Abstract
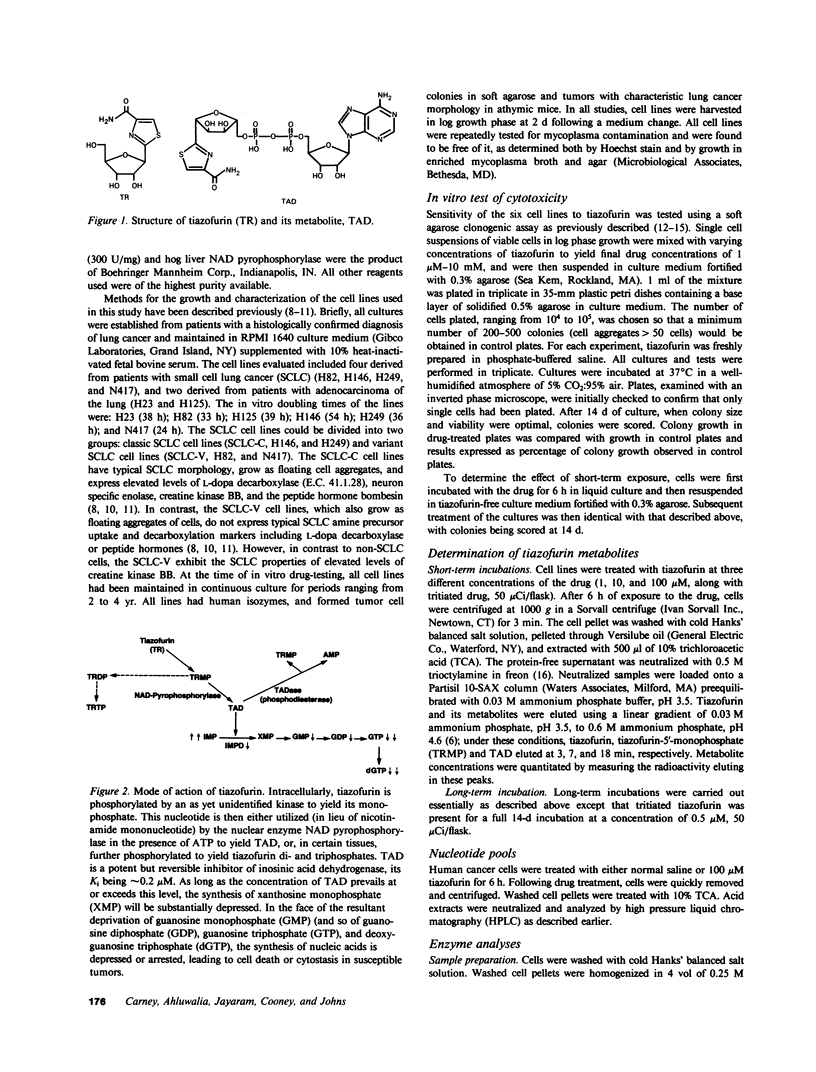
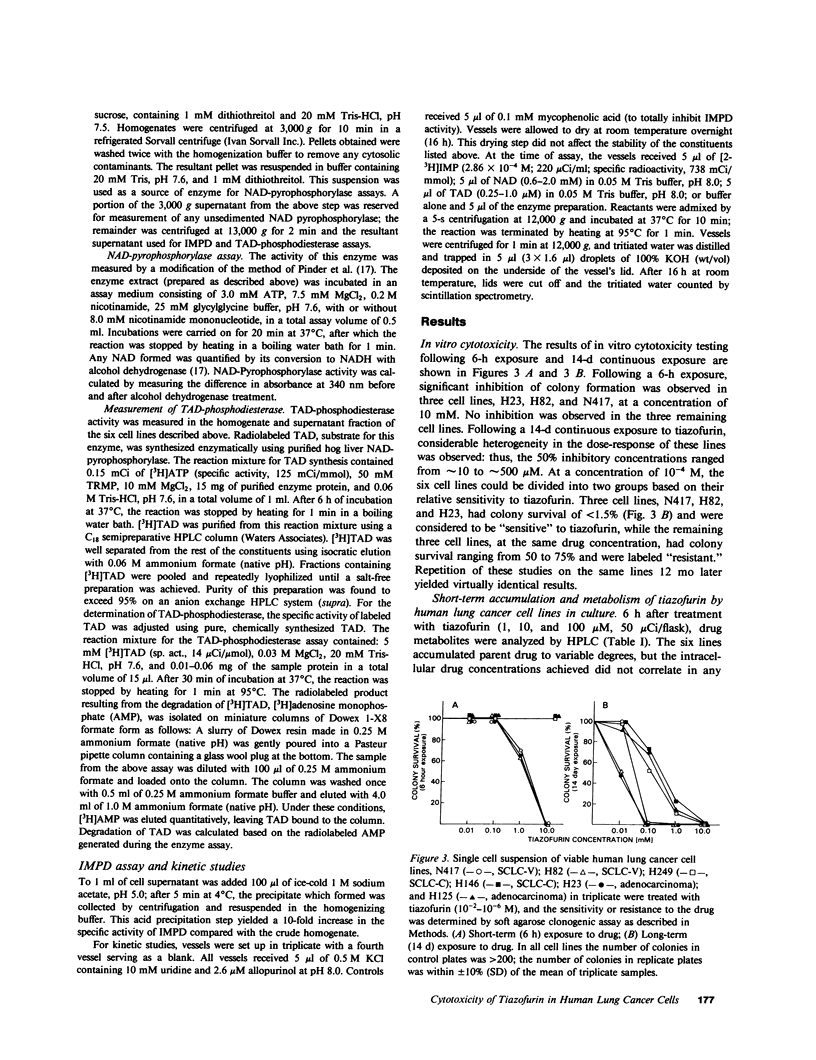
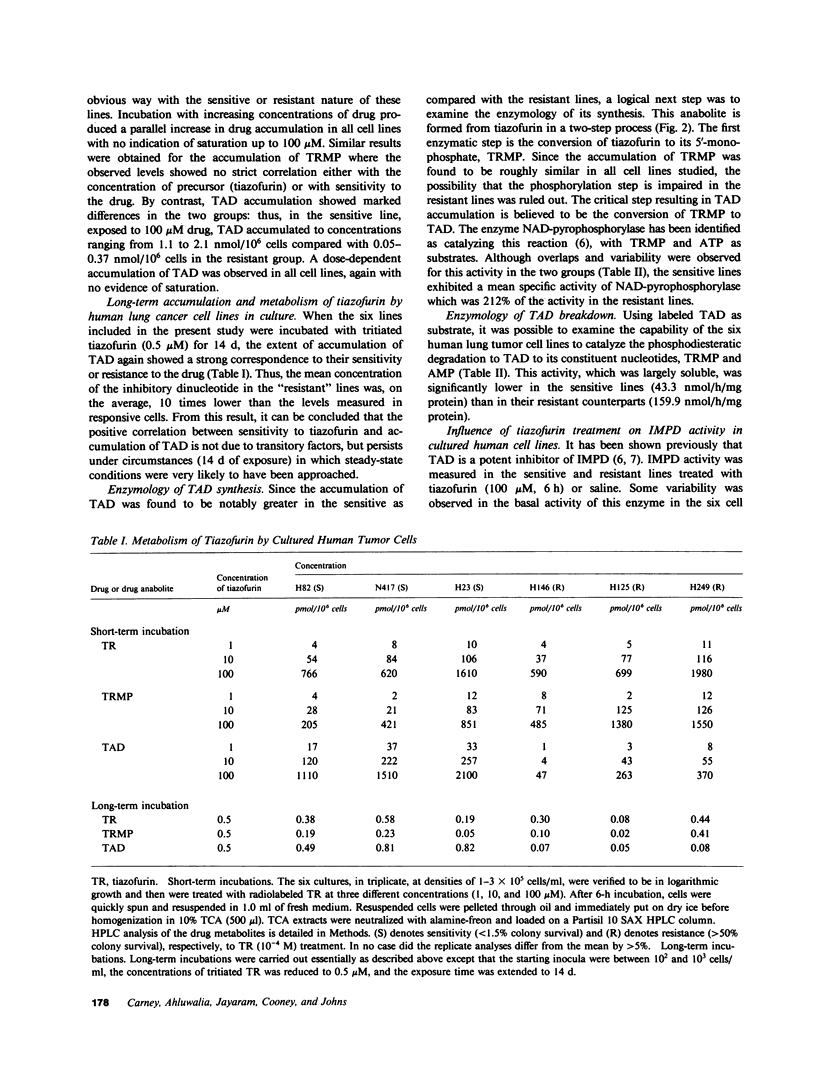
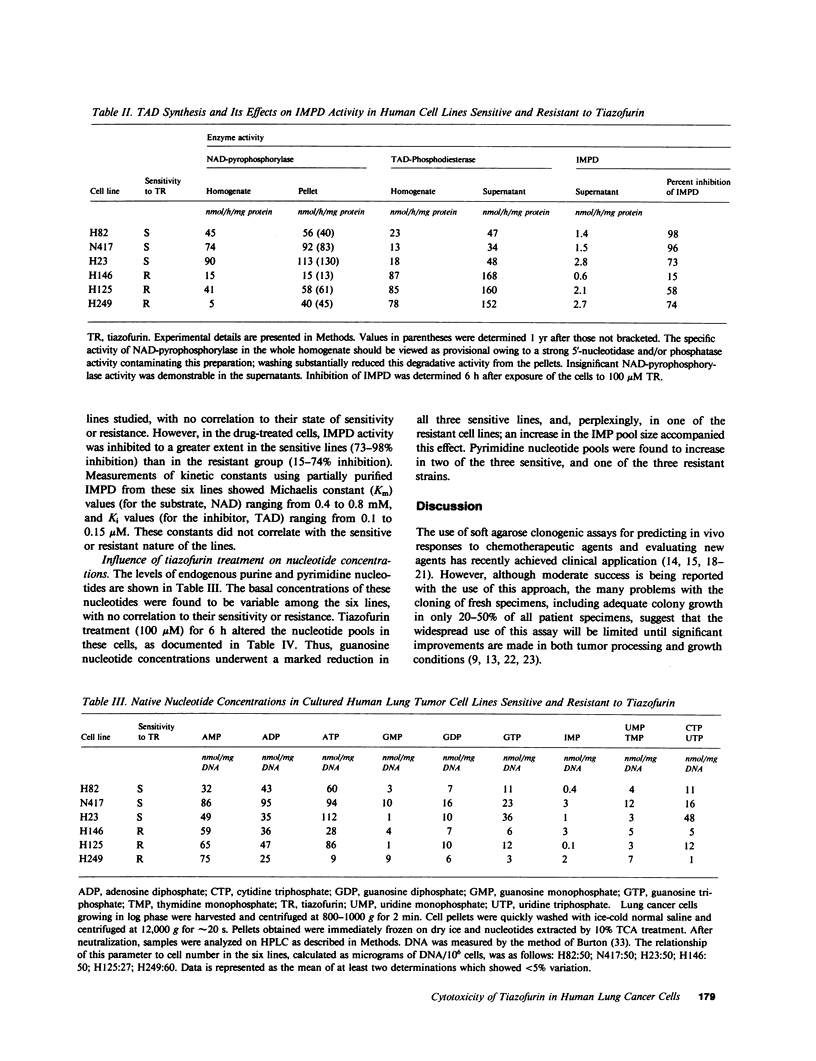
The antitumor activity of the antineoplastic agent, tiazofurin (2-beta-D-ribofuranosylthiazole-4-carboxamide), has previously been shown to require intracellular anabolism of the drug to a nicotinamide adenine dinucleotide (NAD) analog (2-beta-D-ribofuranosylthiazole-4-carboxamide adenine dinucleotide or "tiazofurin adenine dinucleotide"), which then acts as a potent inhibitor of the target enzyme inosine monophosphate (IMP) dehydrogenase. Inhibition of the latter enzyme in turn brings about a profound depletion of intracellular guanosine nucleotides essential for tumor cell growth and replication. In the present study, the cytotoxicity and metabolism of tiazofurin have been examined in six human lung cancer cell lines. At the pharmacologically attainable drug concentration of 100 microM, colony survival was less than 1.5% in three cell lines ("sensitive"), while survival in the remaining three was greater than 50% ("resistant"). The metabolism of tritiated tiazofurin was examined at concentrations ranging from 0.5 to 100 microM following both brief (6 h) and protracted (14 d) exposures. The sensitive lines accumulated concentrations of tiazofurin adenine dinucleotide that were approximately 10 times those achieved by the resistant lines at both time points. We also observed tendencies for the sensitive cell lines to exhibit: (a) higher specific activities of NAD pyrophosphorylase, the enzyme required for the synthesis of tiazofurin adenine dinucleotide, (b) significantly lower levels of a phosphodiesterase which degrades the latter dinucleotide, (c) greater inhibition of the target enzyme IMP dehydrogenase, and (d) greater depressions of guanosine nucleotide pools after drug treatment. By contrast, the basal levels of IMP dehydrogenase and purine nucleotides in these six lines did not correlate in any obvious way with their responsiveness or resistance. The accumulation and monophosphorylation of parent drug were also not prognostic variables. These studies thus suggest that the extent of accumulation of tiazofurin adenine dinucleotide, as regulated by its synthetic and degradative enzyme activities, is the single most predictive determinant of the responsiveness of cultured human lung tumor cells to tiazofurin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold S. T., Jayaram H. N., Harper G. R., Litterst C. L., Malspeis L., DeSouza J. J., Staubus A. E., Ahluwalia G. S., Wilson Y. A., Cooney D. A. The disposition and metabolism of tiazofurin in rodents, rabbits, and dogs. Drug Metab Dispos. 1984 Mar-Apr;12(2):165–173. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoncello I., Bradley T. R., Campbell J. J., Day A. J., McDonald I. A., McLeish G. R., Quinn M. A., Rome R., Hodgson G. S. Limitations of the clonal agar assay for the assessment of primary human ovarian tumour biopsies. Br J Cancer. 1982 Jun;45(6):803–811. doi: 10.1038/bjc.1982.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. N., Broder L., Edelstein M., Gazdar A. F., Hansen M., Havemann K., Matthews M. J., Sorenson G. D., Videløv L. Experimental studies of the biology of human small cell lung cancer. Cancer Treat Rep. 1983 Jan;67(1):27–35. [PubMed] [Google Scholar]
- Carney D. N., Bunn P. A., Jr, Gazdar A. F., Pagan J. A., Minna J. D. Selective growth in serum-free hormone-supplemented medium of tumor cells obtained by biopsy from patients with small cell carcinoma of the lung. Proc Natl Acad Sci U S A. 1981 May;78(5):3185–3189. doi: 10.1073/pnas.78.5.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bunn P. A., Jr, Guccion J. G. Demonstration of the stem cell nature of clonogenic tumor cells from lung cancer patients. Stem Cells. 1982;1(3):149–164. [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Minna J. D. Positive correlation between histological tumor involvement and generation of tumor cell colonies in agarose in specimens taken directly from patients with small-cell carcinoma of the lung. Cancer Res. 1980 Jun;40(6):1820–1823. [PubMed] [Google Scholar]
- Carney D. N., Mitchell J. B., Kinsella T. J. In vitro radiation and chemotherapy sensitivity of established cell lines of human small cell lung cancer and its large cell morphological variants. Cancer Res. 1983 Jun;43(6):2806–2811. [PubMed] [Google Scholar]
- Cooney D. A., Jayaram H. N., Gebeyehu G., Betts C. R., Kelley J. A., Marquez V. E., Johns D. G. The conversion of 2-beta-D-ribofuranosylthiazole-4-carboxamide to an analogue of NAD with potent IMP dehydrogenase-inhibitory properties. Biochem Pharmacol. 1982 Jun 1;31(11):2133–2136. doi: 10.1016/0006-2952(82)90436-1. [DOI] [PubMed] [Google Scholar]
- Cooney D. A., Jayaram H. N., Glazer R. I., Kelley J. A., Marquez V. E., Gebeyehu G., Van Cott A. C., Zwelling L. A., Johns D. G. Studies on the mechanism of action of tiazofurin metabolism to an analog of NAD with potent IMP dehydrogenase-inhibitory activity. Adv Enzyme Regul. 1983;21:271–303. doi: 10.1016/0065-2571(83)90019-5. [DOI] [PubMed] [Google Scholar]
- Curt G. A., Carney D. N., Cowan K. H., Jolivet J., Bailey B. D., Drake J. C., Chien Song K. S., Minna J. D., Chabner B. A. Unstable methotrexate resistance in human small-cell carcinoma associated with double minute chromosomes. N Engl J Med. 1983 Jan 27;308(4):199–202. doi: 10.1056/NEJM198301273080406. [DOI] [PubMed] [Google Scholar]
- Earle M. F., Glazer R. I. Activity and metabolism of 2-beta-D-ribofuranosylthiazole-4-carboxamide in human lymphoid tumor cells in culture. Cancer Res. 1983 Jan;43(1):133–137. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Minna J. D. The biology of non-small cell lung cancer. Semin Oncol. 1983 Mar;10(1):3–19. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Russell E. K., Sims H. L., Baylin S. B., Bunn P. A., Jr, Guccion J. G., Minna J. D. Establishment of continuous, clonable cultures of small-cell carcinoma of lung which have amine precursor uptake and decarboxylation cell properties. Cancer Res. 1980 Oct;40(10):3502–3507. [PubMed] [Google Scholar]
- Jayaram H. N., Dion R. L., Glazer R. I., Johns D. G., Robins R. K., Srivastava P. C., Cooney D. A. Initial studies on the mechanism of action of a new oncolytic thiazole nucleoside, 2-beta-D-ribofuranosylthiazole-4-carboxamide (NSC 286193). Biochem Pharmacol. 1982 Jul 15;31(14):2371–2380. doi: 10.1016/0006-2952(82)90532-9. [DOI] [PubMed] [Google Scholar]
- Jayaram H. N., Smith A. L., Glazer R. I., Johns D. G., Cooney D. A. Studies on the mechanism of action of 2-beta-D-ribofuranosylthiazole-4-carboxamide (NSC 286193)--II. Relationship between dose level and biochemical effects in P388 leukemia in vivo. Biochem Pharmacol. 1982 Dec 1;31(23):3839–3845. doi: 10.1016/0006-2952(82)90300-8. [DOI] [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- Kornblith P. L., Smith B. H., Leonard L. A. Response of cultured human brain tumors to nitrosoureas: correlation with clinical data. Cancer. 1981 Jan 15;47(2):255–265. doi: 10.1002/1097-0142(19810115)47:2<255::aid-cncr2820470209>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Little C. D., Nau M. M., Carney D. N., Gazdar A. F., Minna J. D. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983 Nov 10;306(5939):194–196. doi: 10.1038/306194a0. [DOI] [PubMed] [Google Scholar]
- Ozols R. F., Willson J. K., Weltz M. D., Grotzinger K. R., Myers C. E., Young R. C. Inhibition of human ovarian cancer colony formation by adriamycin and its major metabolites. Cancer Res. 1980 Nov;40(11):4109–4112. [PubMed] [Google Scholar]
- Radice P. A., Matthews M. J., Ihde D. C., Gazdar A. F., Carney D. N., Bunn P. A., Cohen M. H., Fossieck B. E., Makuch R. W., Minna J. D. The clinical behavior of "mixed" small cell/large cell bronchogenic carcinoma compared to "pure" small cell subtypes. Cancer. 1982 Dec 15;50(12):2894–2902. doi: 10.1002/1097-0142(19821215)50:12<2894::aid-cncr2820501232>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Robins R. K., Srivastava P. C., Narayanan V. L., Plowman J., Paull K. D. 2-beta-D-Ribofuranosylthiazole-4-carboxamide, a novel potential antitumor agent for lung tumors and metastases. J Med Chem. 1982 Feb;25(2):107–108. doi: 10.1021/jm00344a002. [DOI] [PubMed] [Google Scholar]
- Rupniak H. T., Hill B. T. The poor cloning ability in agar of human tumour cells from biopsies of primary tumours. Cell Biol Int Rep. 1980 May;4(5):479–486. doi: 10.1016/0309-1651(80)90035-1. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Hamburger A. W., Soehnlen B., Durie B. G., Alberts D. S., Moon T. E. Quantitation of differential sensitivity of human-tumor stem cells to anticancer drugs. N Engl J Med. 1978 Jun 15;298(24):1321–1327. doi: 10.1056/NEJM197806152982401. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Meyskens F. L., Jr, Alberts D. S., Soehnlen B., Young L. New drugs in ovarian cancer and malignant melanoma: in vitro phase II screening with the human tumor stem cell assay. Cancer Treat Rep. 1981 Jan-Feb;65(1-2):1–12. [PubMed] [Google Scholar]
- Siegel M. M., Chung H. S., Rucker N., Siegel S. E., Seeger R. C., Isaacs H., Jr, Benedict W. F. In vitro and in vivo preclinical chemotherapy studies of human neuroblastoma. Cancer Treat Rep. 1980 Aug-Sep;64(8-9):975–979. [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Pihl A. The usefulness of human tumor cell lines in the study of chemosensitivity. A study of malignant melanomas. Int J Cancer. 1981 Oct 15;28(4):403–408. doi: 10.1002/ijc.2910280402. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Casper J., Bradley E., Sandbach J., Jones D., Makuch R. Association between human tumor colony-forming assay results and response of an individual patient's tumor to chemotherapy. Am J Med. 1981 May;70(5):1027–1041. doi: 10.1016/0002-9343(81)90859-7. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Coltman C. A., Jr, Forseth B. Activity of mitoxantrone in a human tumor cloning system. Cancer Res. 1981 May;41(5):1853–1855. [PubMed] [Google Scholar]
- Von Hoff D. D., Gutterman J., Portnoy B., Coltman C. A., Jr Activity of human leukocyte interferon in a human tumor cloning system. Cancer Chemother Pharmacol. 1982;8(1):99–103. doi: 10.1007/BF00292879. [DOI] [PubMed] [Google Scholar]
- Witkowski J. T., Robins R. K., Sidwell R. W., Simon L. N. Design, synthesis, and broad spectrum antiviral activity of 1- -D-ribofuranosyl-1,2,4-triazole-3-carboxamide and related nucleosides. J Med Chem. 1972 Nov;15(11):1150–1154. doi: 10.1021/jm00281a014. [DOI] [PubMed] [Google Scholar]


