Abstract
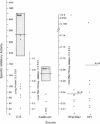
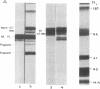
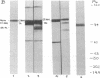
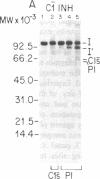
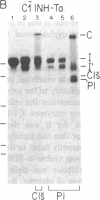
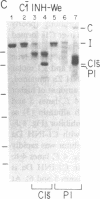
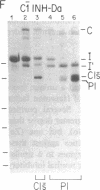
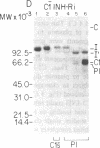
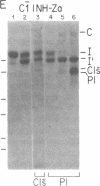
C1(-)-inhibitor (C1(-)-INH) proteins from normal persons and members of eight different kindred with dysfunctional C1(-)-INH proteins associated with hereditary angioneurotic edema (HANE) were compared with respect to their inhibitory activity against purified preparations of C1s-, plasma kallikrein, activated forms of Hageman factor, and plasmin. Each dysfunctional C1(-)-INH protein showed a unique spectrum of inhibitory activity against these enzymes. Although none of the dysfunctional C1(-)-INH proteins significantly impaired amidolysis by plasmin, all but one inhibited activated Hageman factor. One purified dysfunctional C1(-)-INH (Ta) inhibited purified C1s- to a normal degree. Another C1(-)-INH (Za) had almost seven times as much inhibitory activity as normal C1(-)-INH against activated Hageman factor, but had decreased activity against C1s- and no activity against plasmin. Analyses of mixtures of plasmin and C1(-)-INH proteins in SDS gel electrophoresis revealed variability in the patterns of complex formation and cleavage of dysfunctional proteins after exposure to C1s- and plasmin. Some bound to plasmin and were cleaved, even though none significantly impaired the amidolytic activity of plasmin. Two were cleaved by C1s-, whereas neither normal or other dysfunctional C1(-)-INH were cleaved. Dysfunctional C1(-)-INH proteins from patients with HANE are thus heterogeneous in their inhibitory properties and there must be different structural requirements for the inhibition of the various plasma enzymes that can be regulated by normal C1(-)-INH. The data suggest that in addition to common sites of interactions between these proteases and C1(-)-INH, there are also points of contact that are specific for each protease. Genetic mutations leading to structural changes at some of these sites may have differing effects on the interaction between individual proteases and abnormal C1(-)-INH proteins. These alterations may allow these proteins to serve as probes for structural requirements for inhibitory actions of normal C1(-)-INH.
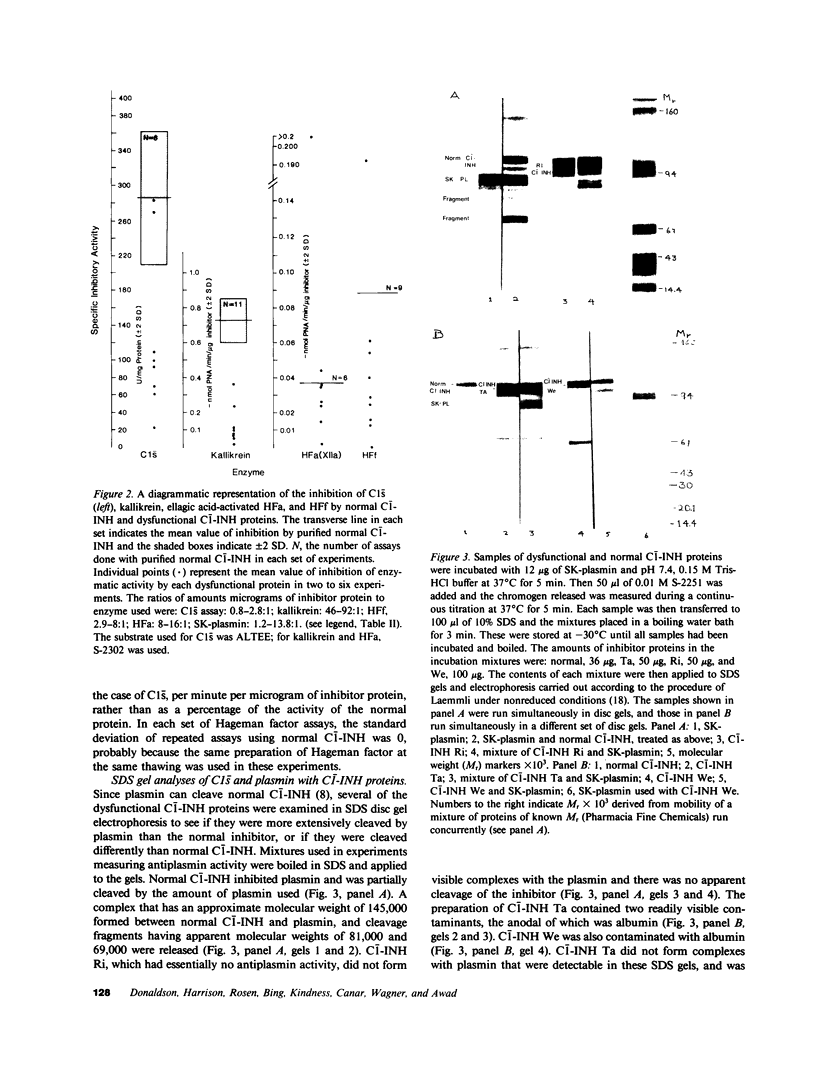
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimeh S. N., Bing D. H., Painter R. H. A simple method for the isolation of the subcomponents of the first component of complement by affinity chromatography. J Immunol. 1974 Jul;113(1):225–234. [PubMed] [Google Scholar]
- Bing D. H. Purification of the first component of human complement by affinity chromatography on human globulin linked to sepharose. J Immunol. 1971 Nov;107(5):1243–1249. [PubMed] [Google Scholar]
- Champion R. H., Lachmann P. J. Hereditary angio-oedema treated with E-aminocaproic acid. Br J Dermatol. 1969 Oct;81(10):763–765. doi: 10.1111/j.1365-2133.1969.tb15938.x. [DOI] [PubMed] [Google Scholar]
- DONALDSON V. H., EVANS R. R. A BIOCHEMICAL ABNORMALITY IN HEREDIATRY ANGIONEUROTIC EDEMA: ABSENCE OF SERUM INHIBITOR OF C' 1-ESTERASE. Am J Med. 1963 Jul;35:37–44. doi: 10.1016/0002-9343(63)90162-1. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Donaldson V. H., Harrison R. A. Complexes between C1-inhibitor, kallikrein, high molecular weight kininogen, plasma thromboplastin antecedent, and plasmin in normal human plasma and hereditary angioneurotic edema plasmas containing dysmorphic C1-inhibitors: role of cold activation. Blood. 1982 Jul;60(1):121–129. [PubMed] [Google Scholar]
- FINKENSTADT W. R., LASKOWSKI M., Jr PEPTIDE BOND CLEAVAGE ON TRYPSINTRYPSIN INHIBITOR COMPLEX FORMATION. J Biol Chem. 1965 Feb;240:962–963. [PubMed] [Google Scholar]
- Gigli I., Mason J. W., Colman R. W., Austen K. F. Interaction of plasma kallikrein with the C1 inhibitor. J Immunol. 1970 Mar;104(3):574–581. [PubMed] [Google Scholar]
- Hadjiyannaki K., Lachmann P. J. Hereditary angio-oedema: a review with particular reference to pathogenesis and treatment. Clin Allergy. 1971 Jun;1(2):221–233. doi: 10.1111/j.1365-2222.1971.tb03021.x. [DOI] [PubMed] [Google Scholar]
- Harpel P. C., Cooper N. R. Studies on human plasma C1 inactivator-enzyme interactions. I. Mechanisms of interaction with C1s, plasmin, and trypsin. J Clin Invest. 1975 Mar;55(3):593–604. doi: 10.1172/JCI107967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C., Rosenberg R. D. Alpha 2-macroglobulin and antithrombin-heparin cofactor: modulators of hemostatic and inflammatory reactions. Alpha 2-macroglobulin. Prog Hemost Thromb. 1976;3:145–189. [PubMed] [Google Scholar]
- Harrison R. A. Human C1 inhibitor: improved isolation and preliminary structural characterization. Biochemistry. 1983 Oct 11;22(21):5001–5007. doi: 10.1021/bi00290a019. [DOI] [PubMed] [Google Scholar]
- LEVY L. R., LEPOW I. H. Assay and properties of serum inhibitor of C'l-esterase. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:608–611. doi: 10.3181/00379727-101-25034. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Rosen F. S. The catabolism of C1(-)-inhibitor and the pathogenesis of hereditary angio-edema. Acta Pathol Microbiol Immunol Scand Suppl. 1984;284:35–39. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. H., Iammarino R. M., Spero J. A., Hasiba U. Antithrombin Pittsburgh: an alpha1-antitrypsin variant causing hemorrhagic disease. Blood. 1978 Jan;51(1):129–137. [PubMed] [Google Scholar]
- Nagase H., Barrett A. J. Human plasma kallikrein. A rapid purification method with high yield. Biochem J. 1981 Jan 1;193(1):187–192. doi: 10.1042/bj1930187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. C., Brennan S. O., Lewis J. H., Carrell R. W. Mutation of antitrypsin to antithrombin. alpha 1-antitrypsin Pittsburgh (358 Met leads to Arg), a fatal bleeding disorder. N Engl J Med. 1983 Sep 22;309(12):694–698. doi: 10.1056/NEJM198309223091203. [DOI] [PubMed] [Google Scholar]
- Quastel M., Harrison R., Cicardi M., Alper C. A., Rosen F. S. Behavior in vivo of normal and dysfunctional C1 inhibitor in normal subjects and patients with hereditary angioneurotic edema. J Clin Invest. 1983 Apr;71(4):1041–1046. doi: 10.1172/JCI110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D., CRUM J. D. ACTIVATION OF HAGEMAN FACTOR BY SOLUTIONS OF ELLAGIC ACID. J Lab Clin Med. 1964 Mar;63:359–377. [PubMed] [Google Scholar]
- ROSEN F. S., PENSKY J., DONALDSON V., CHARACHE P. HEREDITARY ANGIONEUROTIC EDEMA: TWO GENETIC VARIANTS. Science. 1965 May 14;148(3672):957–958. doi: 10.1126/science.148.3672.957. [DOI] [PubMed] [Google Scholar]
- Ratnoff O. D., Saito H. Interactions among Hageman factor, plasma prekallikrein, high molecular weight kininogen, and plasma thromboplastin antecedent. Proc Natl Acad Sci U S A. 1979 Feb;76(2):958–961. doi: 10.1073/pnas.76.2.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnoff O. D. The relative amidolytic activity of Hageman factor (factor XII) and its fragments. The effect of high-molecular-weight kininogen and kaolin. J Lab Clin Med. 1980 Aug;96(2):267–277. [PubMed] [Google Scholar]
- Remold-O'Donnell E., Lewandrowski K. Macrophage surface component gp160: sensitivity to plasmin and other proteases. J Immunol. 1982 Apr;128(4):1541–1544. [PubMed] [Google Scholar]
- Rosen F. S., Alper C. A., Pensky J., Klemperer M. R., Donaldson V. H. Genetically determined heterogeneity of the C1 esterase inhibitor in patients with hereditary angioneurotic edema. J Clin Invest. 1971 Oct;50(10):2143–2149. doi: 10.1172/JCI106708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Ratnoff O. D., Donaldson V. H. Defective activation of clotting, fibrinolytic, and permeability-enhancing systems in human Fletcher trait plasma. Circ Res. 1974 May;34(5):641–651. doi: 10.1161/01.res.34.5.641. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Arlaud G. J., Colomb M. G. Kinetics of reaction of human C1-inhibitor with the human complement system proteases C1r and C1s. Biochim Biophys Acta. 1980 Apr 11;612(2):433–449. doi: 10.1016/0005-2744(80)90126-6. [DOI] [PubMed] [Google Scholar]
- Weiss V., Engel J. Heparin-stimulated modification of C1-inhibitor by subcomponent C1s of human complement. Hoppe Seylers Z Physiol Chem. 1983 Mar;364(3):295–301. [PubMed] [Google Scholar]