Abstract
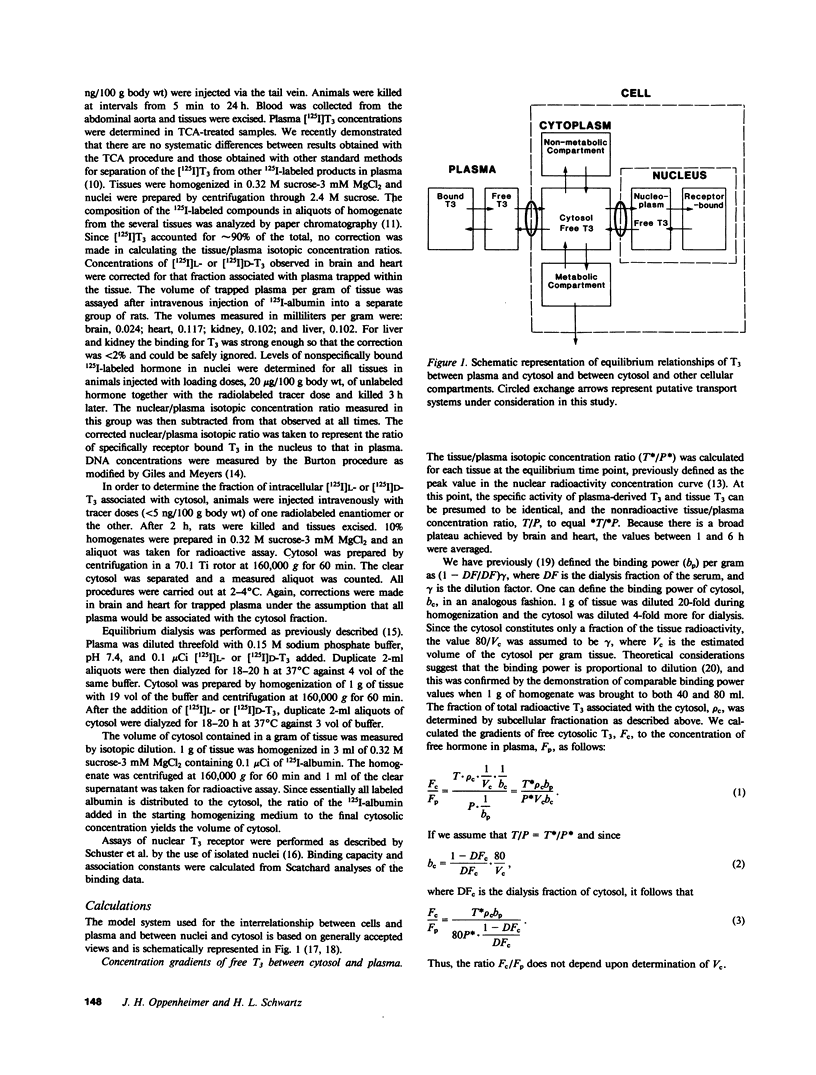
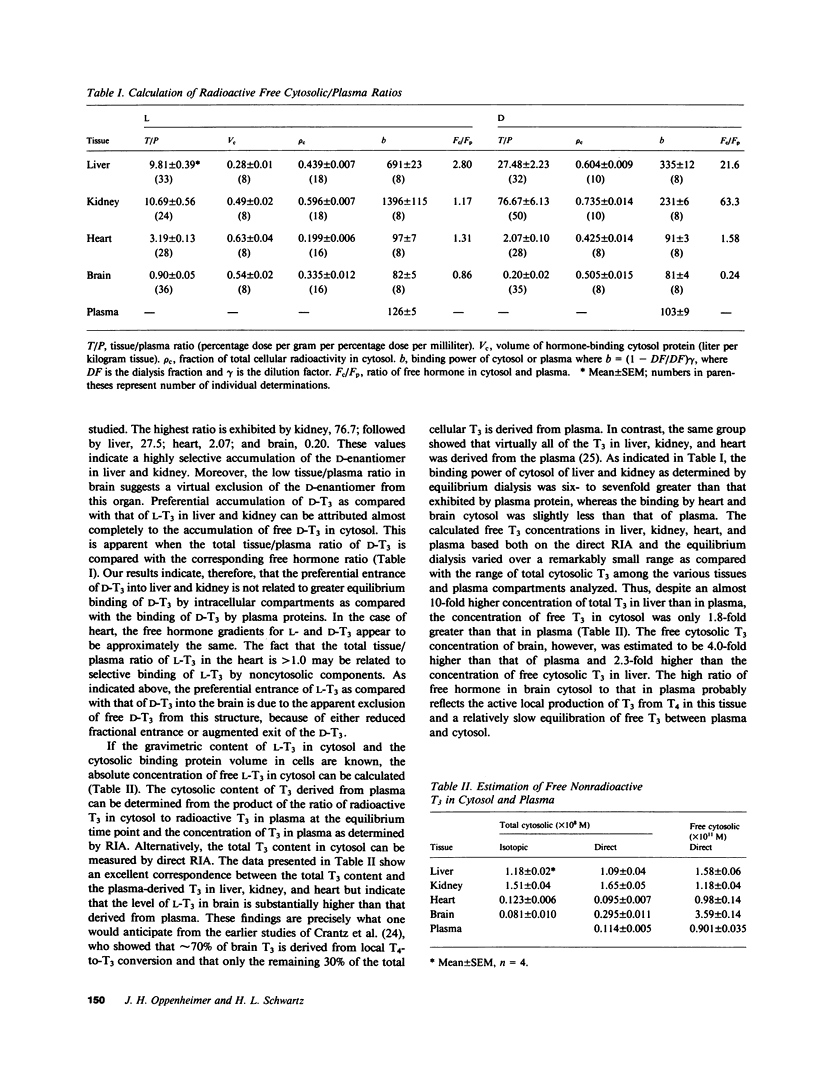
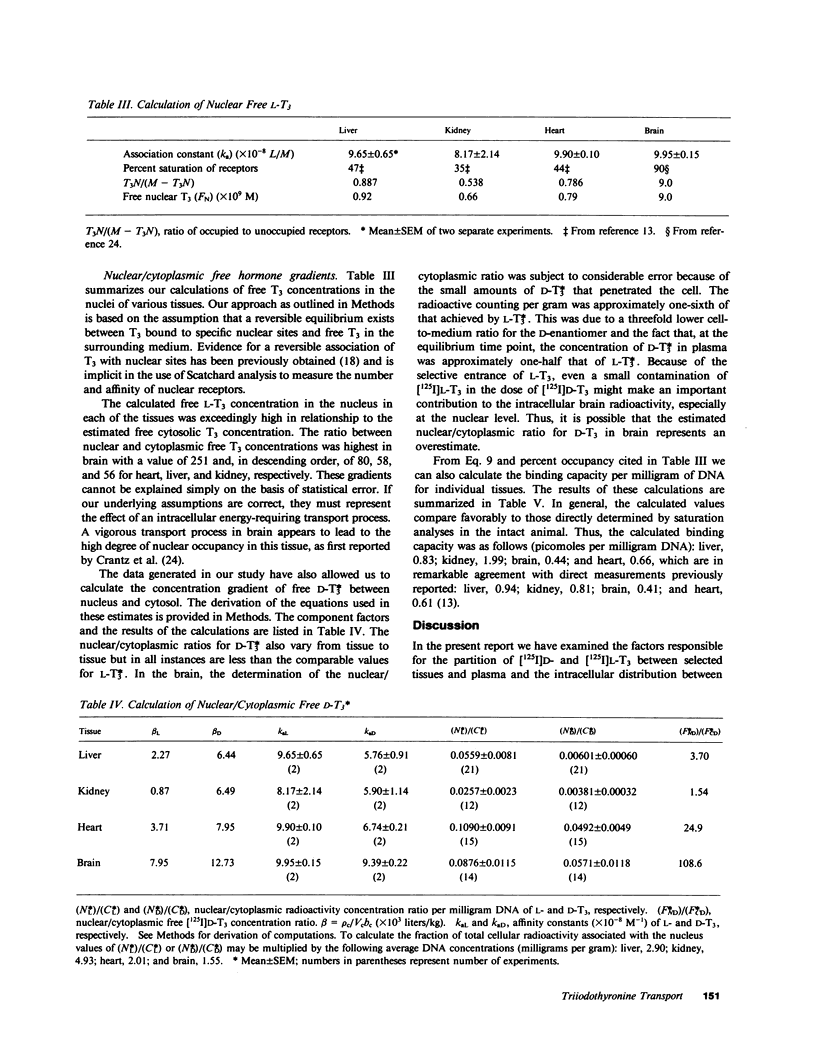
We have investigated the transport of L- and D-triiodothyronine (T3) from plasma to cellular cytoplasm and from cytoplasm to nucleus by estimating the concentration of free hormone in these compartments in rat liver, kidney, brain, and heart. We assessed the distribution of T3 in various tissues and its metabolism by standard isotopic techniques and measured plasma and cytosolic tissue T3 by radioimmunoassay. In addition, we determined the fraction of radiosensitive T3 associated with the cytosol in individual tissues and estimated the cytosolic volume per gram of tissue. Equilibrium dialysis allowed us to determine the binding power of cytosols and plasma, and in vitro saturation techniques provided values for the affinity (ka) for L- and D-T3 of isolated nuclei in aqueous solution at 37 degrees C. We calculated the free cytosolic hormone from the product of cytosolic T3 and the binding power of cytosol for T3, and the free intranuclear T3 from the ka and previously determined ratio of occupied-to-unoccupied binding sites under steady state conditions in euthyroid animals. Our results showed that the free cytosolic/free plasma concentrations for L-T3 and D-T3, respectively, were: liver 2.8, 21.6; kidney 1.17, 63.3; heart 1.31, 1.58; brain 0.86, 0.24. The free nuclear/free cytosolic ratios for L-T3 and D-T3, respectively, were: liver 58.2, 3.70; kidney 55.9, 1.54; heart 80.6, 24.9; and brain 251, 108.6. Our findings suggest that stereospecific transport occurs both from plasma to cytosol and from cytosol to nucleus. The high gradients from cytosol to nucleus imply that there is an energy-dependent process and appear to account for the differences in the nuclear association constant determined in vivo and in vitro.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., MEUDT R., HOPKINS J. W., MIRSKY A. E. Sodium-dependent "transport" reactions in the cell nucleus and their role in protein and nucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Jul 15;47:907–932. doi: 10.1073/pnas.47.7.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- Chait A., Kanter R., Green W., Kenny M. Defective thyroid hormone action in fibroblasts cultured from subjects with the syndrome of resistance to thyroid hormones. J Clin Endocrinol Metab. 1982 Apr;54(4):767–772. doi: 10.1210/jcem-54-4-767. [DOI] [PubMed] [Google Scholar]
- Cheng S. Y., Maxfield F. R., Robbins J., Willingham M. C., Pastan I. H. Receptor-mediated uptake of 3,3',5-triiodo-L-thyronine by cultured fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3425–3429. doi: 10.1073/pnas.77.6.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crantz F. R., Silva J. E., Larsen P. R. An analysis of the sources and quantity of 3,5,3'-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology. 1982 Feb;110(2):367–375. doi: 10.1210/endo-110-2-367. [DOI] [PubMed] [Google Scholar]
- DeGroot L. J., Torresani J. Triiodothyronine binding to isolated liver cell nuclei. Endocrinology. 1975 Feb;96(2):357–359. doi: 10.1210/endo-96-2-357. [DOI] [PubMed] [Google Scholar]
- Hay I. D., Annesley T. M., Jiang N. S., Gorman C. A. Simultaneous determination of D- and L-thyroxine in human serum by liquid chromatography with electrochemical detection. J Chromatogr. 1981 Dec 11;226(2):383–390. doi: 10.1016/s0378-4347(00)86072-7. [DOI] [PubMed] [Google Scholar]
- Horiuchi R., Cheng S. Y., Willingham M., Pastan I. Inhibition of the nuclear entry of 3,3',5'-triiodo-L-thyronine by monodansylcadaverine in GH3 cells. J Biol Chem. 1982 Mar 25;257(6):3139–3144. [PubMed] [Google Scholar]
- Krenning E. P., Docter R., Bernard H. F., Visser T. J., Hennemann G. Active transport of triiodothyronine (T3) into isolated rat liver cells. FEBS Lett. 1978 Jul 1;91(1):113–116. doi: 10.1016/0014-5793(78)80029-5. [DOI] [PubMed] [Google Scholar]
- Krenning E., Docter R., Bernard B., Visser T., Hennemann G. Characteristics of active transport of thyroid hormone into rat hepatocytes. Biochim Biophys Acta. 1981 Sep 4;676(3):314–320. doi: 10.1016/0304-4165(81)90165-3. [DOI] [PubMed] [Google Scholar]
- LEIN A., DOWBEN R. M. Uptake and binding of thyroxine and triiodothyronine by rat diaphragm in vitro. Am J Physiol. 1961 May;200:1029–1031. doi: 10.1152/ajplegacy.1961.200.5.1029. [DOI] [PubMed] [Google Scholar]
- Mann D. N., Surks M. I. 5,5'-Diphenylhydantoin decreases specific 3,5,3'-triiodothyronine (T3) binding by rat hepatic nuclear T3 receptors. Endocrinology. 1983 May;112(5):1723–1731. doi: 10.1210/endo-112-5-1723. [DOI] [PubMed] [Google Scholar]
- OPPENHEIMER J. H., SQUEF R., SURKS M. I., HAUER H. BINDING OF THYROXINE BY SERUM PROTEINS EVALUATED BY EQUILIBRUM DIALYSIS AND ELECTROPHORETIC TECHNIQUES. ALTERATIONS IN NONTHYROIDAL ILLNESS. J Clin Invest. 1963 Nov;42:1769–1782. doi: 10.1172/JCI104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPPENHEIMER J. H., SURKS M. I. DETERMINATION OF FREE THYROXINE IN HUMAN SERUM: A THEORETICAL AND EXPERIMENTAL ANALYSIS. J Clin Endocrinol Metab. 1964 Aug;24:785–793. doi: 10.1210/jcem-24-8-785. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Shapiro H. C., Bernstein G., Surks M. I. Differences in primary cellular factors influencing the metabolism and distribution of 3,5,3'-L-triiodothyronine and L-thyroxine. J Clin Invest. 1970 May;49(5):1016–1024. doi: 10.1172/JCI106301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Surks M. I., Schwartz H. L. The metabolic significance of exchangeable cellular thyroxine. Recent Prog Horm Res. 1969;25:381–422. doi: 10.1016/b978-0-12-571125-8.50011-9. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H. Thyroid hormone action at the cellular level. Science. 1979 Mar 9;203(4384):971–979. doi: 10.1126/science.218285. [DOI] [PubMed] [Google Scholar]
- Rao G. S., Eckel J., Rao M. L., Breuer H. Uptake of thyroid hormone by isolated rat liver cells. Biochem Biophys Res Commun. 1976 Nov 8;73(1):98–104. doi: 10.1016/0006-291x(76)90502-7. [DOI] [PubMed] [Google Scholar]
- Rao G. S., Rao M. L., Thilmann A., Quednau H. D. Study of fluxes at low concentrations of L-tri-iodothyronine with rat liver cells and their plasma-membrane vesicles. Evidence for the accumulation of the hormone against a gradient. Biochem J. 1981 Sep 15;198(3):457–466. doi: 10.1042/bj1980457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Casanova J., Stanley F. Thyroid hormone action: in vitro characterization of solubilized nuclear receptors from rat liver and cultured GH1 cells. J Clin Invest. 1974 Oct;54(4):853–865. doi: 10.1172/JCI107825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster L. D., Schwartz H. L., Oppenheimer J. H. Nuclear receptors for 3,5,3'-triiodothyronine in human liver and kidney: characterization, quantitation, and similarities to rat receptors. J Clin Endocrinol Metab. 1979 Apr;48(4):627–632. doi: 10.1210/jcem-48-4-627. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Trence D., Oppenheimer J. H., Jiang N. S., Jump D. B. Distribution and metabolism of L- and D-triiodothyronine (T3) in the rat: preferential accumulation of L-T3 by hepatic and cardiac nuclei as a probable explanation of the differential biological potency of T3 enantiomers. Endocrinology. 1983 Oct;113(4):1236–1243. doi: 10.1210/endo-113-4-1236. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Dick T. E., Larsen P. R. The contribution of local tissue thyroxine monodeiodination to the nuclear 3,5,3'-triiodothyronine in pituitary, liver, and kidney of euthyroid rats. Endocrinology. 1978 Oct;103(4):1196–1207. doi: 10.1210/endo-103-4-1196. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Koerner D. H., Oppenheimer J. H. In vitro binding of L-triiodothyronine to receptors in rat liver nuclei. Kinectics of binding, extraction properties, and lack of requirement for cytosol proteins. J Clin Invest. 1975 Jan;55(1):50–60. doi: 10.1172/JCI107917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Oppenheimer J. H. A new radioimmunoassay for plasma L-triiodothyronine: measurements in thyroid disease and in patients maintained on hormonal replacement. J Clin Invest. 1972 Dec;51(12):3104–3113. doi: 10.1172/JCI107137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortsman J., Premachandra B. N., Williams K., Burman K. D., Hay I. D., Davis P. J. Familial resistance to thyroid hormone associated with decreased transport across the plasma membrane. Ann Intern Med. 1983 Jun;98(6):904–909. doi: 10.7326/0003-4819-98-6-904. [DOI] [PubMed] [Google Scholar]


