.Brown G, Albers JJ, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323(19):1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 95.Lee PY, Yun AJ, Bazar KA. Acute coronary syndromes and heart failure may reflect maladaptations of trauma physiology that was shaped during pre-modern evolution. Med Hypotheses. 2004;62(6):861–867. doi: 10.1016/j.mehy.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Fletcher EC. Cardiovascular disease associated with obstructive sleep apnea. Monaldi Arch Chest Dis. 2003;59(3):254–261. [PubMed] [Google Scholar]
- 97.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169(3):361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 98.Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25(9):728–734. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 99.Sajkov D, Wang T, Saunders NA, Bune AJ, Neill AM, Douglas MR. Daytime pulmonary hemodynamics in patients with obstructive sleep apnea without lung disease. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1518–1526. doi: 10.1164/ajrccm.159.5.9805086. [DOI] [PubMed] [Google Scholar]
- 100.Harsch IA, Hahn EG, Konturek PC. Insulin resistance and other metabolic aspects of the obstructive sleep apnea syndrome. Med Sci Monit. 2005;11(3):RA70–RA75. [PubMed] [Google Scholar]
- 101.Eastwood PR, Malhotra A, Palmer LJ, et al. Obstructive sleep apnoea: from pathogenesis to treatment: current controversies and future directions. Respirology. 2010;15(4):587–595. doi: 10.1111/j.1440-1843.2009.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sanner M, Ko N, Oberauemr C, Thomaws EBS, Walzide KM. Sleep-disordered breathing in patients referred for angina evaluation – association with left ventricular dysfunction. Clin Cardiol. 2001;24:146–150. doi: 10.1002/clc.4960240209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schäfer H, Koehler U, Ewig S, Hasper E, Tasci S, Lüderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology. 1999;92(2):79–84. doi: 10.1159/000006952. [DOI] [PubMed] [Google Scholar]
- 104.Einhorn D, Stewart DA, Erman MK, Gordon N, Philis-Tsimikas A, Casal E. Prevalence of sleep apnea in a population of adults with Type 2 diabetes mellitus. Endocr Pract. 2007;13(4):355–362. doi: 10.4158/EP.13.4.355. [DOI] [PubMed] [Google Scholar]
- 105.Sjöström C, Lindberg E, Elmasry A, Hägg A, Svärdsudd K, Janson C. Prevalence of sleep apnoea and snoring in hypertensive men: a population based study. Thorax. 2002;57(7):602–607. doi: 10.1136/thorax.57.7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 107.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118(10):1034–1040. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19(12):2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
Websites
- 201.The National Commission on Sleep Disorders Research. [Accessed 20 August 2008]; www.stanford.edu/~dement/overview-ncsdr.html.
- 202.The American Academy of Sleep Medicine (Practice Parameters) [Accessed 24 August 2008]; www.aasmnet.org/practiceparameters.htm.
- 203.The National Institutes of Diabetes & Digestive & Kidney Diseases. [Accessed 20 October 2009];National Institutes of Health. www2.niddk.nih.gov/